Introduction
Fascin is a cytoskeletal protein, is one of the
important members of the Fascin family of proteins and is located
on chromosome 7q22(1). An N-terminal
serine participates in actin binding, which is also the
phosphorylation site of protein kinase C. The phosphorylation of
this site regulates the binding between Fascin and actin, and also
increased the formation of pseudopodia on the surface of the cell
membrane (1). Fascin is an
actin-binding protein that can alter the cytoskeleton to form
pseudopodia and microspores, increase the motility of epithelial
cells, and reduce adhesion between cells and the extracellular
matrix, thereby promoting cell migration (2), and also partakes in signal transduction
(3). Fascin can catalyze the
polymerization of actin and regulate the formation of
microfilaments and filamentous pseudopodia on the cell surface,
increasing the mobility of the epithelium and increasing cell
surface projections, to increase cell migration, invasion and
metastasis of tumor cells (4).
Progression and invasion of colorectal cancer are closely
associated with the increase in the motility of cancer cells, and
the structural changes of the kinetic protein cytokine skeleton and
the structure and function of the three-dimensional actuation
microfilament are regulated and controlled by numerous kinetic
protein binding proteins (5).
Findings have shown that an increased expression of Fascin is
closely associated with malignant biological behaviors, such as
poor survival and metastasis in patients with colorectal cancer
(6).
Fascin is involved in progression, survival and
migration, and the expression of MMP-2 and collagen genes in human
hepatic stellate cells, via a FAK-PI3K-AKT signaling pathway
(7). PI3K may also regulate
Skp2-mediated degradation of p27 through downstream signaling
molecules (such as PKC, p70S6K and SGK, amongst others), and thus
regulate the proliferation of liver cancer cells (8). Fascin is involved in STAT3 signaling in
response to oncostatin M and interleukin-6 in human breast cancer
cells, STAT3 directly increases the expression of Fascin, and this
underlies the migration of breast cancer cells (9). The STAT3-SKP2 molecular network
controls the development of cervical cancer (10). Fascin serves an important role in
laryngeal cancer cell transition from G1 to the S phase (11).
Fascin expression is upregulated in lung cancer,
thyroid, ovarian and liver cancer (12-15),
and is associated with a poor prognosis, Tumor-Node-Metastasis
(TNM) staging and lymph node and distant metastases (16). Simultaneously, the expression of
Fascin-1 in the outer layer of the tumor is higher compared with
the inner tumor, suggesting that it may be associated with tumor
invasion (17). The upregulation of
Fascin in cells can result in increased in cell membrane process
formation, dissociation of the cell from the extracellular matrix,
and thus an increase in cell motility, which serves a key role in
invasion and lymph node metastasis (18-20).
Hayashi et al (21) detected
the expression of Fascin-1 in the early stages of liver formation
using immunohistochemistry. In particular, in the embryonic stage,
the expression of Fascin-1 is significantly increased in liver buds
and hepatocytes. These results suggest that the expression of
Fascin-1 may be associated with migratory activity of hepatocytes
during the early hepatogenesis (21). In the present study, a meta-analysis
and bioinformatics analysis was performed to provide evidence of
the association between Fascin expression and clinicopathological
factors in patients with colorectal cancer.
Materials and methods
Literature search and selection
criteria
Articles included in the present analysis were
searched for in PubMed, Web of Science, Wanfang data, SinoMed and
CNKI (June, 2019) using the following key words and modifiers:
Fascin OR Fascin-1 OR FSCN1 AND colorectal OR colon OR rectum OR
rectal AND cancer OR carcinoma OR tumor OR adenocarcinoma.
Inclusion criteria for studies were: i) Studies using
immunohistochemistry to detect the expression of Fascin or
Fascin-1; ii) articles which included an association between Fascin
expression and prognosis in colorectal cancer; and iii) articles
assessing the association between Fascin expression and
clinicopathological parameters, such as depth of invasion, lymph
node metastasis and TNM stages, amongst others. The exclusion
criteria were: i) Abstracts, case reports, reviews and meeting
notes; ii) studies with a small sample size (n<30); iii) repeat
publications or repeat data; and iv) and animal-based studies.
Data extraction and quality
assessment
The information regarding all eligible publications
was extracted by two reviewers, and the authors, year of
publication, nationality of the patients, antibody companies,
number of cases and controls, risks for cancer and follow-up
outcomes are presented in Table I.
Any disagreements regarding any of these data were resolved by
discussion. The quality of the studies was independently assessed
by two reviewers according to Newcastle Ottawa Oncomine Scale (NOS;
ohri.ca/programs/clinical_epidemiology/oxford.htm).
The methods were assessed based on consistency of sample selection,
comparability and ascertainment of outcomes.
 | Table IPrimary characteristics of the
eligible studies. |
Table I
Primary characteristics of the
eligible studies.
| Author, year | Country | Ethnicity | Antibody
supplier | Cases | Control | Risk to cancer | Outcome | Quality | (Refs.) |
|---|
| Zhao, 2014 | China | China | | 126 | 126 | Increased | - | 7 | (31) |
| Yi, 2013 | China | China | Sunbio | 60 | 60 | Increased | - | 8 | (34) |
| Xue, 2010 | China | USA | Dako | 28 | 5 | Increased | - | 8 | (28) |
| Song, 2010 | China | China | Mxb | 40 | 20 | Increased | - | 8 | (32) |
| Chan, 2010 | Australia | USA | Dako | 446 | 433 | Increased | Negative | 9 | (29) |
| Yao, 2014 | China | China | Mxb | 40 | 20 | Increased | Negative | 8 | (30) |
| Yang, 2006 | China | USA | Dako | 41 | 20 | Increased | - | 8 | (33) |
| Seung, 2012 | Korea | USA | Dako | 74 | 52 | Increased | - | 8 | (35) |
| Kong, 2016 | China | USA | Clplor | 87 | 28 | Increased | Negative | 8 | (23) |
| Li, 2015 | China | China | Mxb | 86 | 40 | Increased | - | 8 | (24) |
| Liu, 2007 | China | China | Mxb | 80 | 40 | Increased | - | 8 | (25) |
| Ding, 2008 | China | China | Chemicon | 100 | 100 | Increased | - | 8 | (26) |
| Li, 2010 | China | China | Mxb | 60 | 30 | Increased | - | 8 | (27) |
| Jung, 2011 | Korea | USA | Thermo Fisher
Scientific, Inc. | 186 | 24 | Increased | Negative | 8 | (36) |
| Pang, 2011 | China | USA | Neomarker | 60 | 20 | Increased | - | 8 | (22) |
Bioinformatics analysis
FSCN1 gene expression levels were analyzed
using Oncomine (oncomine.org), the largest chip-based
oncogene database and integrated data mining platform. Multiple
analysis (fold change) and the expression ratio of FSCN1 in
the range of 0.5-2.0, and there was no significant differential
expression of the gene. Genes where the T statistic (T-test)
exceeded a specific value was considered an abnormality. Whether
the comparison was statistically significant was determined by
calculating the confidence of the difference. The
differences in FSCN1 mRNA expression levels were compared
between colorectal tissue (including the colon and the rectum),
normal tissue, adenoma and colorectal cancer. All data were
log-transformed, median centered per array, and the standard
deviation was normalized to a single value for each array. The
expression data were obtained (RNA-seqV2) and clinicopathological
data of colorectal cancer from The Cancer Genome Atlas (TCGA)
database (cancer.gov) were analyzed using TCGA-assembler in
R software. The raw data were integrated, FSCN1 expression
in colorectal cancer was analyzed and compared with the
clinicopathological and prognostic data of patients with colorectal
cancer.
Statistical analysis
Revman version 5.3 (cochrane.es) was used for data
analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were
used to estimate the expression of Fascin based on the
clinicopathological parameters of patients with colorectal cancer.
Initially, the heterogeneity of the original documents were
assessed. Statistical significance of the pooled ORs were
determined using Z tests. If there was no significance in the
heterogeneity, a fixed effect model (Mantel-Haenszel method) was
used, otherwise, a random effect model (Der Simonian and Laird
method) was used. The effect of heterogeneity was quantified using
an I2 test. Using the following cut-off values; 25, 50
and 75%, heterogeneity was subdivided into low, medium and high
degrees, respectively. Publication bias was evaluated using funnel
plot and quantified using Begg's test and Egger's test to assess
funnel plot asymmetry. A funnel plot was used to evaluate
publication bias. COX risk regression models were used for
univariate and multivariate analysis. Meta-analyses were performed
using Revman 5.3 and data obtained from TCGA was analyzed using
SPSS version 17.0, and compared using a Student's t-test. Two-sided
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study selection and
characteristics
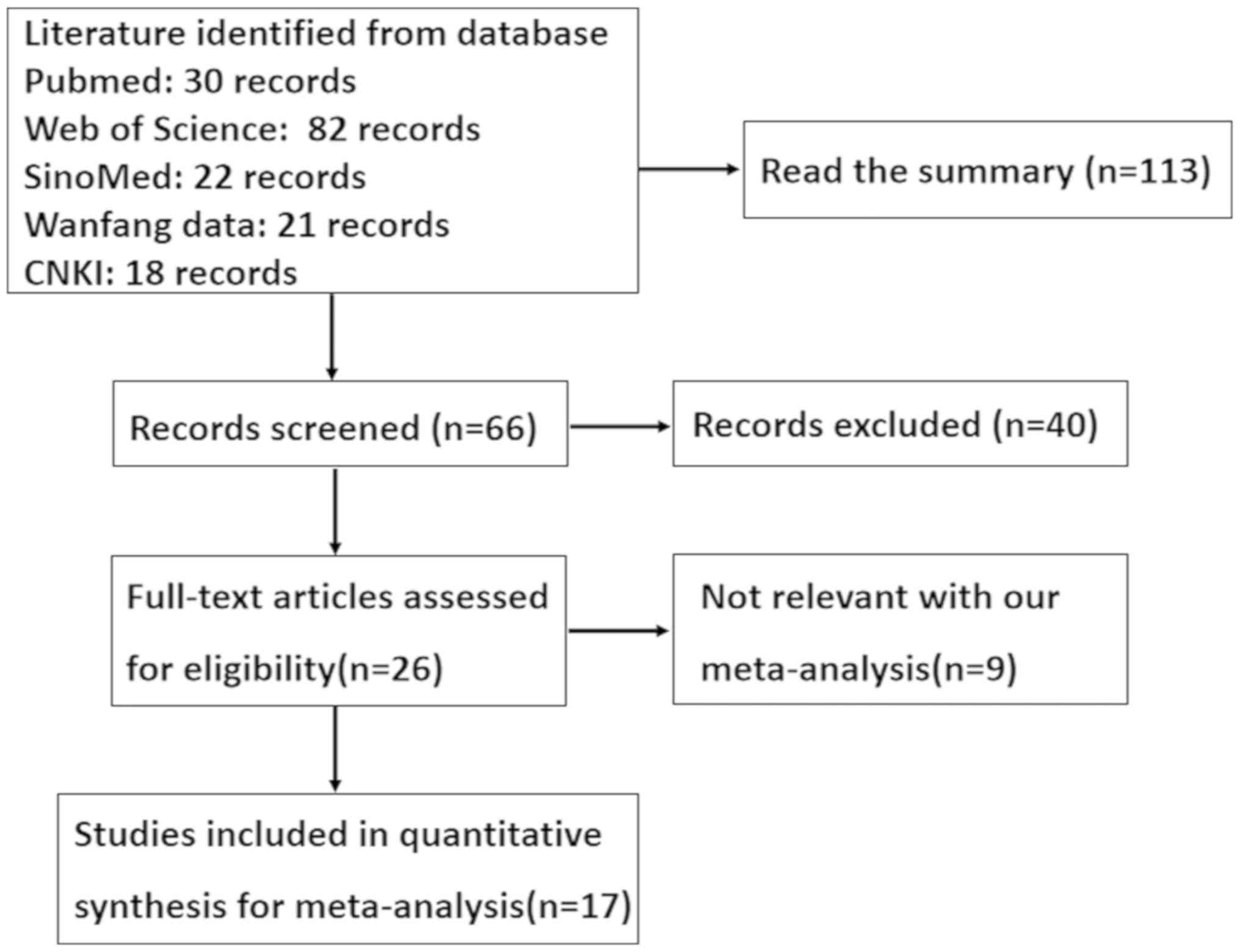
As shown in Fig. 1, a
total of 17 articles were found which assessed the relationship
between Fascin expression and clinical pathology or prognosis of
patients with colorectal cancer. Only 14 articles included analysis
of normal colorectal tissue (22-35),
and 6 included analysis of colorectal adenoma (24,27,29,32-34).
These 15 articles were used in the meta-analysis for comparison
between Fascin expression and clinicopathological features of
colorectal cancer. Additionally, there were 4 articles that
discussed the prognostic significance of Fascin expression and its
relationship clinicopathological or prognostic indicators of
colorectal cancer (23,29,30,36). All
these studies evaluated the expression of Fascin and the risk of
colorectal cancer using immunohistochemistry. The detailed
characteristics of these articles are presented in Table I.
Forest plot of OR for the association
between Fascin expression and clinicopathological parameters of
colorectal cancer
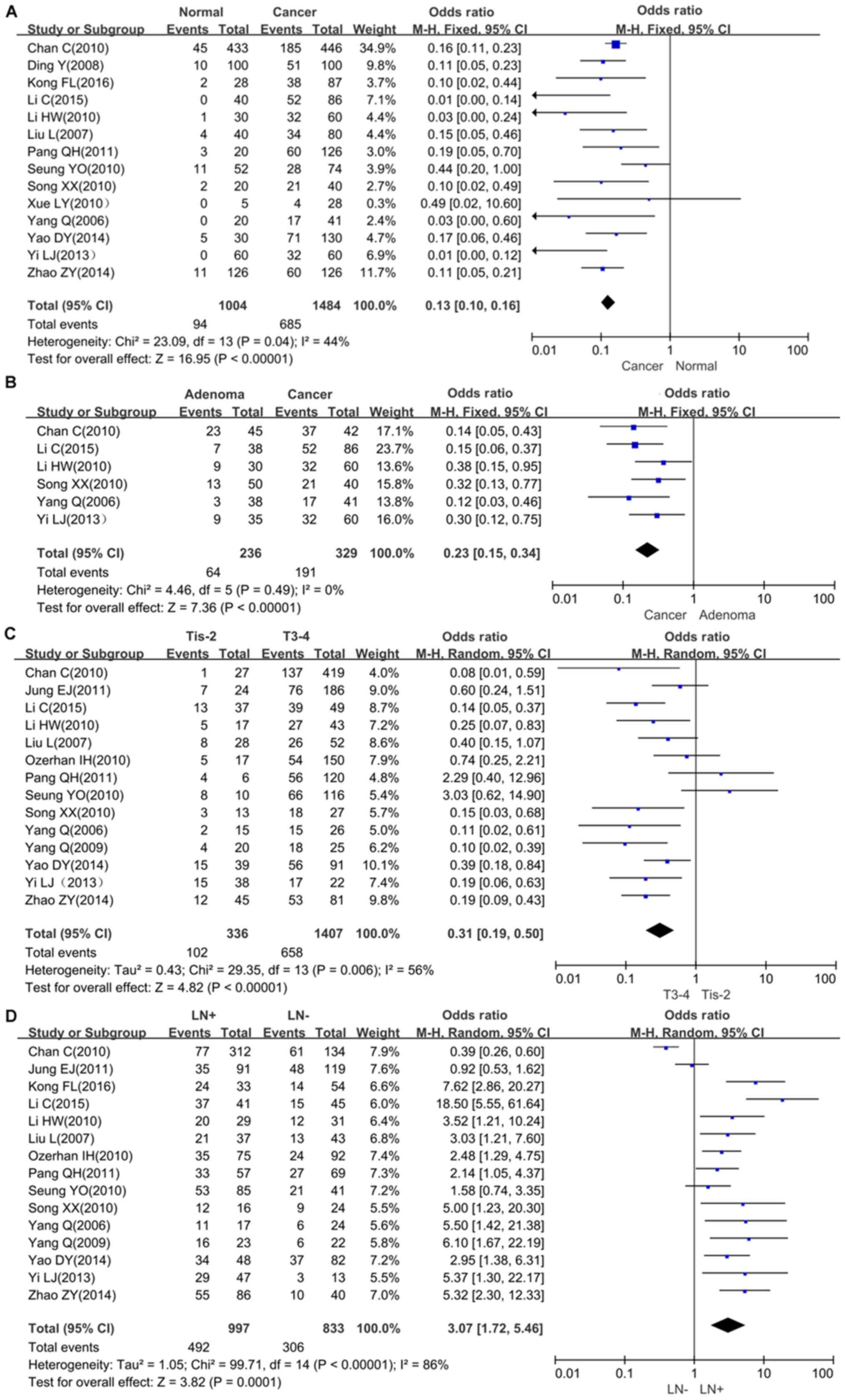
The relationship between Fascin expression and tumor
susceptibility in colorectal normal mucosa tissues were assessed in
14 studies, with 1,484 cancer patients and 1,004 controls
collectively. The expression of Fascin was upregulated in
colorectal cancer compared with normal mucosa (Fig. 2A; OR=7.78, 95% CI=6.14-9.87,
P<0.00001). Additionally, the cancer risk of Fascin-positive
adenoma was also assessed, and the same trend were observed as in
colorectal cancer using 329 cancer cases and 236 adenoma cases
(Fig. 2B; OR=0.23, 0.15-0.34,
P<0.00001). The meta-analysis showed that Fascin expression was
associated with depth of invasion (OR=0.31, 95% CI=0.19-0.50,
P<0.00001), lymph node metastasis (OR=3.07, 95% CI=1.72-5.46,
P=0.0001), Dukes stage (OR=0.14, 95% CI=0.04-0.46, P=0.0001),
dedifferentiation (OR=0.42, 95% CI=0.19-0.94, P=0.04) and TNM stage
(OR=0.38, 95% CI=0.21-0.71, P=0.003) (Fig. 2C-H). However, Fascin was not
associated with distant metastasis (OR=2.26, 95% CI=0.78-6.51,
P=0.13). The survival data are presented in Fig. 2I, and based on the 4 datasets, it was
shown that Fascin expression was associated with a less favorable
prognosis in patients with colorectal cancer (hazard ratio=0.48,
95% CI=0.38-0.60, P<0.00001).
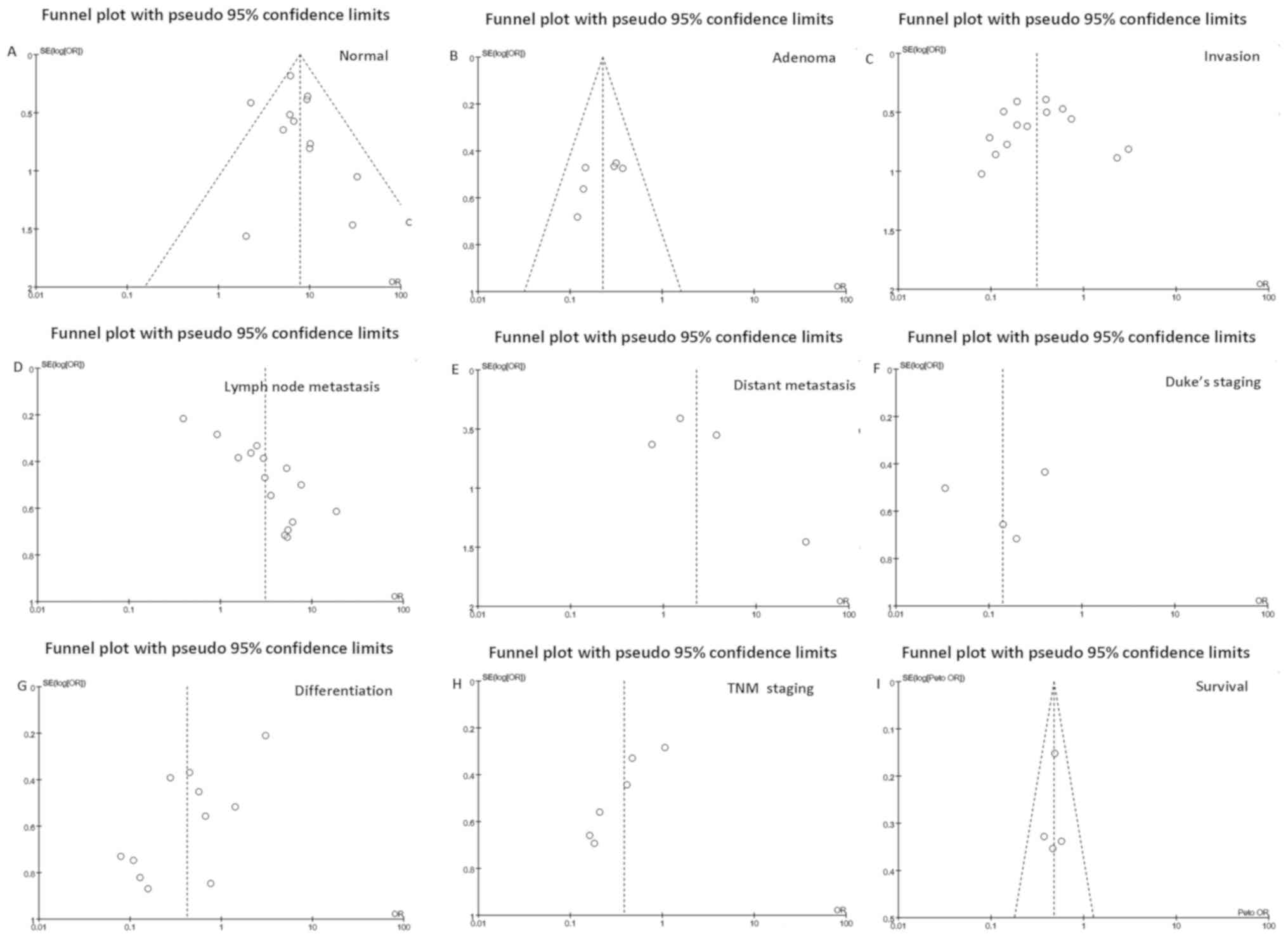
Publication bias
Publication bias was qualitatively determined using
funnel plots (Fig. 3). An individual
study was removed from the pooled analysis, and then sensitivity
analysis was used to assess the impact of that individual study on
the aggregated results. According to Egger's test, the present
meta-analysis had no apparent publication bias.
Relationship between Fascin expression
and bioinformatics analysis in patients with colorectal cancer
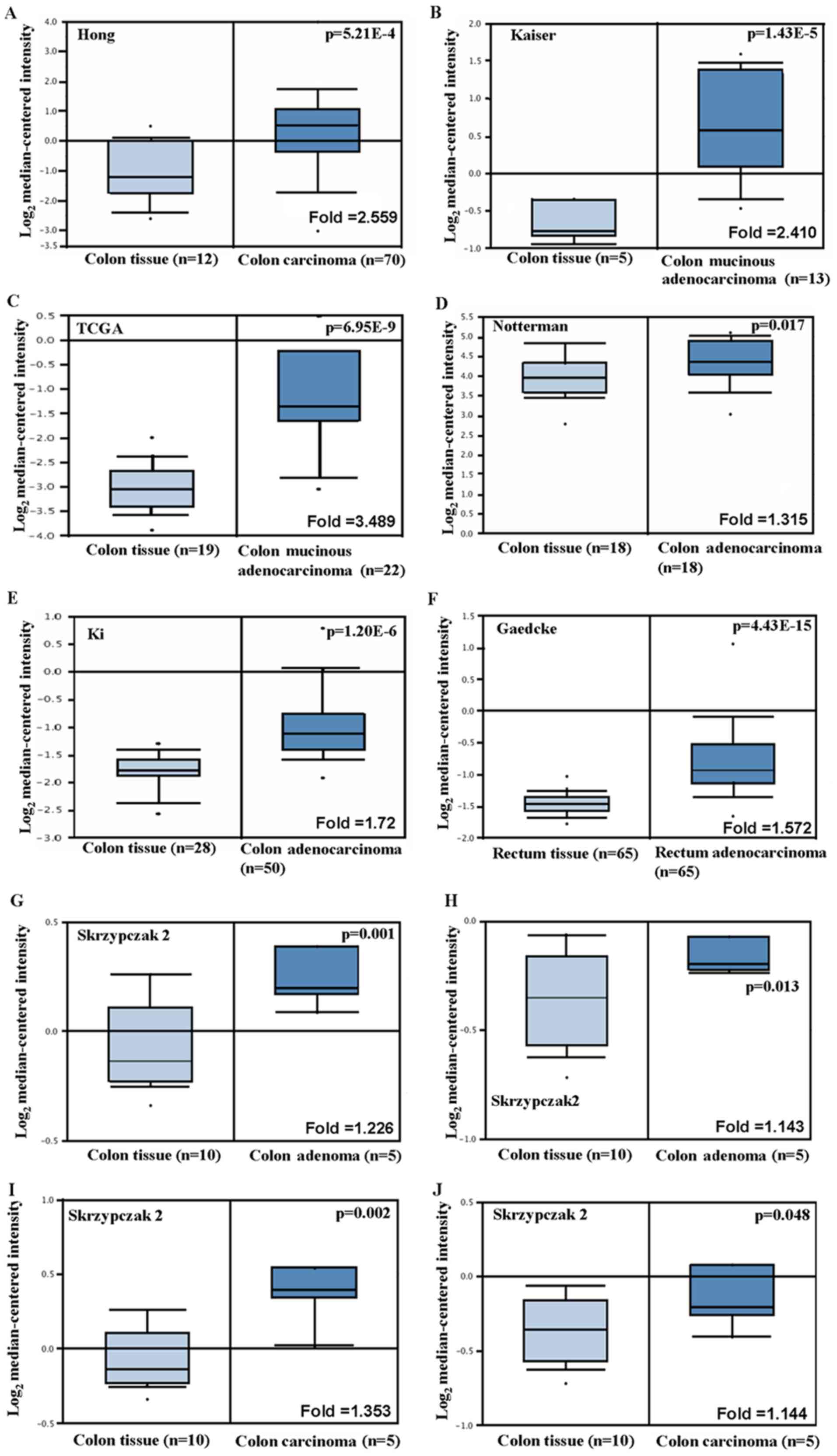
According to Hong's datasets, FSCN1 mRNA
expression was upregulated in colon carcinoma tissues compared with
the normal tissues (Fig. 4A,
P<0.05). In Kaiser and TCGA dataset, FSCN1 mRNA
expression was also upregulated in colon mucinous adenocarcinoma
tissues compared with colon tissues (Fig. 4B and C, P<0.05). Notterman's and Ki's data
showed increased FSCN1 mRNA expression levels in colon
adenocarcinoma tissues compared with the normal colon tissue
(Fig. 4D and E, P<0.05). According to Gaedcke's data,
the expression of FSCN1 mRNA in rectal adenocarcinoma was
higher compared with normal tissue (Fig.
4F, P<0.05). Skrzypczak's data showed that the expression of
FSCN1 mRNA was higher in colon adenoma and colon carcinoma
compared with normal tissue (Fig.
4G-J, P<0.05), and also showed that FSCN1 mRNA
expression was upregulated in colorectal carcinoma compared with
colorectal tissue (Fig. 4K,
P<0.05).
According to the data obtained from TCGA, univariate
analysis showed a positive correlation between FSCN1 mRNA
expression and overall prognosis in patients with colorectal cancer
(Fig. 4L, P<0.05). Multivariate
analysis was performed using a Cox risk scale model, which showed
that distant metastasis was an independent prognostic factor for
colorectal cancer (Table II,
P<0.05). Fascin expression was positively correlated with depth
of invasion and microsatellite instability (MSI) of colorectal
cancer (Fig. 4M and N, P<0.05). These data also showed that
FSCN1 mRNA expression levels were higher in the colon
compared with the rectum (Fig. 4O,
P<0.05), and that upregulated FSCN1 mRNA expression
levels were negatively correlated with serum carcinoembryonic
antigen (CEA) levels in all patients with cancer (Fig. 4P, P<0.05).
 | Table IIMultivariate analysis of hazardous
factors in the prognosis of patients with colorectal cancer. |
Table II
Multivariate analysis of hazardous
factors in the prognosis of patients with colorectal cancer.
| Clinicopathological
features | Hazard ratio (95%
CI) | P-value |
|---|
| T-stage,
Tis-2/T3-4 | 1.265
(0.477-3.354) | 0.636 |
| Lymph node status,
-/+ | 0.774
(0.176-3.391) | 0.734 |
| Distant metastasis,
-/+ | 2.247
(1.212-4.165) | 0.010 |
| TNM staging,
I-II/III-IV | 2.674
(0.541-13.212) | 0.227 |
| FSCN mRNA
expression | 1.430
(0.877-2.330) | 0.151 |
Discussion
The Fascin family of proteins consists of 3 members;
Fascin-1-3 (37-39).
Fascin-1 is widely expressed in smooth muscle tissues, vascular
endothelium, fibroblasts and in neural crest cells (40,41).
Fascin-2 expression is confined to retinal photoreceptor cells, and
Fascin-3 is only expressed in the testis (38,39).
Fascin has been studied in different types of tumors, such as
breast cancer, colon cancer, brain cancer, esophageal cancer,
stomach cancer, lung cancer, urinary bladder cancer and
hematological malignancies (42-48).
In normal epithelia of the bile duct, breast, colon, ovary,
pancreas and stomach, the expression of Fascin is usually
completely negative (49). Increased
expression of Fascin is associated with a less favorable prognosis
in patients with lung, gastric, esophageal or breast cancer
(45,46,50,51). To
demonstrate the association between Fascin expression and its
clinicopathological features, a meta-analysis of 17 articles was
performed which were selected based on the NOS scores.
Fascin expression is upregulated in colorectal
cancer, but is not expressed in normal colorectal tissues. The
studies showed that the expression of Fascin was associated with an
invasive phenotype and a poor prognosis in patients with colorectal
cancer. Thus, it is hypothesized that Fascin protein is universally
expressed in epithelial tumors (35). Fascin expression was negatively
associated with distant metastases. In cell culture, in cells
treated with recombinant Fascin, the migratory and invasive
capacities of colorectal cancer cells are increased (40). Similar findings have been obtained in
other types of epithelial cells, suggesting that Fascin may
facilitate a more aggressive tumor phenotype (18,51). In
the present study, Fascin protein expression was found to be
positively associated with depth of invasion, lymph node
metastasis, Dukes stage, dedifferentiation and TNM stage.
The mechanism by which Fascin is upregulated during
tumorigenesis, invasion and metastasis may be associated with
multiple signaling pathways. Previous reports suggest that
amplification or overexpression of c-erbB-2/HER-2 may affect the
upregulation of Fascin expression (52). A study found that Wnt signaling may
also have an effect on Fascin activity, suggesting that anomalies
in the Wnt signaling pathway may result in upregulation of Fascin
expression in tumor cells (53).
Exogenous TGF-β and EGF induce the expression of Fascin, and this
induction is dependent on the activation of ERK (54,55), a
downstream molecule of the Ras signaling pathway. Inhibition of
phosphorylation of ERK results in a decrease in the expression
levels of Fascin (54), suggesting
that activation of the downstream molecules of the ERK-Ras pathway
results in increased Fascin expression in tumor cells. Fu et
al (54) found a significant
upregulation of Fascin expression in experimental autoimmune
neuritis sciatic nerves which was associated with disease severity.
The interaction between Fascin and PKC may be regulated by
cannabinoid signaling, which controlled neuroblast migration in
vivo. It was also shown that Fascin upregulation marked a shift
to the migratory neuroblast stage and was a key regulator of
neuroblast activity (55). Fascin
can stabilize actin bundles in the invasive pseudopod structure,
which may promote the metastatic potential of tumor cells (56,57).
Additionally, Fascin promotes epithelial-mesenchymal transition
through the formation of pseudopodium, and formation of a
mesothelial cell layer (58).
Previous studies showed that LMP1 mediated the upregulation of
Fascin, and this was dependent on NF-κB, where both NF-κB and
Fascin contributed to an invasion and migration in LMP1-expressing
lymphocytes (59). Fascin expression
is significantly associated with Tiam1 expression. Tiam1 activates
Rac, and activated Rac affects the movement of cytoskeleton by
inducing actin aggregation at the plasma membrane, resulting in
formation of filopodia, and thus inducing invasion and metastasis
of tumor cells (26).
Zhang et al (60) showed there was a positive correlation
between Twist and Fascin mRNA expression levels in colorectal
cancer, suggesting that they both served a synergistic role in the
development and progression of colorectal cancer. In the present
study, it was shown that there was a positive correlation between
FSCN1 mRNA expression levels and overall prognosis in
patients with colorectal cancer, and distant metastasis was an
independent prognostic factor for colorectal cancer. However,
Fascin protein expression levels were not associated with distant
metastasis, and this may be due to the sample size. Simultaneously,
Fascin expression was positively correlated with depth of invasion.
In the univariate analysis, there may be false or indirect
associations between independent and dependent variables, which are
adjusted for and thus may disappear in the multivariate analysis.
The purpose of multivariate analysis is to determine the
influencing factors with independent effects, and to estimate the
effect size by controlling the influence of other confounding
factors.
The present study has some limitations. First,
potential publication bias stems from the fact that published
results were predominantly positive. Second, the patients included
in the studies were only from Asia and America. Different levels of
medical development in different areas may influence the results.
Third, extraction of survival data, such as incomplete extraction,
may affect results. Fourth, small sample sizes may influence
associations in some articles. Finally, the difference in the
expression of Fascin between normal tissues and adenoma was not
assessed.
In conclusion, Fascin expression is upregulated in
colorectal cancer and adenoma. Fascin expression was positively
associated with depth of invasion, lymph node metastasis, Dukes
stage, TNM staging, dedifferentiation and a less favorable
prognosis in patients with colorectal cancer. FSCN1 mRNA
expression was positively associated with depth of invasion, MSI,
low serum CEA levels in colorectal cancer and with a less favorable
prognosis in patients with colorectal cancer as an independent
factor.
Acknowledgements
Not appliable.
Funding
Not funding was received.
Availability data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS and ZGZ performed the meta-analysis, and wrote
the manuscript. HCZ analyzed the data from TCGA. All authors
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li LJ, Xu L and Xia D: Progress of Fascin
in colorectal cancer. J Mil Surg Southwest China. 14:753–756.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhou C and Li TY: Research progress of the
relationship between fascin protein and tumor. J Nanchang Univ
(Medical Sciences). 53:94–96. 2013.
|
|
3
|
Hashimoto Y, Kim DJ and Adams JC: The
roles of fascins in health and disease. J Pathol. 224:289–300.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Swierczynski SL, Maitra A, Abraham SC,
Iacobuzio-Donahue CA, Ashfaq R, Cameron JL, Schulick RD, Yeo CJ,
Rahman A, Hinkle DA, et al: Analysis of novel tumor markers in
pancreatic and biliary carcinomas using tissue microarrays. Hum
Pathol. 35:357–366. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Buda A and Pignatelli M: Cytoskeletal
network in colon cancer: From genes to clinical application. Int J
Biochem Cell Biol. 36:759–765. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsai WC, Chao YC, Sheu LF, Chang JL, Nieh
S and Jin JS: Overexpression of fascin-1 in advanced colorectal
adenocarcinoma: Tissue microarray analysis of immunostaining scores
with clinicopathological parameters. Dis Markers. 23:153–160.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Uyama N, Iimuro Y, Kawada N, Reynaert H,
Suzumura K, Hirano T, Kuroda N and Fujimoto J: Fascin, a novel
marker of human hepatic stellate cells, may regulate their
proliferation, migration, and collagen gene expression through the
FAK-PI3K pathway. Lab Invest. 92:57–71. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gungor-Ordueri NE, Celik-Ozenci C and
Cheng CY: Fascin 1 is an actin filament-bundling protein that
regulates ectoplasmic specialization dynamics in the rat testis. Am
J Physiol Endocrinol Metab. 307:E738–E753. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Snyder M, Huang XY and Zhang JJ: Signal
transducers and activators of transcription 3 (STAT3) directly
regulates cytokine-induced fascin expression and is required for
breast cancer cell migration. J Biol Chem. 286:38886–38893.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang H, Zhao W and Yang D: Stat3 induces
oncogenic Skp2 expression in human cervical carcinoma cells.
Biochem Biophys Res Commun. 418:186–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang J, Li X, Liu X and Pi L: The
regulation of stat3 signal transduction pathway to G1 to S phase of
laryngocarcinoma cell. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 22:699–703. 2008.(In Chinese). PubMed/NCBI
|
|
12
|
Zhang N, Shen Y and Hu G: Expression of
fascin protein in human lung cancer and its clinical significance.
J Nanjing Med Univ (Natural Sciences). 30:309–311. 2010.
|
|
13
|
Chen G, Zhang FR, Ren J, Tao LH, Shen ZY,
Lv Z, Yu SJ, Dong BF, Xu LY and Li EM: Expression of fascin in
thyroid neoplasms: A novel diagnostic marker. J Cancer Res Clin
Oncol. 134:947–951. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kostopoulou E, Daponte A, Terzis A, Nakou
M, Chiotoglou I, Theodosiou D, Chatzichristodoulou C, Messinis IE
and Koukoulis G: Fascin in ovarian epithelial tumors. Histol
Histopathol. 23:935–944. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Iguchi T, Aishima S, Umeda K, Sanefuji K,
Fujita N, Sugimachi K, Gion T, Taketomi A, Maehara Y and Tsuneyoshi
M: Fascin expression in progression and prognosis of hepatocellular
carcinoma. J Surg Oncol. 100:575–579. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kostopoulou E, Daponte A, Terzis A, Nakou
M, Chiotoglou I, Theodosiou D, Chatzichristodoulou C, Messinis IE
and Koukoulis G: Fascin in ovarian epithelial tumors. Histol
Histopathol. 23:935–944. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao X and Wu DH: Expression of FSCN1
protein in human epithelial tumor and its clinical significance.
Nan Fang Yi Ke Da Xue Xue Bao. 28:953–955. 2008.(In Chinese).
|
|
18
|
Yamashiro S, Yamakita Y, Ono S and
Matsumura F: Fascin, an actin-bundling protein, induces membrane
protrusions and increases cell motility of epithelial cells. Mol
Biol Cell. 9:993–1006. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xing P, Li JG, Jin F, Zhao TT, Liu Q, Dong
HT and Wei XL: Fascin, an actin-bundling protein, promotes breast
cancer progression in vitro. Cell Biochem Funct. 29:303–310.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Vignjevic D, Schoumacher M, Gavert N,
Janssen KP, Jih G, Laé M, Louvard D, Ben-Ze'ev A and Robine S:
Fascin, a novel target of beta-catenin-TCF signaling, is expressed
at the invasive front of human colon cancer. Cancer Res.
67:6844–6853. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hayashi Y, Toda K, Saibara T, Okamoto S,
Osanai M, Enzan H and Lee GH: Expression of fascin-1, an
actin-bundling protein, in migrating hepatoblasts during rat
developmentliver. Cell Tissue Res. 334:219–226. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pang QH, Dai XL, Wu WX and Zhang HX:
Expression of fascin and CXCR4 in colorectal carcinoma tissues and
their clinical significance. Chin J Cancer Prev Treat.
18:1175–1185. 2011.
|
|
23
|
Kong FL, Hu LX, Li CF, Huang MQ, Jiang YJ,
Liao HW, Wu Y and Wang FX: The expression of fascin-1 in colorectal
cancer and its clinical significance. Chongqing Yixue.
45:1519–1521. 2016.
|
|
24
|
Li C, Cai L, Zhang N and Zhang RQ:
Expression and significance of fascin-1 in colorectal adenoma and
colorectal carcinoma. Health Magazine. 9:11–12. 2015.
|
|
25
|
Liu L and Ding YQ: Expression of fascin-1
in human colorectal cancer and its significance. J Fourth MiI Med
Univ. 28:108–110. 2007.
|
|
26
|
Ding Y, Liu L, Jiang HY and Ding YQ:
Expression and significance of Tiam1, fascin-1, and HSPB1 in human
colorectal cancer. Guangdong Med J. 29:230–232. 2008.
|
|
27
|
Li HW, Wang TS, Huang Y, Li YH and Hu HX:
Expression of fascin-1 protein in colorectal adenocarcinoma and
relation with clinicopathologic characteristics. Chin J Bas Clin
Gen Surg. 17:362–364. 2010.
|
|
28
|
Xue LY, Zou SM, Zheng S, Xie YQ, Wen F,
Liu XY, Lin DM and Lü N: Expression of fascin and CK14 in different
histological types of cancer and its differential diagnostic
significance. Zhonghua Zhong Liu Za Zhi. 32:838–844. 2010.(In
Chinese). PubMed/NCBI
|
|
29
|
Chan C, Jankova L, Fung CL, Clarke C,
Robertson G, Chapuis PH, Bokey L, Lin BP, Dent OF and Clarke S:
Fascin expression predicts survival after potentially curative
resection of node-positive colon cancer. Am J Surg Pathol.
34:656–666. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yao DY, Zhang HY and Xu X: Expression of
fascin in colorectal carcinoma and its correlation with prognosis.
Hainan Med J. 25:3089–3091. 2014.
|
|
31
|
Zhao ZY: Expression and significance of
fascin and iNOS in colorectal adenoma carcinogenesis. Medicine
People. 7(66)2014.
|
|
32
|
Song XX: Expression and significance of
fascin in canceration of colorectal adenoma. Heibei Med J.
32:2370–2372. 2010.
|
|
33
|
Yang Q, Lu ZH, Luo YF, Wan JW, Cao JL, Gao
J, Wu SF and Chen J: Significance of fascin expression in
colorectal adenoma and carcinoma. Chin J Diagn Pathol. 13:359–361.
2006.
|
|
34
|
Yi LJ: Expression and significance of
fascin and P27 in colorectal carcinoma. Luzhou Medical College,
2013.
|
|
35
|
Oh SY, Kim YB, Suh KW, Paek OJ and Moon
HY: Prognostic impact of fascin-1 expression is more significant in
advanced colorectal cancer. J Surg Res. 172:102–108.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jung EJ, Lee JH, Min BW, Kim YS and Choi
JS: Clinicopathologic significance of fascin, extracellular matrix
metalloproteinase inducer, and ezrin expressions in colorectal
adenocarcinoma. Indian J Pathol Microbiol. 54:32–36.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Duh FM, Latif F, Weng Y, Geil L, Modi W,
Stackhouse T, Matsumura F, Duan DR, Linehan WM, Lerman MI, et al:
cDNA cloning and expression of the human homolog of the sea urchin
fascin and Drosophila singed genes which encodes an actin-bundling
protein. DNA Cell Biol. 13:821–827. 1994.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tubb BE, Bardien-Kruger S, Kashork CD,
Shaffer LG, Ramagli LS, Xu JP, Siciliano MJ and Bryan J:
Characterization of human retinal fascin gene (FSCN2) at 17q25:
Close physical linkage of fascin and cytoplasmic actin genes.
Genomics. 65:146–156. 2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tubb B, Mulholland DJ, Vogl W, Lan ZJ,
Niederberger C, Cooney A and Bryan J: Testis fascin (FSCN3): A
novel paralog of the actin-bundling protein fascin expressed
specifically in the elongate spermatid head. Exp Cell Res.
275:92–109. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jawhari AU, Buda A, Jenkins M, Shehzad K,
Sarraf C, Noda M, Farthing MJ, Pignatelli M and Adams JC: Fascin,
an actin-bundling protein, modulates colonic epithelial cell
invasiveness and differentiation in vitro. Am J Pathol. 162:69–80.
2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mosialos G, Birkenbach M, Ayehunie S,
Matsumura F, Pinkus GS, Kieff E and Langhoff E: Circulating human
dendritic cells differentially express high levels of a 55-kd
actin-bundling protein. Am J Pathol. 148:593–600. 1996.PubMed/NCBI
|
|
42
|
Grothey A, Hashizume R, Sahin AA and
Mccrea PD: Fascin, an actin-bundling protein associated with cell
motility, is upregulated in hormone receptor negative breast
cancer. Br J Cancer. 83:870–873. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Roma AA and Prayson RA: Fascin expression
in 90 patients with glioblastoma multiforme. Ann Diagn Pathol.
9:307–311. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xie JJ, Xu LY, Zhang HH, Cai WJ, Mai RQ,
Xie YM, Yang ZM, Niu YD, Shen ZY and Li EM: Role of fascin in the
proliferation and invasiveness of esophageal carcinoma cells.
Biochem Biophys Res Commun. 337:355–362. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hashimoto Y, Shimada Y, Kawamura J,
Yamasaki S and Imamura M: The prognostic relevance of fascin
expression in human gastric carcinoma. Oncology. 67:262–270.
2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pelosi G, Pastorino U, Pasini F,
Maissoneuve P, Fraggetta F, Iannucci A, Sonzogni A, De Manzoni G,
Terzi A, Durante E, et al: Independent prognostic value of fascin
immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer.
88:537–547. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tong GX, Yee H, Chiriboga L, Hernandez O
and Waisman J: Fascin-1 expression in papillary and invasive
urothelial carcinomas of the urinary bladder. Hum Pathol.
36:741–746. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fan G, Kotylo P, Neiman RS and Braziei RM:
Comparison of fascin expression in anaplastic large cell lymphoma
and Hodgkin disease. Am J Clin Pathol. 119:199–204. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hashimoto Y, Skacel M and Adams JC: Roles
of fascin in human carcinoma motility and signaling: Prospects for
a novel biomarker? Int J Biochem Cell Biol. 37:1787–1804.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Adams JC: Fascin protrusions in cell
interactions. Trends Cardiovasc Med. 14:221–226. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hashimoto Y, Ito T, Inoue H, Okumura T,
Tanaka E, Tsunoda S, Higashiyama M, Watanabe G, Imamura M and
Shimada Y: Prognostic significance of fascin overexpression in
human esophageal squamous cell carcinoma. Clin Cancer Res.
11:2597–2605. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kabukcuoglu S, Onedr U, Ozalp SS, Dundar
E, Yalcin OT and Colak E: Prognostic significance of fascin
expression in endometrioid carcinoma. Eur J Gynaecol Oncol.
27:481–486. 2006.PubMed/NCBI
|
|
53
|
Xue LY, Hu N, Song YM, Zou SM, Shou JZ,
Qian LX, Ren LQ, Lin DM, Tong T, He ZG, et al: Tissue microarray
analysis reveals a tight correlation between protein expression
pattern and progression of esophageal squamous cell carcinoma. BMC
Cancer. 6(296)2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fu H, Hu Z, Wen J, Wang K and Liu Y:
TGF-beta promotes invasion and metastasis of gastric cancer cells
by increasing fascin1 expression via ERK and JNK signal pathways.
Acta Biochim Biophys Sin (Shanghai). 41:648–656. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lu XF, Li EM, Du ZP, Xie JJ, Guo ZY, Gao
SY, Liao LD, Shen ZY, Xie D and Xu LY: Specificity protein 1
regulates fascin expression in esophageal squamous cell carcinoma
as the result of the epidermal growth factor/extracellular
signal-regulated kinase signaling pathway activation. Cell Mol Life
Sci. 67:3313–3329. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li A, Dawson JC, Forero-Vargas M, Spence
HJ, Yu X, König I, Anderson K and Machesky LM: The actin-bundling
protein fascin stabilizes actin in invadopodia and potentiates
protrusive invasion. Curr Biol. 20:339–345. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Van Audenhove I, Boucherie C, Pieters L,
Zwaenepoel O, Vanloo B, Martens E, Verbrugge C,
Hassanzadeh-Ghassabeh G, Vandekerckhove J, Cornelissen M, et al:
Stratifying fascin and cortactin function in invadopodium formation
using inhibitory nanobodies and targeted subcellular
delocalization. FASEB J. 28:1805–1818. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Li A, Morton JP, Ma Y, Karim SA, Zhou Y,
Faller WJ, Woodham EF, Morris HT, Stevenson RP, Juin A, et al:
Fascin is regulated by slug, promotes progression of pancreatic
cancer in mice, and is associated with patient outcomes.
Gastroenterology. 146:1386–1396.e1-e17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–130. 2007.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang JS, Li YC and Qi F: Expression of
Twist and Fascin mRNA in colorectal carcinoma tissue. Shandong Med
J. 27:42–43. 2015.(In Chinese).
|