Introduction
Endometrial cancer is one of the most common
gynecological cancers and its incidence has increased worldwide in
recent years (1). Although low-risk
and early stage endometrial cancer patients have a favorable
prognosis, there are few effective chemotherapy options for
high-risk patients. Although Pembrolizumab (a PD-1 inhibitor) was
approved for use in patients with microsatellite instability-high
tumors, which are often seen in endometrial cancer, no molecular
targeted therapies for endometrial cancer have been approved to
date (2,3). Thus, it is important to diagnose
endometrial cancer at an early stage. Dilation and curettage
(D&C) is a common diagnostic procedure. Although almost all
institutions perform D&C for examination of endometrial cancer
(4), there is a weakness about using
D&C for the diagnosis because this blind procedure might miss
endometrial cancer (5). Therefore,
this procedure has a high rate of false negatives. For example, Liu
et al showed that failure rates with D&C were 7.75%
(6). In contrast, hysteroscopy is
generally the common standard procedure for examining endometrial
lesions, evaluating the uterine cavity directly, and performing the
biopsy. In addition, office hysteroscopy is a minimally invasive
procedure and easier to schedule than hysteroscopic biopsy in
hospitalization. Recently, some reports indicated that hysteroscopy
is a useful procedure for diagnosing endometrial cancer (3)
Here, we report the case of a patient with suspected
endometrial cancer; however, it could not be diagnosed via D&C
and hysteroscopic biopsy during hospitalization, and she was
diagnosed with the condition via office hysteroscopic biopsy.
Case report
The patient was a 40-year-old woman with no history
of pregnancy or delivery. Her menstruation is irregular. She
visited the local clinic for hypermenorrhea. Endometrial polyp was
detected by transvaginal echo and transcervical resection was
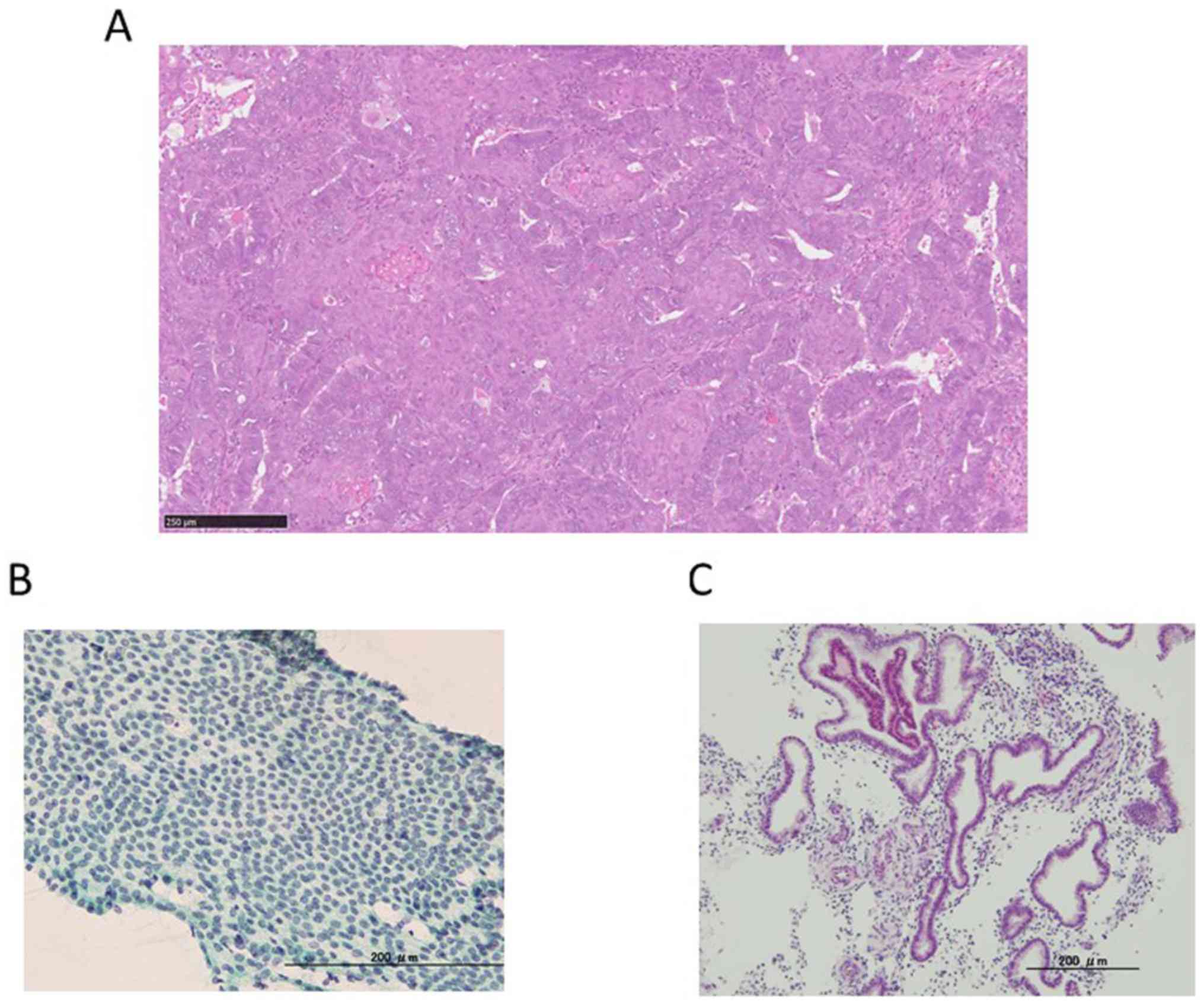
performed. Pathological finding was atypical glands of uterine
corpus. Atypical glands might suggest atypical endometrioid
hyperplasia complex or endometrioid adenocarcinoma (Fig. 1A). It is necessary to rule out
atypical polypoid adenomyoma. She visited our hospital for further
examination. During gynecological examination, abnormal findings
were not detected. Endometrial thickness was determined via
ultrasound. Blood test, cytological and histological examination
did not reveal abnormal findings (Fig.
1B and C).
Additionally,pathological examination was not abnormal in our
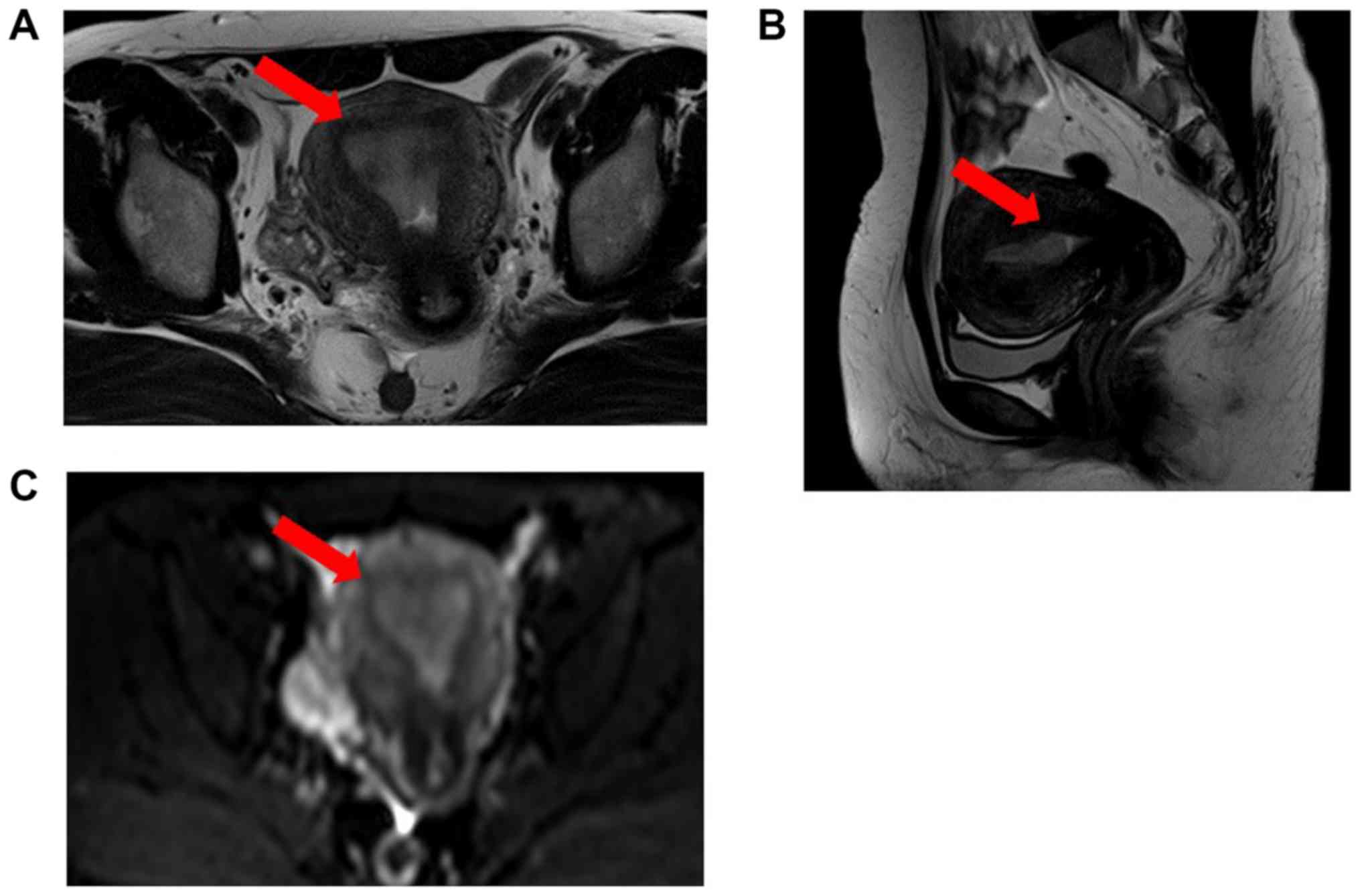
hospital. Magnetic resonance imaging (MRI) was performed in our
hospital, and it showed endometrial thickening (10 mm) (Fig. 2). There was no evidence of invasion
to the wall of the uterus. On computed tomography scan, there were
no metastatic lesions or enlarged lymph nodes. Therefore, we
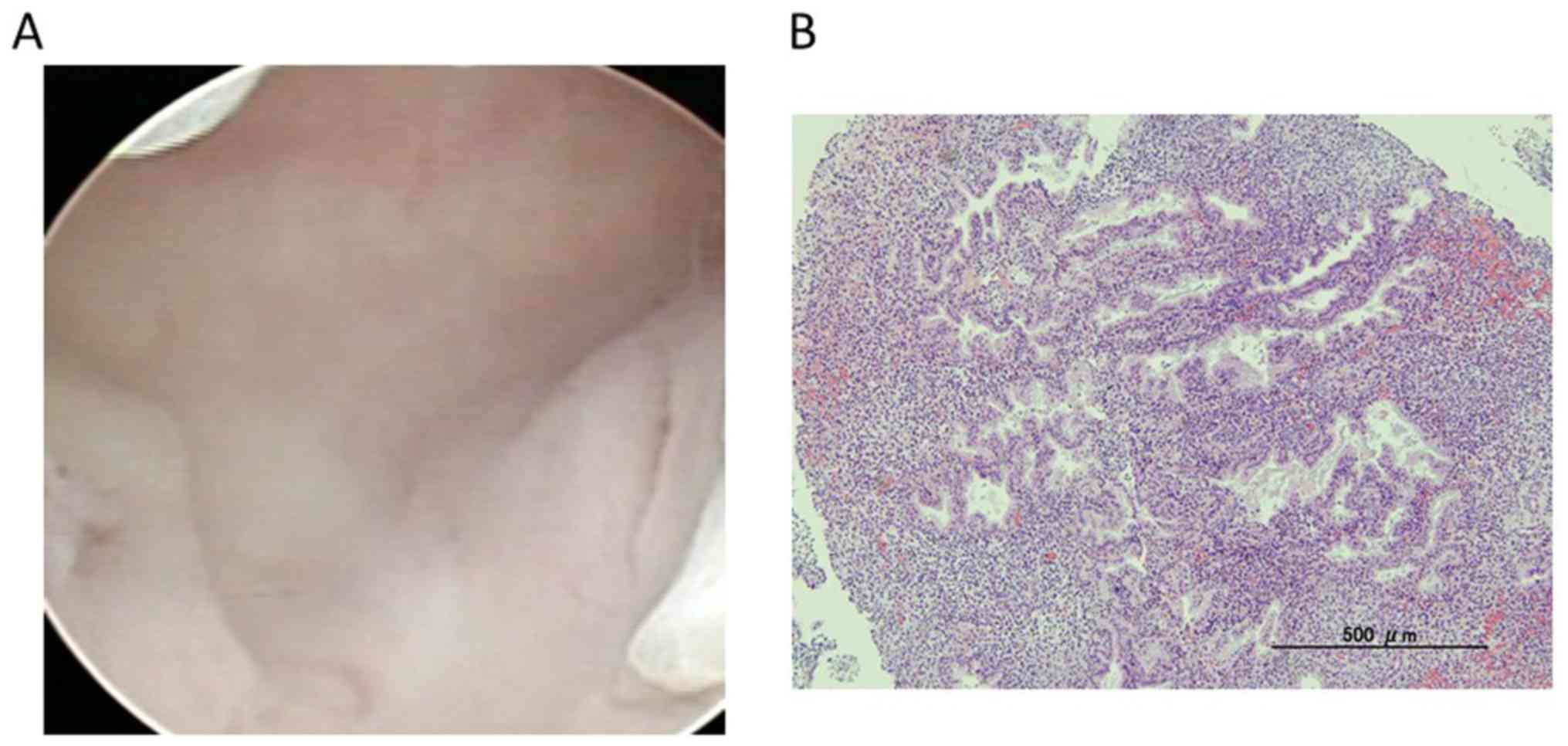
performed hysteroscopic biopsy and D&C under anesthesia after
admission. However, there were no hysteroscopic findings, and
pathological findings showed the endometrium to be in secretory
phase without malignancy (Fig. 3).
Although we followed up the patient to perform cytological
examination of the endometrium in outpatient clinic after the
operation, we did not detect abnormal findings. We planned to
perform hysteroscopic biopsy in the outpatient clinic 5 months
after the first hysteroscopy since a previous transcervical
resection biopsy performed at another hospital found atypical
glands in the uterine corpus, which are suggestive of complex
atypical endometrial hyperplasia or endometrioid adenocarcinoma. We
scheduled the patient to come to our hospital in the proliferative
phase. We used TROPHYscope® CAMPO (KARL STORZ) as a
hysteroscope. At the start of the examination, the primary access
into the uterine cavity was performed with an outer diameter of 2.9
mm with the corresponding sheath in the passive position. The
hysteroscope was introduced into the uterine cavity through this
sheath. For endometrial biopsy, an extra operating sheath with a
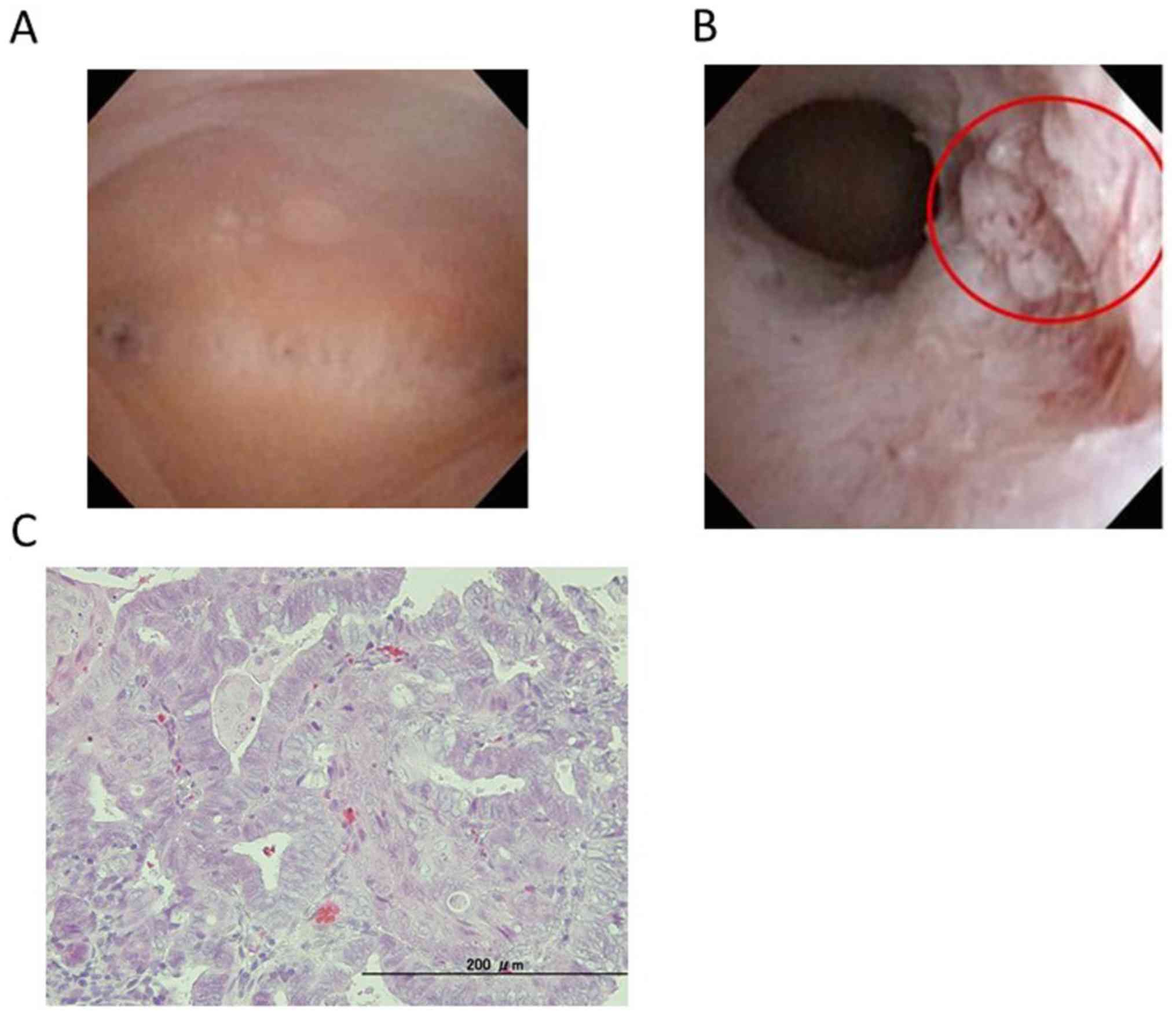
working channel for 5 Fr. instruments was used (7). Office hysteroscopy showed that the
suspicious endometrial cancerous lesion was minimal at the isthmus
of the uterus with atypical vessels and a white spot, for which
biopsy was performed twice (Fig. 4A
and B). The pathological finding was
endometrioid adenocarcinoma, G1 (Fig.
4C). Our department usually performs pelvic lymphadenectomy in
low-risk patients for precise staging according to the guidelines
of the Japan Society of Gynecologic Oncology. Therefore, total
laparoscopic hysterectomy, bilateral salpingo-oophorectomy, and
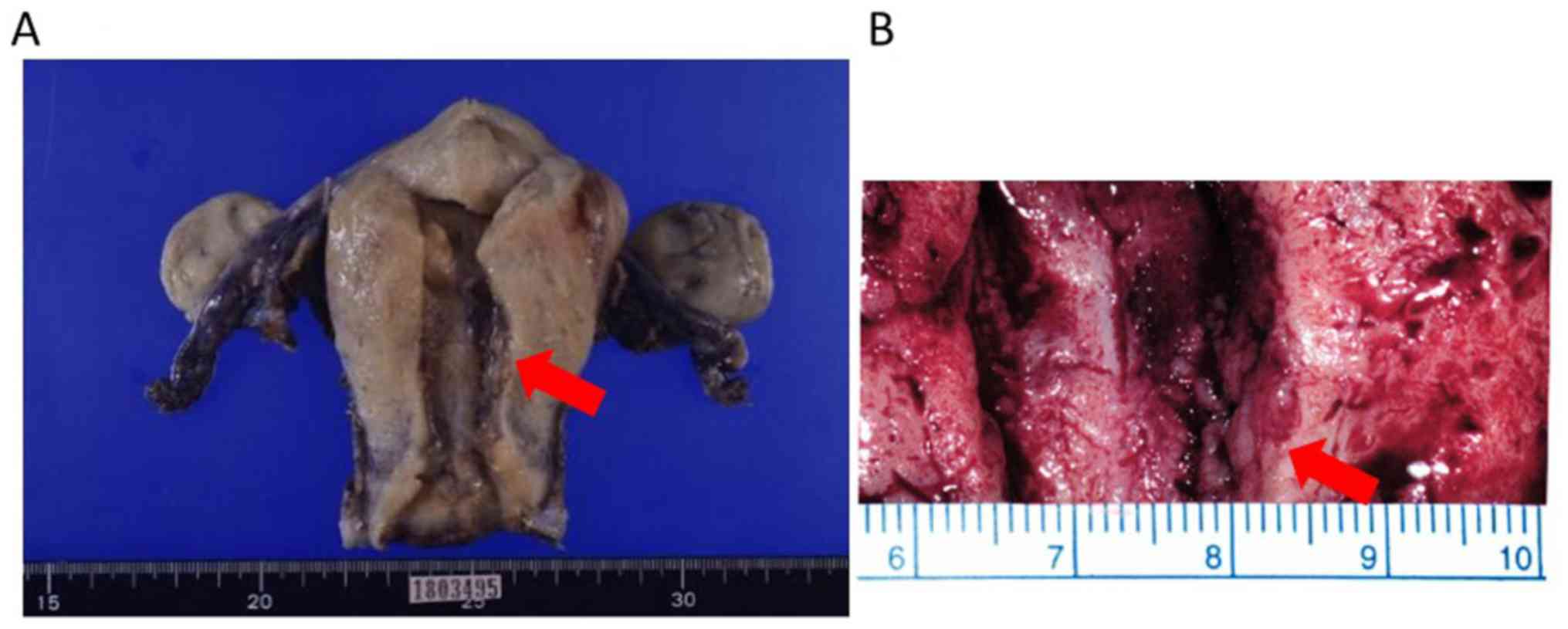
pelvic lymphadenectomy were performed. Pathological diagnosis was
endometrioid carcinoma with squamous differentiation, G1, 9x7x6 mm,
pT1a, ly(-), v(-), LN(0/39) (Fig.
5). The patient is making steady progress two years after
surgery.
Discussion
We report the case of a 40-year-old woman with
suspected endometrial cancer; however, it could not be diagnosed by
D&C and biopsy using hysteroscopy in hospitalization. The
patient was diagnosed with endometrial cancer via office
hysteroscopic biopsy. Increasing tumor size is one of the reasons
that enable office hysteroscopy to detect endometrial cancer. In
this case, we believe that small polyps suggestive of atypical
hyperplasia and endometrial cancer that was revealed in the
proliferative phase were the main reason for outpatient
hysteroscopy. Lately, the number of patients with endometrial
cancer is increasing in developed countries. There are a few
methods for the treatment of advanced endometrial cancer (8). It is necessary to diagnose endometrial
cancer early to ensure good prognosis. Although endometrial cancer
with tumor formation can be easily diagnosed, it is difficult to
diagnose it when it is in the early stages. Therefore, the negative
predictive value of normal endometrial biopsy is low at 51%.
Approximately 3% of endometrial cancer is missed because of blinded
D&C (9). Some gynecologists used
several types of hysteroscopic features of endometrial lesions for
diagnosing malignancy, including irregularity of endometrial
glands, polypoid pattern, uneven surface, and abnormal endometrial
vessels (5). Additionally, some
reports suggested that normal hysteroscopic findings do not prove
the absence of endometrial lesion. It is necessary to perform
endometrial biopsy when there is increased endometrial thickness,
with abnormal bleeding (5). The use
of blind sampling of endometrium, such as is done via D&C, is
not an accurate method for diagnosing endometrial cancer. We surely
can biopsy the malignant endometrial lesion with the use of
hysteroscopy with directed biopsy (7). However, we did not detect endometrial
lesion in the first hysteroscopy because of endometrial thickness
in the secretory phase. Therefore, we performed office hysteroscopy
and succeeded in biopsy of malignant endometrial lesion. One of the
important advantages of office hysteroscopy is that this procedure
does not need hospitalization and anesthesia. The patients can
avoid from discomfort of going into the operating room. Therefore,
this procedure is considered as minimally invasive and highly
tolerable by the patients while still sufficiently accurate for
diagnostic purposes. These factors explain why patients tend to
prefer the diagnosis of endometrial cancer by office hysteroscopy.
Another important point is that it is easier to schedule office
hysteroscopy than hysteroscopy during hospitalization. In high
volume hospitals such as ours, it is difficult to schedule surgical
procedures when compared to a local hospital. We consider the ease
of examination an important advantage of office hysteroscopy. In
this case, it was difficult to detect minimal lesion in the
secretory phase because the endometrial thickness hid the
endometrial cancer. It is easy to schedule office surgery in the
proliferative phase even if her menstruation is irregular. There
are issues with diagnosing endometrial cancer using hysteroscopy. A
previous report indicated that hysteroscopy could increase the
probability of positive peritoneal cytology (10). However, hysteroscopy does not induce
the risk of ovarian or abdominal metastasis. This result suggested
that the iatrogenic peritoneal spillage cannot induce metastasis
(11,12). In addition, there is the possibility
that intracavitary pressure by hysteroscopic fluid induces
iatrogenic peritoneal spillage of endometrial cells. No spillage
has been reported to occur at pressures <70 mmHg (13). Another examination method for
endometrial cancer is transvaginal ultrasonography. Berretta et
al suggested that transvaginal ultrasonography is a useful
method for assessing myometrial invasion by endometrial cancer
(14). Other reports suggested that
tumor size is a useful marker for the surgical staging of
endometrial cancer (15).
Taken together, we suggest that office hysteroscopy
with directed biopsy is useful for the diagnosis of minimal
endometrial cancer.
Acknowledgements
No applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS prepared the manuscript and figures. KS, SE, KA,
KO, YO, TF and OHW provided medical care for the patients and
collected the data. KS, YMi and OHW performed the operation. KS,
SE, KA, FI, YMi, MT, TT and MMU participated in the conception and
design of the case report. YMa, OHW, KO, YO, and TF revised the
manuscript for intellectual content. FI also retrieved the
pathology images. KO, YO, and TF supervised the entire project. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient in this case. The study design was approved by the Ethics
Committee at The University of Tokyo.
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson AS, Key TJ, Norat T, Scoccianti
C, Cecchini M, Berrino F, Boutron-Ruault MC, Espina C, Leitzmann M,
Powers H, et al: European code against cancer 4th edition: Obesity,
body fatness and cancer. Cancer Epidemiol. 39:S34–S45.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lachance JA, Darus CJ and Rice LW:
Surgical management and postoperative treatment of endometrial
carcinoma. Rev Obstet Gynecol. 1:97–105. 2008.PubMed/NCBI
|
|
3
|
Ott PA, Bang YJ, Berton-Rigaud D, Elez E,
Pishvaian MJ, Rugo HS, Puzanov I, Mehnert JM, Aung KL, Lopez J, et
al: Safety and antitumor activity of pembrolizumab in advanced
programmed death ligand 1-positive endometrial cancer: Results from
the KEYNOTE- 028 Study. J Clin Oncol. 35:2535–2541. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang B, Xu Y, Zhu Q, Xie L, Shan W, Ning
C, Xie B, Shi Y, Luo X, Zhang H and Chen X: Treatment efficiency of
comprehensive hysteroscopic evaluation and lesion resection
combined with progestin therapy in young women with endometrial
atypical hyperplasia and endometrial cancer. Gynecol Oncol.
153:55–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Trojano G, Damiani GR, Casavola VC,
Loiacono R, Malvasi A, Pellegrino A, Siciliano V, Cicinelli E,
Salerno MG and Battini L: The role of hysteroscopy in evaluating
postmenopausal asymptomatic women with thickened endometrium.
Gynecol Minim Invasive Ther. 7:6–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu H, Wang FL, Zhao YM, Yao YQ and Li YL:
Comparison of Pipelle sampler with conventional dilatation and
curettage (D&C) for Chinese endometrial biopsy. J Obstet
Gynaecol. 35:508–511. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De Wilde RL: Office Hysteroscopy:
TROPHYscope CAMPO compact hysteroscope®: Manufacturer:
KARL STORZ, Tuttlingen, Germany. J Obstet Gynaecol India.
64:301–303. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Thigpen JT, Brady MF, Homesley HD,
Malfetano J, DuBeshter B, Burger RA and Liao S: Phase III trial of
doxorubicin with or without cisplatin in advanced endometrial
carcinoma: A gynecologic oncology group study. J Clin Oncol.
22:3902–3908. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yen CF, Chou HH, Wu HM, Lee CL and Chang
TC: Effectiveness and appropriateness in the application of office
hysteroscopy. J Formos Med Assoc. 118:1480–1487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dovnik A, Crnobrnja B, Zegura B, Takac I
and Pakiz M: Incidence of positive peritoneal cytology in patients
with endometrial carcinoma after hysteroscopy vs. dilatation and
curettage. Radiol Oncol. 51:88–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ben-Arie A, Tamir S, Dubnik S, Gemer O,
Ben Shushan A, Dgani R, Peer G, Barnett-Griness O and Lavie O: Does
hysteroscopy affect prognosis in apparent early-stage endometrial
cancer? Int J Gynecol Cancer. 18:813–819. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen J, Clark LH, Kong WM, Yan Z, Han C,
Zhao H, Liu TT, Zhang TQ, Song D, Jiao SM and Zhou C: Does
hysteroscopy worsen prognosis in women with type II endometrial
carcinoma? PLoS One. 12(e0174226)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baker VL and Adamson GD: Threshold
intrauterine perfusion pressures for intraperitoneal spill during
hydrotubation and correlation with tubal adhesive disease. Fertil
Steril. 64:1066–1069. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Berretta R, Merisio C, Piantelli G, Rolla
M, Giordano G, Melpignano M and Nardelli GB: Preoperative
transvaginal ultrasonography and intraoperative gross examination
for assessing myometrial invasion by endometrial cancer. J
Ultrasound Med. 27:349–355. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Berretta R, Patrelli TS, Migliavacca C,
Rolla M, Franchi L, Monica M, Modena AB and Gizzo S: Assessment of
tumor size as a useful marker for the surgical stagingof
endometrial cancer. Oncol Rep. 31:2407–2412. 2014.PubMed/NCBI View Article : Google Scholar
|