Introduction
Breast cancer with distant metastasis at first
presentation (stage IV disease) is often encountered in the
outpatient setting. With the recent advances in multimodal
therapies for breast cancer, long-term survival may be expected,
even in stage IV breast cancer with distant metastasis (1). However, one of the goals in treating
metastatic disease is prolongation of survival while maintaining
good quality of life (QOL). Endocrine therapy is suitable for this
purpose. Adverse events are less severe with endocrine therapy
compared with those with chemotherapy; therefore, due to its
ability to maintain QOL, endocrine therapy is the preferred
first-line treatment for non-life-threatening hormone
receptor-positive advanced breast cancer (Hortobagyi's algorithm)
(2). However, although endocrine
therapy is useful for certain types of hormone receptor-positive
breast cancer (HRBC), there are other types for which it is not
very effective. In neoadjuvant endocrine therapy for early-stage
breast cancer, the use of the preoperative endocrine prognostic
index score and Ki67 as predictive markers has been reported
(3,4). However, few studies to date have
investigated the prediction of the therapeutic effect of endocrine
therapy in stage IV breast cancer. Stage IV breast cancer is
advanced and, if it cannot be controlled with initial drug therapy,
it is life-threatening. Therefore, the ability to predict
therapeutic efficacy would be of considerable value for selecting
the initial drug therapy (endocrine therapy or chemotherapy).
Cancer cells harbor various gene abnormalities that
allow them to proliferate spontaneously and survive, but they are
also affected by the surrounding environment, which is involved in
the intrinsic characteristics of cancer (5). The tumor immune environment not only
modulates the effects of immunotherapy, but also the effects of
other anticancer drugs and treatment outcomes (6,7). Thus,
the importance of inhibiting and improving the tumor immune
microenvironment is well-recognized. These immune responses may be
evaluated by tumor-infiltrating lymphocytes (TILs), which has been
demonstrated clinically (8-10).
We also previously reported the prediction of the therapeutic
efficacy of neoadjuvant chemotherapy (NAC) by TILs (11). The aim of the present study was to
clinically verify the prediction of the therapeutic efficacy of
endocrine therapy for stage IV breast cancer using TILs.
Patients and methods
Patient background
The same methods as those used in the present study
were previously used to investigate the significance of NAC for
early breast cancer (11-14).
Data from 40 patients who underwent endocrine therapy as the
initial drug therapy for stage IV breast cancer at Osaka City
University Hospital between June 2004 and December 2013 were used.
The median follow-up time was 155 weeks (range, 13-553 weeks). The
overall response rate (ORR), clinical benefit rate (CBR), disease
control rate (DCR), overall survival (OS), time to treatment
failure (TTF) and progression-free survival (PFS) were calculated
to determine the efficacy of this regimen. Additionally, tumor
stage and T and N factors were stratified based on the TNM
Classification of Malignant Tumors, Union for International Cancer
Control (UICC), 7th edition (15).
Breast cancer was histologically confirmed by core needle biopsy
and staged by systemic imaging studies using computed tomography
(CT), ultrasonography (US) and bone scintigraphy. Breast cancer was
classified into subtypes according to the immunohistochemical
expression of estrogen receptors (ERs), progesterone receptors
(PgRs), human epidermal growth factor receptor (HER)2 and Ki67.
Based on their immunohistochemical expression, the tumors were
categorized into the following immunophenotypes: Luminal A
(ER+ and/or PgR+, HER2-,
Ki67-low), luminal B (ER+ and/or PgR+,
HER2+; or ER+ and/or PgR+,
HER2-, Ki67-high), HER2-enriched (HER2BC;
ER-, PgR-, HER2+) and
triple-negative breast cancer (TNBC; ER-,
PgR-, HER2-) (16). In the present study, luminal A and B
were considered as HRBC.
Endocrine therapy was administered on an outpatient
basis in all cases. This protocol was repeated until progressive
disease (PD) was detected or a severe adverse event requiring the
discontinuation of the scheduled endocrine therapy was reported.
The therapeutic antitumor effects were assessed according to the
Response Evaluation Criteria in Solid Tumors (17). All clinical analyses in the present
study were based on imaging assessment.
TTF was evaluated on a daily basis and was defined
as the period from the date of treatment initiation to
discontinuation for any reason, including disease progression,
treatment toxicity and death. OS was evaluated on a weekly basis
and was defined as the period from the date of treatment initiation
to the date of death from any cause. PFS was evaluated on a weekly
basis and was defined as the period from the date of treatment
initiation to the date of death or the date of confirmation of
PD.
Histopathological evaluation of
TILs
Histopathological assessment for predictive factors
was performed using core needle biopsy specimens at the time of
breast cancer diagnosis. The histopathological parameters examined
included nuclear grade, histological type, presence of TILs, and
correlation of these parameters with intrinsic subtypes and
pathological complete response. Histopathological analysis of the
percentage of TILs was evaluated on a single full-face hematoxylin
and eosin (HE)-stained tumor section using the criteria described
by Salgado et al (18). TILs
were defined as the infiltrating lymphocytes within the tumor
stroma and were expressed as a proportion of the field
investigated; additionally, the number of TILs in the stroma
surrounding the stained cancer cells was quantitatively measured in
each field at a magnification, x400 (19,20).
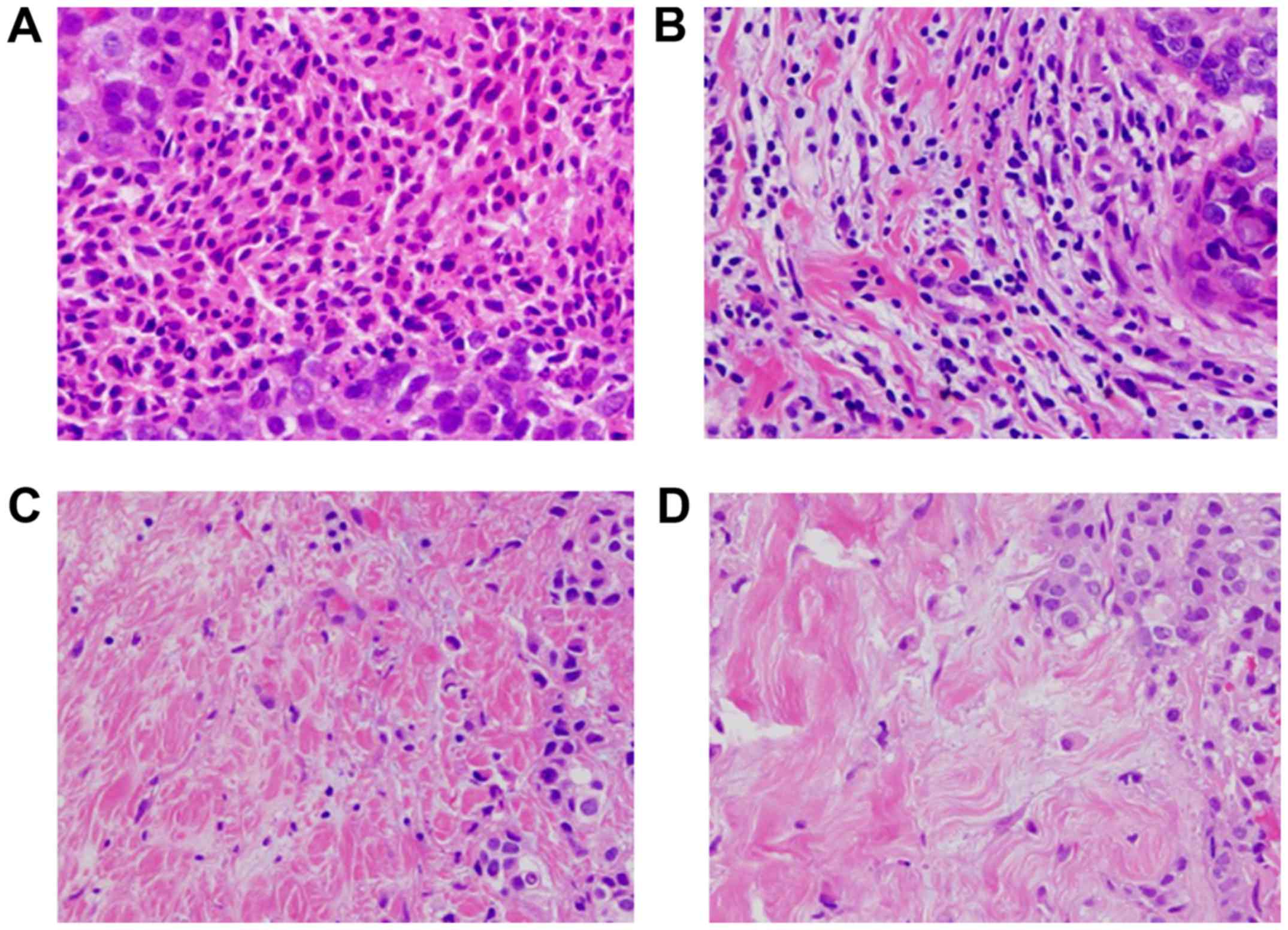
Areas of carcinoma in situ and crush artifacts were not
included. Proportional scores were defined as 3, 2, 1 and 0 if the
area of stroma with lymphoplasmacytic infiltration around invasive
tumor cell nests was >50, 10-50, ≤10% and absent, respectively
(Fig. 1). TILs were considered
‘high’ for scores ≥2, and ‘low’ for scores 1 and 0. Patients with
≥50% lymphocytic infiltration were considered to have
lymphocyte-predominant breast cancer (LPBC) (21). Histopathological evaluation of TILs
was jointly performed by two breast pathologists who were blinded
to clinical information, including treatment allocation and
outcomes.
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 (SPSS Inc.). The associations between TILs, LPBC and
clinicopathological variables were analyzed using Chi-squared or
Fisher's exact tests, as appropriate. OS, TTF and PFS were
estimated using the Kaplan-Meier method and compared using log-rank
tests. Univariate and multivariate hazard ratios (HRs) were
computed for the study parameters with 95% confidence intervals
(CIs) using a Cox proportional hazards model, and used in a
backward stepwise method for variable selection in multivariate
analyses. P<0.05 was considered to indicate statistically
significant differences.
Ethics approval
The present study was conducted at Osaka City
University Graduate School of Medicine (Osaka, Japan), according to
the Reporting Recommendations for Tumour Marker Prognostic Studies
(REMARK) guidelines and following a retrospectively written
research, pathological evaluation, and statistical plan (22). The research protocol conformed to the
provisions of the Declaration of Helsinki as revised in 2013. All
patients were informed of the investigational nature of the study
and provided written informed consent. The study protocol was
approved by the Ethics Committee of Osaka City University (approval
no. 926).
Results
Endocrine therapy in stage IV breast
cancer
The background characteristics of 40 patients who
underwent endocrine therapy as the initial drug therapy for stage
IV breast cancer are shown in Table
I. All patients were women with a median age of 64 years
(range, 44-88 years); the bone or soft tissue was the site of
metastasis in 21 patients (52.5%), and visceral metastasis was
present in 19 patients (47.5%). A total of 33 patients (82.5%) were
strongly positive and 7 (17.5%) were weakly positive for ER
expression; a total of 14 patients (35.0%) were strongly positive
and 26 (65.0%) were weakly positive for PgR expression; a total of
4 patients (10.0%) were positive and 36 (90.0%) were negative for
HER2 expression; as regards Ki67 expression, 7 patients (17.5%)
exhibited high levels and 33 patients (82.5%) exhibited low levels.
Endocrine therapy consisted of letrozole in 22 patients (55.0%),
anastrozole in 11 patients (27.5%), tamoxifen ± luteinizing
hormone-releasing hormone agonist in 6 patients (15.0%), and
exemestane in 1 patient (2.5%). As regards tumor response, the ORR
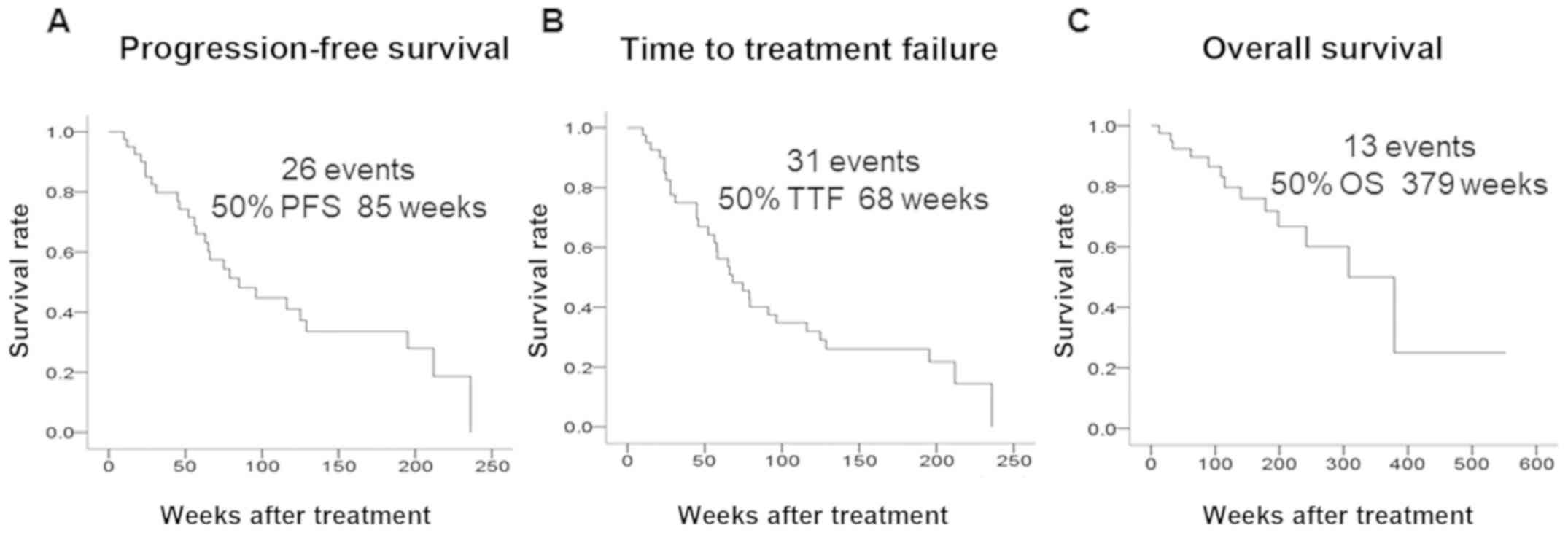
was 75.0%, the CBR was 85.0%, and the DCR was 90.0%. The median PFS
was 85 weeks, the median TTF was 68 weeks, and the median OS was
379 weeks (Fig. 2).
 | Table IDemographic data of 40 patients
receiving endocrine therapy for stage IV breast cancer. |
Table I
Demographic data of 40 patients
receiving endocrine therapy for stage IV breast cancer.
| Parameters
(n=40) | Patient no. (%) |
|---|
| Age (years) | 64 (range,
44-88) |
| Menopause |
|
Post-/premenopausal | 34 (85.0)/6
(15.0) |
| Degree of progression
(metastasis) |
|
Bone or soft
tissue/visceral | 21 (52.5)/19
(47.5) |
| Estrogen
receptor |
|
Strongly
positive/weakly positive | 33 (82.5)/7
(17.5) |
| Progesterone
receptor |
|
Strongly
positive/weakly positive | 14 (35.0)/26
(65.0) |
| HER2 |
|
Negative/positive | 36 (90.0)/4
(10.0) |
| Ki67 |
|
≤14/>14% | 33 (82.5)/7
(17.5) |
| Treatment |
|
Letrozole/anastrozole/tamoxifen
± LHRH agonist/exemestane | 22 (55.0)/11 (27.5)/6
(15.0)/1 (2.5) |
| Clinical
response |
|
CR/PR/SD ≥24
weeks/SD <24 weeks/PD | 0 (0.0)/30 (75.0)/4
(10.0)/2 (5.0)/4 (10.0) |
| Clinical
response |
|
ORR/CBR/DCR | 30 (75.0)/30 (85.0)/4
(90.0) |
Prediction of therapeutic effect using
TILs
Among the 40 patients, TIL levels were high
(>10%) in 13 (32.5%) and low (≤10%) in 27 (67.5%) patients. A
total of 9 patients (22.5%) had LPBC (≥50% lymphocyte
infiltration), and 31 patients (77.5%) had non-LPBC. Investigation
of the clinicopathological characteristics of the patients revealed
no significant differences between the high TIL and low TIL groups
(Table II). There were also no
significant differences between LPBC and non-LPBC patients. An
analysis of outcomes revealed no difference in PFS (P=0.171,
log-rank), TTF (P=0.054, log-rank), or OS (P=0.641, log-rank)
between the high and low TIL groups (Fig. 3). LPBC patients had significantly
prolonged PFS (P=0.005, log-rank), TTF (P=0.001, log-rank) and OS
(P=0.027, log-rank) compared with non-LPBC patients. Univariate
analysis of PFS found that responding to treatment (HR=0.179,
P<0.001) and LPBC (HR=0.158, P=0.013) were factors associated
with a favorable prognosis (Table
III). Multivariate analysis confirmed that responding to
treatment (HR=0.048, P<0.001) and LPBC (HR=0.058, P=0.001) were
independent factors associated with a favorable prognosis.
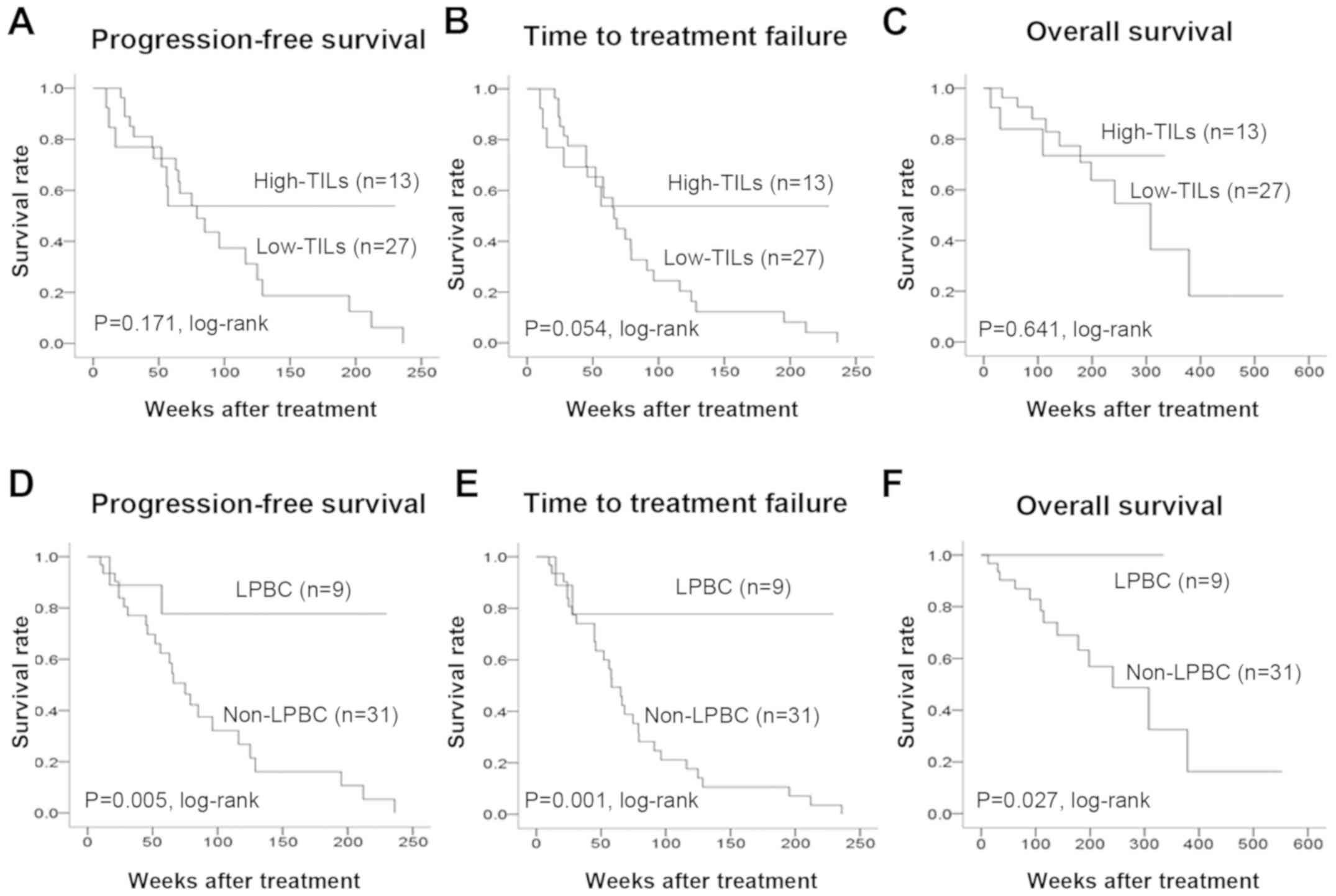 | Figure 3PFS, TTF and OS in stage IV breast
cancer according to TIL levels. An analysis of outcomes of the high
and low TIL groups revealed no difference in (A) PFS (P=0.171,
log-rank), (B) TTF (P=0.054, log-rank), or (C) OS (P=0.641,
log-rank) between the two groups. LPBC patients exhibited a
significant prolongation of the (D) PFS (P=0.005, log-rank), (E)
TTF (P=0.001, log-rank) and (F) OS (P=0.027, log-rank) compared to
non-LPBC patients. PFS, progression-free survival; TTF, time to
treatment failure; OS, overall survival; TILs, tumor-infiltrating
lymphocytes; LPBC, lymphocyte-predominant breast cancer. |
 | Table IICorrelation of clinicopathological
characteristics with TILs and LPBC in 40 stage IV breast cancer
patients. |
Table II
Correlation of clinicopathological
characteristics with TILs and LPBC in 40 stage IV breast cancer
patients.
| | TILs, n (%) | | LPBC, n (%) | |
|---|
| Parameters | High (n=13) | Low (n=27) | P-value | LPBC (n=9) | Νon-LPBC
(n=31) | P-value |
|---|
| Age at surgery,
years |
|
≤64 | 7 (53.8) | 14 (51.9) | 0.906 | 7 (77.8) | 14 (45.2) | 0.088 |
|
>64 | 6 (46.2) | 13 (48.1) | | 2 (22.2) | 17 (54.8) | |
| Menopausal
status |
|
Premenopausal | 2 (15.4) | 4 (14.8) | 0.649 | 2 (22.2) | 4 (12.9) | 0.410 |
|
Postmenopausal | 11 (84.6) | 23 (85.2) | | 7 (77.8) | 27 (87.1) | |
| Estrogen
receptor |
|
Strongly
positive | 9 (69.2) | 24 (88.9) | 0.139 | 7 (77.8) | 26 (83.9) | 0.504 |
|
Weakly
positive | 4 (30.8) | 3 (11.1) | | 2 (22.2) | 5 (16.1) | |
| Progesterone
receptor |
|
Strongly
positive | 4 (30.8) | 10 (37.0) | 0.491 | 4 (44.4) | 10 (32.3) | 0.383 |
|
Weakly
positive | 9 (69.2) | 17 (63.0) | | 5 (55.6) | 21 (67.7) | |
| HER2 |
|
Negative | 12 (92.3) | 24 (88.9) | 0.608 | 8 (88.9) | 28 (90.3) | 0.656 |
|
Positive | 1 (7.7) | 3 (11.1) | | 1 (11.1) | 3 (9.7) | |
| Ki67, % |
|
≤14 | 10 (76.9) | 23 (85.2) | 0.408 | 7 (77.8) | 26 (83.9) | 0.504 |
|
>14 | 3 (23.1) | 4 (14.8) | | 2 (22.2) | 5 (16.1) | |
| Degree of
progression |
|
Bone or soft
tissue metastasis | 7 (53.8) | 14 (51.9) | 0.906 | 4 (44.4) | 17 (54.8) | 0.431 |
|
Visceral
metastases | 6 (46.2) | 13 (48.1) | | 5 (55.6) | 14 (45.2) | |
| Objective response
rate |
|
ORR | 9 (69.2) | 21 (77.8) | 0.414 | 7 (77.8) | 23 (74.2) | 0.601 |
|
Non-ORR | 4 (30.8) | 6 (22.2) | | 2 (22.2) | 8 (25.8) | |
 | Table IIIUnivariate and multivariate analysis
with respect to progression-free survival in 40 stage IV breast
cancer patients. |
Table III
Univariate and multivariate analysis
with respect to progression-free survival in 40 stage IV breast
cancer patients.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Parameters | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, years |
|
≤64 | 1.138 | 0.516-2.511 | 0.749 | | | |
|
>64 | | | | | | |
| Menopausal
status |
|
Premenopausal | 2.209 | 0.515-9.478 | 0.286 | | | |
|
Postmenopausal | | | | | | |
| Degree of
progression |
|
Bone or soft
tissue | 0.605 | 0.271-1.355 | 0.222 | | | |
|
Visceral
metastases | | | | | | |
| TILs |
|
High | 0.527 | 0.208-1.339 | 0.178 | | | |
|
Low | | | | | | |
| Ki67, % |
|
≤14 | 1.049 | 0.391-2.815 | 0.925 | 1.578 | 0.540-4.610 | 0.405 |
|
>14 | | | | | | |
| Response rate |
|
ORR | 0.179 | 0.072-0.443 | <0.001 | 0.048 | 0.011-0.199 | <0.001 |
|
Non-ORR | | | | | | |
| LPBC |
|
Yes | 0.158 | 0.037-0.680 | 0.013 | 0.058 | 0.010-0.333 | 0.001 |
|
No | | | | | | |
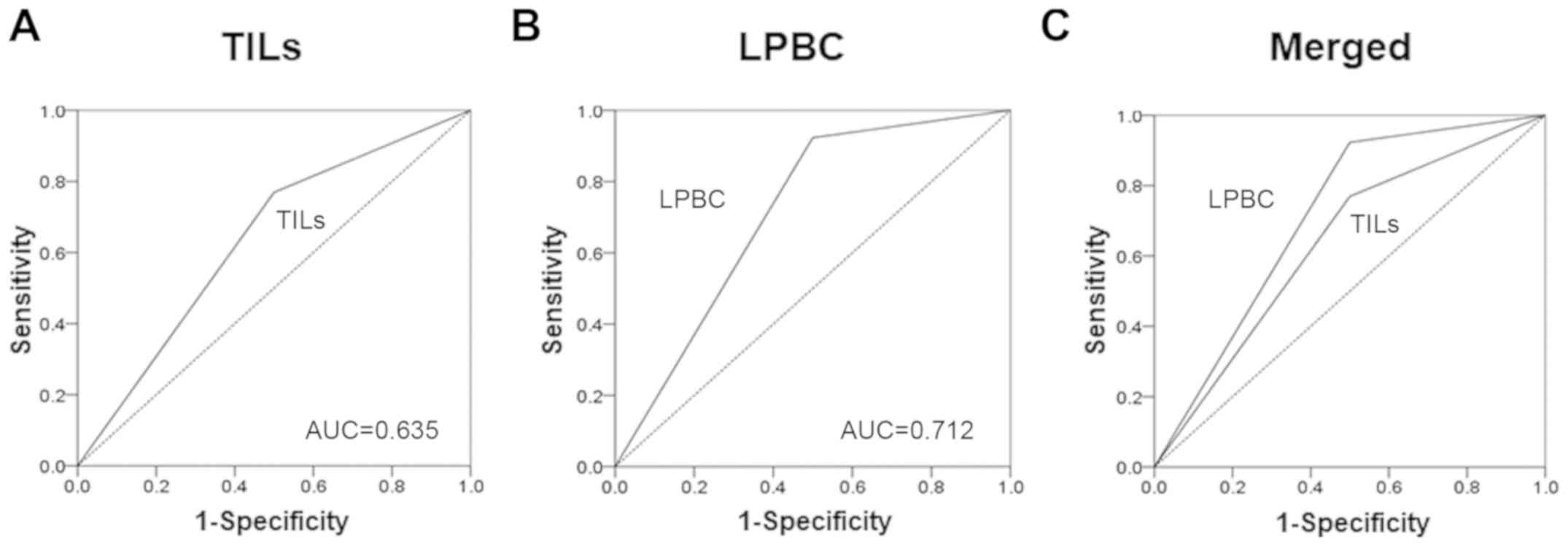
On receiver operating characteristics (ROC) curve
analyses, better results were obtained with LPBCs [area under the
curve (AUC)=0.700] compared with TILs (AUC=0.606) (Fig. 4).
Discussion
The immune system has recently been found to affect
the therapeutic efficacy, and it plays an important role in patient
prognosis, even in the field of breast cancer (5,23,24).
TILs, an indicator for monitoring antitumor immune responses, are
reported to be chemosensitive in breast cancers such as TNBC and
HER2BC, which display a high degree of immune activity (10,25-27).
However, although it is known that there are few TILs in
ER-positive breast cancer, the association of these TILs with
endocrine therapy has yet to be determined (20).
Cancer cells have various gene abnormalities that
allow them to proliferate spontaneously and survive, but they are
also affected by the surrounding environment (cancer
microenvironment), which is involved in the intrinsic
characteristics of cancer (5). On
the other hand, the ER, which is crucial for the development and
progression of breast cancer, is activated not by estrogen alone,
but by a complex signaling cascade, including a pathway that is
mediated by growth factor-stimulated phosphorylation, and its
regulation depends on the microenvironment surrounding the cancer
cells (28). Specifically, even in
endocrine therapy, which exerts an antitumor effect by blocking
estrogen activity and inhibiting estrogen production, the
regulation and improvement of the cancer microenvironment are
crucial to therapy. In the present study, it was possible to
predict the therapeutic effect of endocrine therapy in LPBC, which
displays a high degree of lymphocyte infiltration. In ER-positive
breast cancer, due to the small number of TILs, a high degree of
lymphocytic infiltration is considered predictive of the
therapeutic effect.
In addition, immunogenic cell death (ICD) is induced
by both chemotherapy and radiotherapy (29-31).
Cancer cells that cause ICD release nucleoproteins, such as high
mobility group box 1 protein (HMGB1). HMGB1 acts on toll-like
receptor 4, which is expressed on dendritic cells (DC) to induce DC
maturation, and promotes antigen presentation and T-cell activation
(32). In this manner, anticancer
treatments markedly affect the immune response of the body, and it
is believed that an immune response is activated by a similar
mechanism in endocrine therapy as well. The present findings
suggest that LPBC patients, who display a high level of
immunoactivity, respond to treatment with a greater level of
sensitivity, as the tumor immune microenvironment is modulated by
endocrine therapy.
In the present study, confirmation of LPBC by
evaluating TILs in biopsy samples in stage IV breast cancer was
found to contribute to the selection of the appropriate initial
drug therapy. However, the fact that this was a small-scale
retrospective study is a major limitation. It will be necessary to
perform future analyses of subsets of TILs and to verify the
immunologically relevant genes involved in endocrine therapy. In
the future, it is expected that the suitability of LPBC as a
predictive factor of therapeutic efficacy will be investigated in
other studies, including the PROACT study, in which neoadjuvant
endocrine therapy is currently being investigated, as well as in
the ACOSOG Z1031 study (4,33).
The findings of the present study suggest that a
high level of lymphocytic infiltration in the tumor stroma may
serve as a predictor of the therapeutic efficacy of endocrine
therapy for patients with stage IV ER-positive breast cancer.
Acknowledgements
The authors would like to thank Yayoi Matsukiyo and
Tomomi Okawa (Department of Breast and Endocrine Surgery, Osaka
City University Graduate School of Medicine) for their helpful
advice regarding data management.
Funding
The present study was supported by grants from the
Japan Society for the Promotion of Science (KAKENHI; nos. 19K18038,
26461957 and 17K10559). The funding source had no role in the
design of the study, the collection, analysis and interpretation of
the data, or the writing of this manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA participated in the design of the study and
drafted the manuscript. SK participated in the design of the study
and manuscript editing. WG, KoT, MS, RA and TT helped with data
collection and manuscript preparation. KaT and ST helped with study
data collection and participated in its design. KH and MO conceived
the study, participated in its design and coordination, and helped
to draft the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
This research conformed to the provisions of the
Declaration of Helsinki, 2013. All patients were informed of the
investigational nature of this study and provided their written,
informed consent. The study protocol was approved by the Ethics
Committee of Osaka City University (approval no. 926).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Andre F, Slimane K, Bachelot T, Dunant A,
Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R,
et al: Breast cancer with synchronous metastases: Trends in
survival during a 14-year period. J Clin Oncol. 22:3302–3308.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hortobagyi GN: Treatment of breast cancer.
N Engl J Med. 339:974–984. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ellis MJ, Tao Y, Luo J, A'Hern R, Evans
DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith
I, et al: Outcome prediction for estrogen receptor-positive breast
cancer based on postneoadjuvant endocrine therapy tumor
characteristics. J Natl Cancer Inst. 100:1380–1388. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider
J, Prat A, Parker JS, Luo J, DeSchryver K, Allred DC, et al:
Randomized phase II neoadjuvant comparison between letrozole,
anastrozole, and exemestane for postmenopausal women with estrogen
receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker
outcomes and predictive value of the baseline PAM50-based intrinsic
subtype-ACOSOG Z1031. J Clin Oncol. 29:2342–2349. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zitvogel L, Kepp O and Kroemer G: Immune
parameters affecting the efficacy of chemotherapeutic regimens. Nat
Rev Clin Oncol. 8:151–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dougan M and Dranoff G: Immune therapy for
cancer. Annu Rev Immunol. 27:83–117. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Denkert C, von Minckwitz G, Brase JC, Sinn
BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD,
et al: Tumor-infiltrating lymphocytes and response to neoadjuvant
chemotherapy with or without carboplatin in human epidermal growth
factor receptor 2-positive and triple-negative primary breast
cancers. J Clin Oncol. 33:983–991. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Savas P, Salgado R, Denkert C, Sotiriou C,
Darcy PK, Smyth MJ and Loi S: Clinical relevance of host immunity
in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol.
13:228–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Asano Y, Kashiwagi S, Goto W, Kurata K,
Noda S, Takashima T, Onoda N, Tanaka S, Ohsawa M and Hirakawa K:
Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting
treatment responses to neoadjuvant chemotherapy of aggressive
breast cancer. Br J Surg. 103:845–854. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Asano Y, Kashiwagi S, Onoda N, Kurata K,
Morisaki T, Noda S, Takashima T, Ohsawa M, Kitagawa S and Hirakawa
K: Clinical verification of sensitivity to preoperative
chemotherapy in cases of androgen receptor-expressing positive
breast cancer. Br J Cancer. 114:14–20. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asano Y, Kashiwagi S, Onoda N, Noda S,
Kawajiri H, Takashima T, Ohsawa M, Kitagawa S and Hirakawa K:
Predictive value of neutrophil/lymphocyte ratio for efficacy of
preoperative chemotherapy in triple-negative breast cancer. Ann
Surg Oncol. 23:1104–1110. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Asano Y, Kashiwagi S, Onoda N, Noda S,
Kawajiri H, Takashima T, Ohsawa M, Kitagawa S and Hirakawa K:
Platelet-lymphocyte ratio as a useful predictor of the therapeutic
effect of neoadjuvant chemotherapy in breast cancer. PLoS One.
11(e0153459)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Greene FL and Sobin LH: A worldwide
approach to the TNM staging system: Collaborative efforts of the
AJCC and UICC. J Surg Oncol. 99:269–272. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members. Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
International TILs Working Group 2014. Ann Oncol. 26:259–271.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ono M, Tsuda H, Shimizu C, Yamamoto S,
Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, et
al: Tumor-infiltrating lymphocytes are correlated with response to
neoadjuvant chemotherapy in triple-negative breast cancer. Breast
Cancer Res Treat. 132:793–805. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mao Y, Qu Q, Zhang Y, Liu J, Chen X and
Shen K: The value of tumor infiltrating lymphocytes (TILs) for
predicting response to neoadjuvant chemotherapy in breast cancer: A
systematic review and meta-analysis. PLoS One.
9(e115103)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Loi S: Host antitumor immunity plays a
role in the survival of patients with newly diagnosed
triple-negative breast cancer. J Clin Oncol. 32:2935–2937.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McShane LM, Altman DG, Sauerbrei W, Taube
SE, Gion M and Clark GM: Statistics Subcommittee of the NCI-EORTC
Working Group on Cancer Diagnostics. Reporting recommendations for
tumor marker prognostic studies. J Clin Oncol. 23:9067–9072.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fridman WH, Pages F, Sautes-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Liu H, Zhang T, Ye J, Li H, Huang J, Li X,
Wu B, Huang X and Hou J: Tumor-infiltrating lymphocytes predict
response to chemotherapy in patients with advance non-small cell
lung cancer. Cancer Immunol Immunother. 61:1849–1856.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kocián P, Šedivcová M, Drgáč J, Cerná K,
Hoch J, Kodet R, Bartůňková J, Špíšek R and Fialová A:
Tumor-infiltrating lymphocytes and dendritic cells in human
colorectal cancer: Their relationship to KRAS mutational status and
disease recurrence. Hum Immunol. 72:1022–1028. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee WS, Kang M, Baek JH, Lee JI and Ha SY:
Clinical impact of tumor-infiltrating lymphocytes for survival in
curatively resected stage IV colon cancer with isolated liver or
lung metastasis. Ann Surg Oncol. 20:697–702. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y
and Hayashi S: Estrogen receptor (ER) beta1 and ERbetacx/beta2
inhibit ERalpha function differently in breast cancer cell line
MCF7. Oncogene. 22:5011–5020. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kroemer G, Galluzzi L, Kepp O and Zitvogel
L: Immunogenic cell death in cancer therapy. Annu Rev Immunol.
31:51–72. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tesniere A, Panaretakis T, Kepp O, Apetoh
L, Ghiringhelli F, Zitvogel L and Kroemer G: Molecular
characteristics of immunogenic cancer cell death. Cell Death
Differ. 15:3–12. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kono K, Mimura K and Kiessling R:
Immunogenic tumor cell death induced by chemoradiotherapy:
Molecular mechanisms and a clinical translation. Cell Death Dis.
4(e688)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yamazaki T, Hannani D, Poirier-Colame V,
Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP,
Freudenberg M, et al: Defective immunogenic cell death of
HMGB1-deficient tumors: Compensatory therapy with TLR4 agonists.
Cell Death Differ. 21:69–78. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cataliotti L, Buzdar AU, Noguchi S, Bines
J, Takatsuka Y, Petrakova K, Dube P and de Oliveira CT: Comparison
of anastrozole versus tamoxifen as preoperative therapy in
postmenopausal women with hormone receptor-positive breast cancer:
The Pre-Operative ‘Arimidex’ Compared to Tamoxifen (PROACT) trial.
Cancer. 106:2095–2103. 2006.PubMed/NCBI View Article : Google Scholar
|