Introduction
Sarcomas are malignant tumors of mesenchymal origin,
arising from bones, muscles, cartilage, fat, nerves, blood vessels,
fibrous tissues, or deep skin tissues. Soft tissue sarcomas
(STS)are approximately three to four times as common as bone
sarcomas (1). Osteosarcoma (OS) is
the most common form of primary malignant bone tumor in adolescents
and young adults and is extremely aggressive (2). High-grade OS requires surgery and
systemic chemotherapy. The 5-year survival rate is less than 20%
for patients with localized resectable primary tumors treated with
surgery without chemotherapy (3).Unfortunately, overall survival has not
improved significantly over the past several decades as no new
effective drug regimen has been developed.
Undifferentiated pleomorphic sarcoma (UPS),
previously known as malignant fibrous histiocytoma, is the most
common sarcoma appearing in adult life (4). It is most commonly found in the soft
tissues with a less frequent occurrence in the bone.
Morphologically, these tumors are composed of fibroblasts,
myofibroblasts and histiocytes (5).
UPS has a high rate of local recurrence and metastasis. It is
recommended to treat UPS arising in the bone with a combination of
surgery and chemotherapy similar to OS (6-8).
Synovial sarcoma (SS) is the fourth most common type
of STS and accounts for 5-10% of all STSs (9). SS occurs predominantly in younger
adults with a median age of diagnosis of 35 years (10). Approximately 70% these tumors arise
in the extremities, with significantly better long-term survival
outcomes than those with non-extremity involvement (11,12).
In patients with localized disease, 10-year survival varies from 8
to 88% depending on the tumor size and location (13). Standard treatment for SS is tumor
resection and is frequently accompanied by radiotherapy and
sometimes chemotherapy.
Local anesthetics (LAs) are widely available
medications and relatively inexpensive. LAs are used for various
reasons, including adjunctive pain management to decrease opioid
use in cancer patients (14,15).
They have also been shown to induce apoptosis and arrest cell
growth in certain malignancies such as carcinoma of the thyroid and
breast (16,17). Recently, we have demonstrated the
inhibitory effect of bupivacaine on cartilage-forming tumor cells
which was harvested from patients during tumor resection (18). In addition to this, the potential
benefit of use of LAs during the surgical resection includes a
decrease in the risk of metastasis, cancer recurrence, and
improvement of overall survival (19-21).
LAs may also indirectly influence the long-term outcomes in cancer
patients undergoing surgery by modulation of the neuroendocrine
stress response and attenuation of immune responses, which both may
play a role in tumor metastasis and recurrence (22,23).
Metastasis negatively impacts patient prognosis and significantly
reduces survival outcomes. Most metastases from sarcoma develop in
the lungs (80%), although bone (9.9%) and liver (4.5%) can also
occur (24).
The purpose of this study was to investigate the
effects of bupivacaine on various patient-derived sarcoma tumor
cells. This study allows us to evaluate the treatment effects of a
medication that is currently available, FDA approved, cost
effective, and has an established side-effect profile.
Materials and methods
Cell culture and reagents
Different sarcoma types were evaluated in this study
including: A high-grade conventional OS obtained from a 24-year-old
female with a right distal femur tumor who had undergone
neoadjuvant chemotherapy, a high-grade UPS from a 10-year-old male
with a right distal femur tumor who had undergone neoadjuvant
chemotherapy, and a high-grade SS from a 10-year-old female with a
right forearm tumor. The SS was further classified as a monophasic,
spindle cell type. There was only limited chemotherapy related
tumor necrosis noted in the specimens that were obtained after the
patients had undergone chemotherapy.
All specimens were harvested from patients during
tumor resection. Human tissue collection protocols were reviewed
and approved by the Loma Linda University Institutional Review
Board (IRB, cat. no. 58238) in accordance to the provisions of the
Declaration of Helsinki, and informed, written consent was obtained
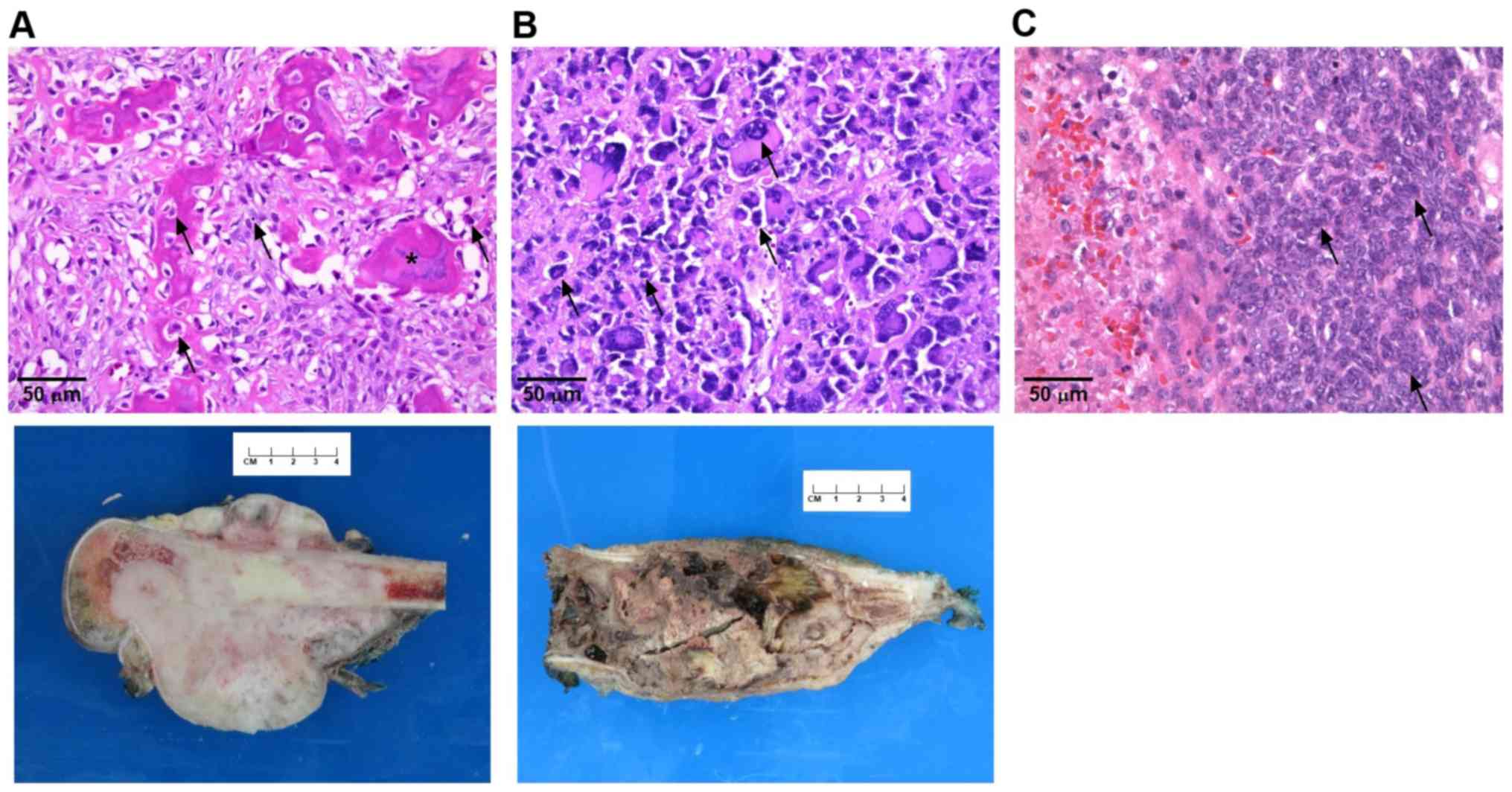
from all patients or their guardians. Diagnosis of the harvested
tumors was confirmed by pathology (Fig.
1).
The tumor cell suspension was prepared by cutting
the tumors into small pieces. Collagenase was added and shaken at
37̊C for several hours until the tissues dispersed into single
cells. The collagenase solution was then centrifuged, and the
precipitate was washed. In addition, a human HTB-93 (SS) cell line
was obtained from the American Type Culture Collection (ATCC).
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
and supplemented with 10% fetal bovine serum, 100 U/ml penicillin G
and streptomycin, and 1% nonessential amino acids. Cell cultures
were maintained in a humidified incubator containing 5%
CO2 at 37̊C. Patient-derived sarcoma cells were used in
experiments after up to 4-5 passages in culture, in order to not
significantly change their gene expressions. The doubling rate was
constant for up to 5 passages and decreased after that.
Preservative-free 0.5% bupivacaine (Marcaine, Hospira) was
purchased from McKesson Medical-Surgical Inc.
Cell viability assay
Cells were seeded in triplicate at a density of
5,000 cells/well in flat bottom, 96-well plates. After cell
confluence reached 80%, the cells were treated with 0.125, 0.25 and
0.5% of bupivacaine (4.33, 8.66 and 17.33 mM, respectively) for 60,
120, 240 and 480 min at 37̊C. These doses replicate the commonly
used doses of bupivacaine in the clinical setting. The untreated
group was exposed to phosphate-buffered saline (PBS) with a pH of
5.5 in order to control for the pH and microenvironment of the
treated cells. After various time durations, the bupivacaine was
removed and fresh media (10% DMEM) was added. Viability was
assessed 48 h after treatment with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT
reagent, Roche Diagnostics) according to the manufacturer's
protocol. The plates were read, and the absorbance at 570 nm was
measured on a microplate reader (model 3550; Bio-Rad). The results
were expressed as a percentage of the untreated control (% of
control). Each experiment was done in triplicate. The mean values
for each individual tumor sample were averaged to yield a single
mean for the separate tumors.
Colony forming assay
UPS, SS and HTB-93 cells were exposed to 0.125, 0.25
and 0.5% bupivacaine for 60 and 480 min. The bupivacaine was then
replaced with fresh media and colony counting was performed to
determine the colony forming potential of the adherent cells.
Colonies were stained with 0.01% crystal violet (Sigma-Aldrich) and
counted under microscopy on day 14. Cell clusters were considered
colonies and therefore counted. Experiments were done in
triplicate.
Microscopic observation of cell
morphology
Cells were exposed to 0.125, 0.25 and 0.5% of
bupivacaine for 1 h and 0.0156, 0.0312, 0.0625 and 0.125% (0.54,
1.08, 2.16 and 4.33 mM, respectively) for 24 h. Morphological
changes in tumor cells were examined by phase-contrast
photomicrograph at 24 h after exposure. Apoptotic cells were
characterized by cell shrinkage and detachment from the plates.
Analysis of apoptosis
Cell death by apoptosis was analyzed using the
Annexin V-fluorescein isothiocyanate (FITC) apoptosis kit (BD
Biosciences). Tumor cells were treated with either 0.25% or 0.5%
bupivacaine for various durations. Initially, 1x105
cells were seeded in 6-well plates, and when confluence reached
80%, they were exposed to either 0.25% or 0.5% bupivacaine. After
24 h, both floating and attached cells were harvested and then
washed. Flow cytometry with FITC-conjugated Annexin-V/propidium
iodide (PI) double staining was used to assess the number of
apoptotic cells. Samples were analyzed by flow cytometry
(MACSQuant; Miltenyi Biotec). Using the FlowJo software (v10;
TreeStar), measurements were displayed as 4 quadrants, in which the
lower right quadrant represented the apoptosis rate during the
early stages, the upper right quadrant indicated advanced apoptosis
rate, the upper left quadrant represented dead cells, and the lower
left quadrant represented living cells. The apoptotic rate was
calculated as early apoptosis (Ann+/PI-) and late apoptosis
(Ann+/PI+). This experiment was repeated 3 times.
Statistical analysis
Each assay was performed in triplicate and the
results were expressed as a mean ± SEM. Statistical comparisons
were performed using analysis of variance followed by the
Bonferroni t-test and done with Prism v5.01 software (GraphPad
Software). A P-value of <0.05 was considered to be statistically
significant.
Results
Bupivacaine reduces the viability of
sarcoma cells
Cell viability data on the cultured tumor cells
showed that bupivacaine had dose- and time-dependent cytotoxic
effect across all tumor types at clinically relevant concentrations
when compared with the controls. Exposure to bupivacaine resulted
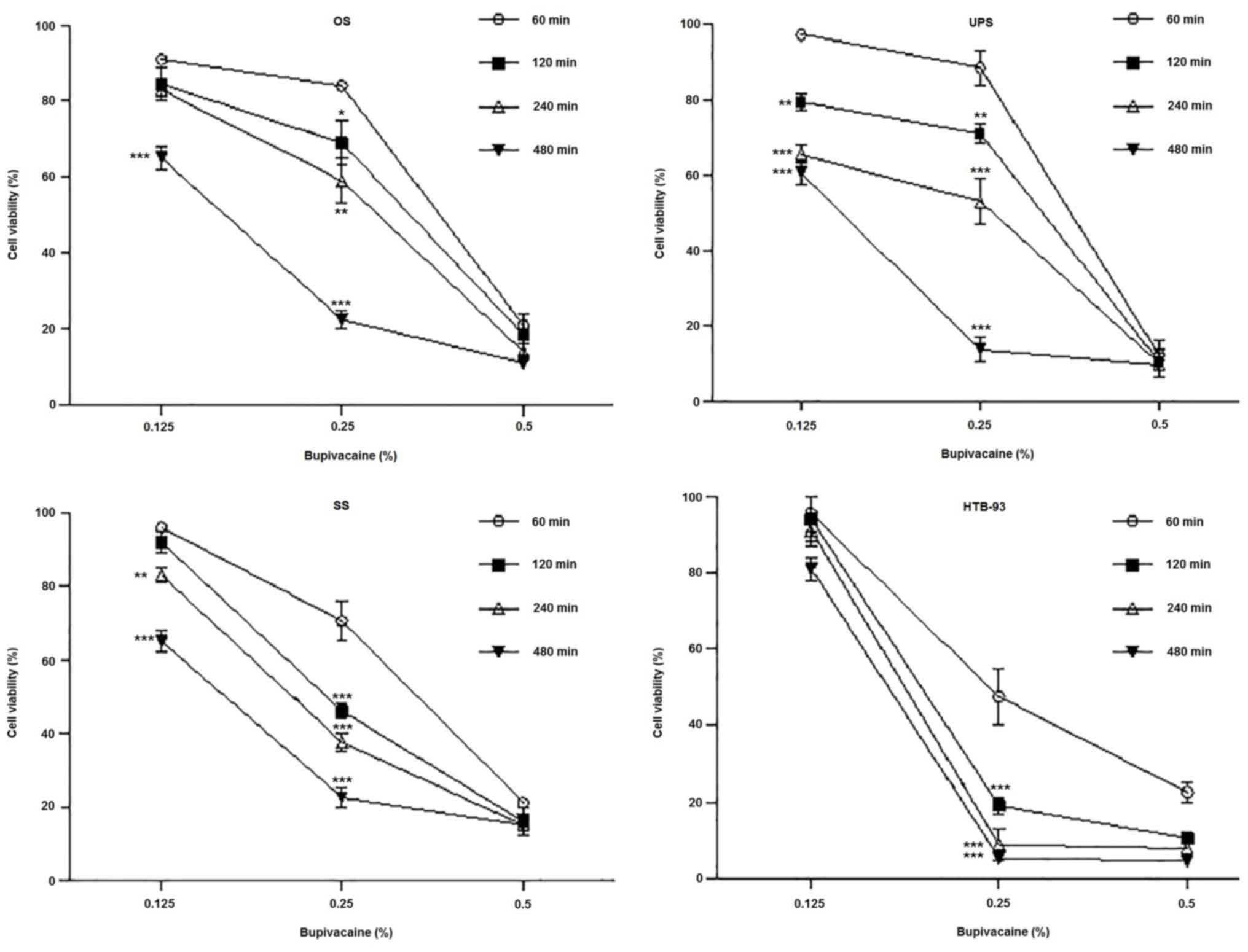
in a dose- and time-dependent decrease in OS cell viability
(Fig. 2). Significantly decreased
viability was observed after exposure to 0.5% of bupivacaine when
compared to 0.125% at 60, 120, 240 and 480 min, and to 0.25% at 60,
120 and 240 min (P<0.001). As the duration of exposure
increased, there was a corresponding decrease in cell viability.
The time-dependent effect was more pronounced after treatment with
0.125 and 0.25% of bupivacaine, with a significant decrease
occurring after 480 min of exposure compared to 60 min
(P<0.001).
Analysis of cell viability data on UPS tumor cells
revealed a similar cytotoxic effect after treatment with different
doses of bupivacaine (Fig. 2). Cell
death occurred more rapidly after treatment with 0.5% bupivacaine,
with a significant reduction in cell viability observed after 60
min. (P<0.001). The difference in viability with 0.125 and 0.25%
bupivacaine was significant at 120, 240 and 480 min compared to 60
min (P<0.01, P<0.001 and P<0.001, respectively).
All concentrations of bupivacaine caused a
significant decrease in the viability of SS tumor cells (Fig. 2). The difference in cell viability
with 0.125 and 0.25% bupivacaine compared to 0.5% bupivacaine was
significant at all the time points (P<0.001). Moreover, the
difference in cell viability with 0.125% bupivacaine was
significant at 240 and 480 min compared to 60 min (P<0.05,
P<0.001 respectively) but was not significant at 120 min
(P>0.5). The difference in viability was significant at 120, 240
and 480 min compared to 60 min (P<0.001) in cells that were
treated with 0.25% bupivacaine. The cytotoxicity of 0.25%
bupivacaine on SS tumor cells at 60, 120 and 240 min was more
pronounced compared to OS and UPS cells at the same doses (70%
viability vs. 84 and 88%, 46% vs. 69 and 71% and 37% vs. 59 and
53%, respectively). The cell viability data on HTB-93 cells
revealed a similar cytotoxic effect after treatment with different
doses of bupivacaine (Fig. 2).
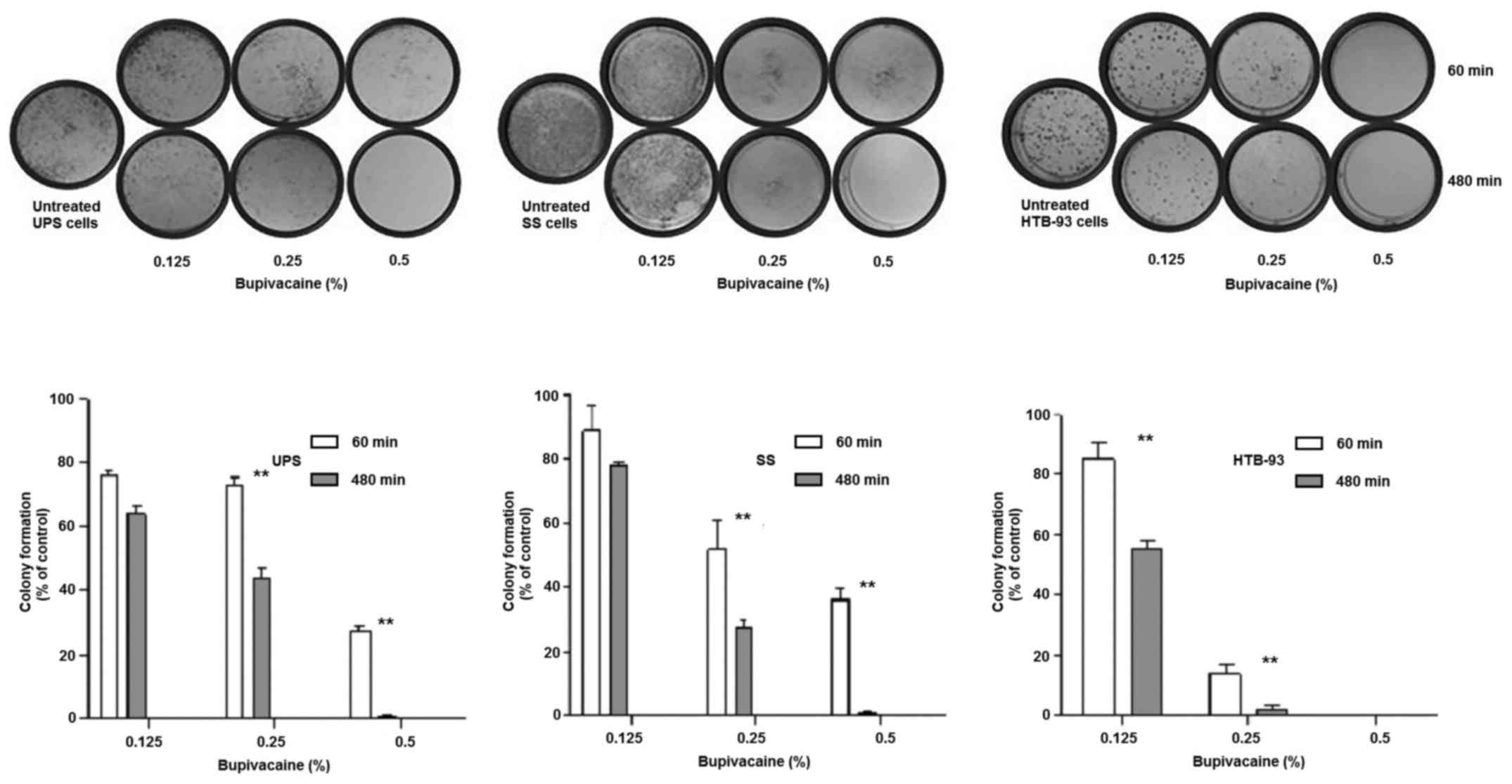
Bupivacaine was also found to disrupt colony forming
ability in UPS and SS in a heterologous population as well in
HTB-93 cells which are a homologous cell population when compared
with the controls. The results showed that the colony formation
ability of tumor cells was reduced after exposure to bupivacaine,
in a time- and dose-dependent manner (Fig. 3). The UPS cells had a significant
decrease in colony formation at both 0.25 and 0.5% bupivacaine
between 60 and 480 min (P<0.01) with minimal colonies remaining
after 480 min at 0.5%. SS demonstrated similar results. HTB-93 had
a significant difference between 60 and 480 min at both 0.125 and
0.25%. No colonies remained after treatment with 0.5% at both 60
and 480 min.
Bupivacaine induces abnormal
morphologic changes in sarcoma cells
To verify the cytotoxicity of the drug, the
morphological changes in OS, UPS and HTB-93 cells were examined by
phase-contrast photomicrograph at 24 h after exposure to
bupivacaine. Fig. 4A shows that OS
and UPS cells exhibited abnormal morphological changes, which are
associated with programmed cell death (apoptosis) and characterized
by cellular shrinkage, turning round, floating and eventually death
when compared to untreated tumor cells after 1 h in a
dose-dependent manner. Similar results were observed for SS cells
(data not shown). These results occurred at clinically relevant
doses of bupivacaine.
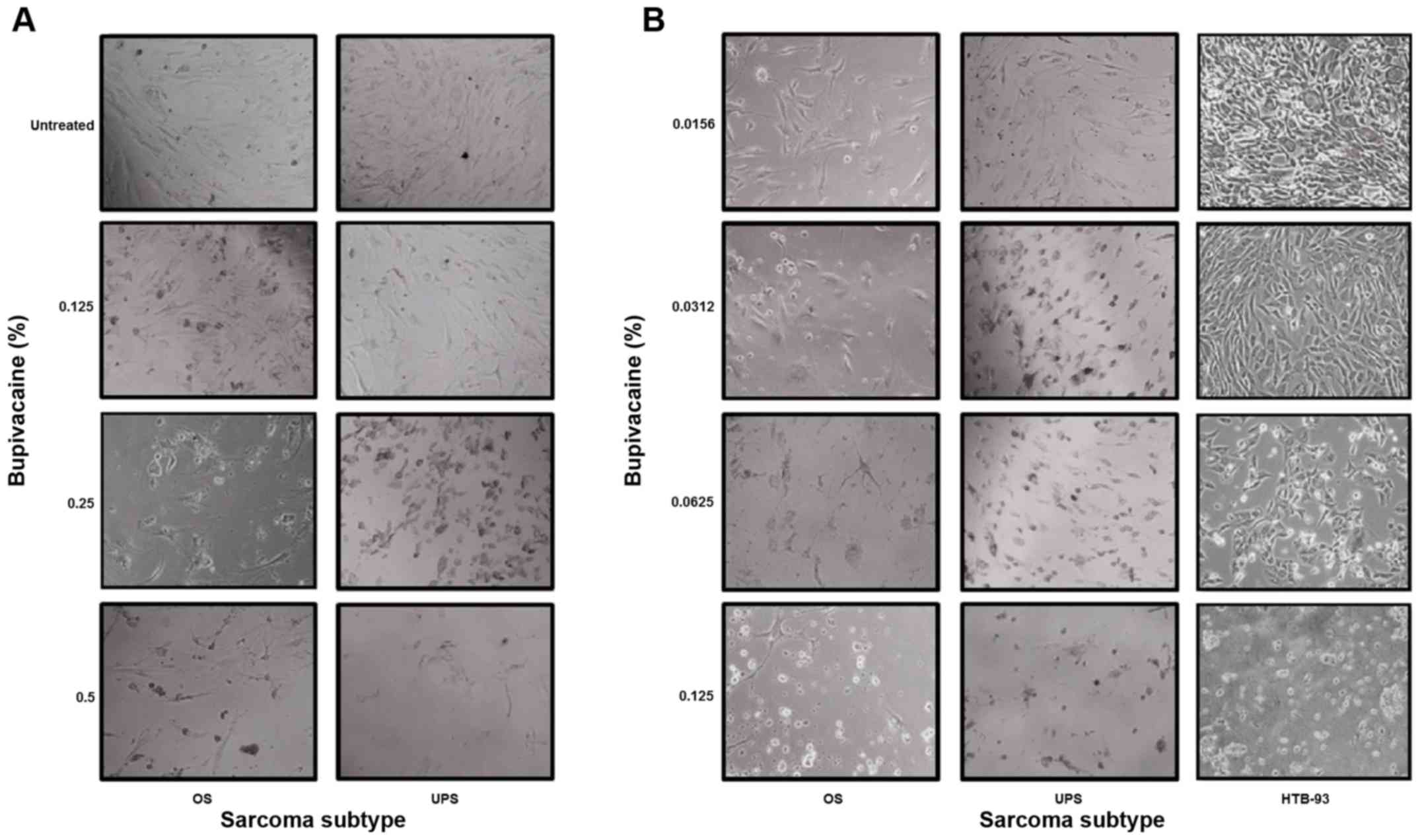 | Figure 4Bupivacaine induces abnormal
morphological changes in sarcoma tumor cells. (A) OS and UPS tumor
cells were treated with bupivacaine for 1 h at the following
concentrations: 0.125, 0.25 and 0.5% (4.33, 8.66 and 17.33 mM,
respectively). (B) OS, UPS and HTB-93 cells were treated with
bupivacaine at concentrations of 0.0156, 0.0312, 0.0625 and 0.125%
(0.54, 1.08, 2.16 and 4.33 mM, respectively) for 24 h. Morphologic
changes of the tumor cells were examined by phase-contrast
photomicrograph after 24 h. The treated cells underwent cellular
shrinkage, turned round, floated and eventually death when compared
to untreated tumor cells. SS, synovial sarcoma; UPS,
undifferentiated pleomorphic sarcoma; OS, osteosarcoma. |
OS, UPS and HTB-93 cells also exhibited abnormal
morphological changes, when exposed to 0.0156, 0.0312, 0.0625 and
0.125% (0.54, 1.08, 2.16 and 4.33 mM) of bupivacaine after 24 h in
a dose-dependent manner compared to untreated tumor cells (Fig. 4B). The cell viability assay showed
that the IC50 was between 0.04 and 0.05% (data not
shown). All together it shows that the cytotoxicity of bupivacaine
occurs in a time- and dose-dependent manner.
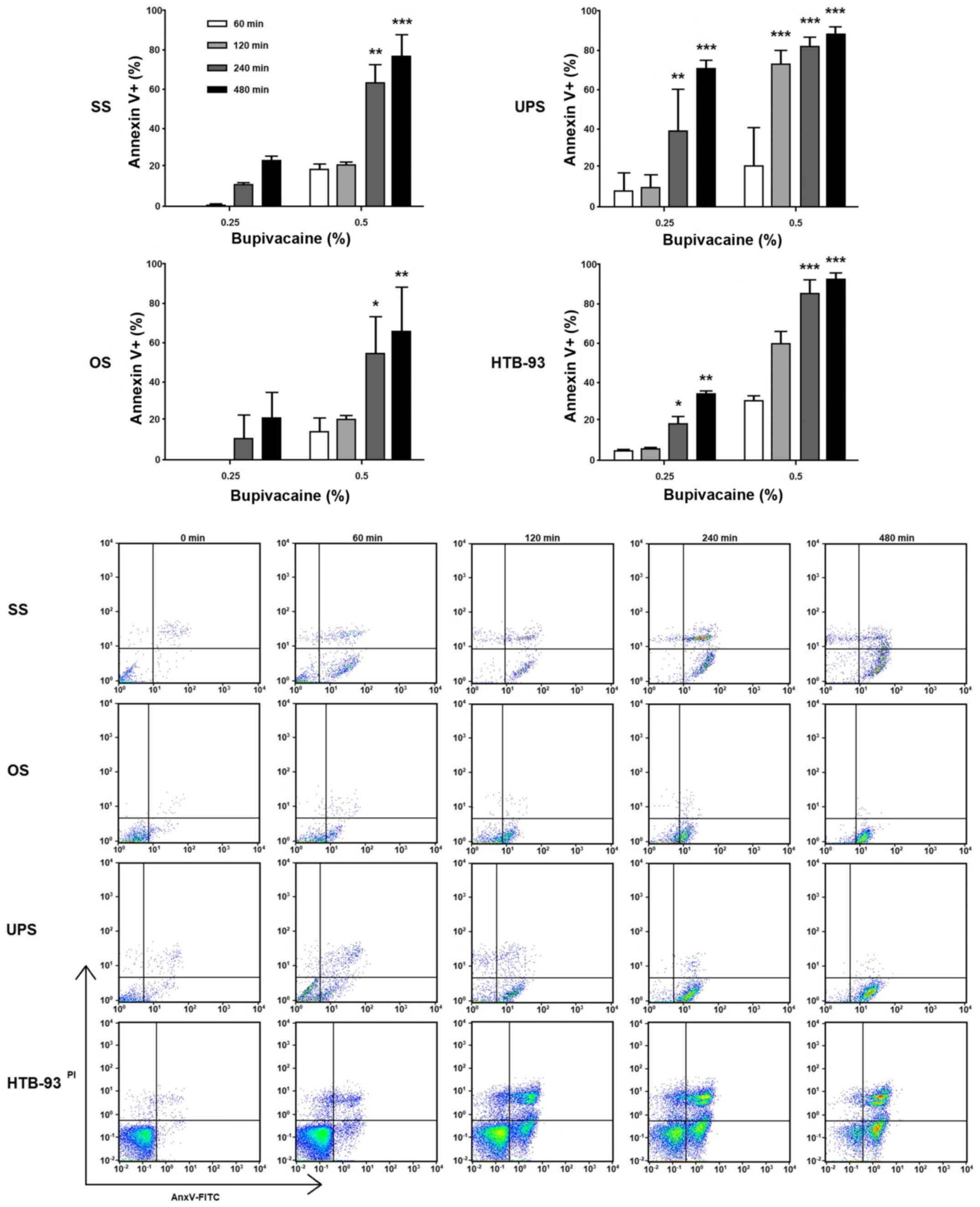
Bupivacaine induces apoptosis in tumor
cells
During apoptosis, translocation of
phosphatidylserine (PS) from the internal leaflet of the
cellular membrane to the external leaflet is a common feature and
key step (25). To investigate the
mechanism underlying the decreased viability, tumor cells were
exposed to bupivacaine at various doses for different time
durations. After 24 h, Annexin-V and PI staining was performed.
Similar to the results obtained in the cell viability assay, an
increase in apoptotic cells occurred across all tumor groups with
increased bupivacaine concentration and duration of exposure when
compared with the controls (Fig.
5). For the OS cells, an increase in apoptotic cells was seen
after 240 and 480 min of exposure to 0.25%. After exposure to 0.5%
bupivacaine an increase was seen at 60 and 120 min, with a
significant increase occurring after 240 (P<0.01) and 480 min
(P<0.001), and a range of 40-70% apoptotic cells present. In the
UPS tumor cells, an increase in apoptosis was seen with both 0.25
and 0.5% of bupivacaine. A significant increase occurred after 240
and 480 min of exposure to 0.25% of bupivacaine, with a range of
70-90% apoptotic cells present (P<0.01 and P<0.001,
respectively). A significant increase occurred after 120, 240 and
480 min of exposure to 0.5% of bupivacaine, with a range of 40-70%
apoptotic cells present (P<0.001; Fig. 5). For the SS tumor cells, an
increase in apoptotic cells was seen with both concentrations, with
a significant difference occurring after 240 min (P<0.05) and
480 min (P<0.01) of exposure to 0.5% of bupivacaine. The
percentage of apoptotic cells remained below 20 with 0.25 and 0.5%
of bupivacaine at the remaining time points and did not represent a
significant difference (P>0.05). The HTB-93 cells had a
significant increase in the number of apoptotic cells at both
concentrations. At 0.25%, a difference was noted at 240 (P<0.05)
and 480 (P<0.01) min. After exposure to 0.5% bupivacaine, a
significant difference was noted at 240 and 480 min
(P<0.001).
Discussion
At clinically relevant concentrations, in
vitro exposure to bupivacaine caused a decrease in cellular
viability and an increase in the induction of apoptosis. These
effects were seen in all tumor types evaluated in this study. The
results from both the viability and apoptosis assays indicate that
longer exposure to bupivacaine causes greater toxicity.
Additionally, the cytotoxicity of 0.5% of bupivacaine was greater
than that of 0.125 and 0.25% of bupivacaine at all time points for
each sarcoma subtype. The data also suggests that apoptosis may
play a role in the bupivacaine-induced cytotoxicity observed among
the different sarcoma tumors.
The concentrations of bupivacaine (4.33, 8.66, and
17.33 mM) which were used in this study may raise the question
whether these concentrations are applicable in a clinical context.
Other studies regarding the anti-cancer properties of bupivacaine
have used 1-5 mM and tumor cells were exposed for longer times
(24-72 h) (26,27). However, in our study, cells were
exposed to these concentrations for only 1-8 h. In addition,
Fig. 4 shows that the inhibitory
effect of bupivacaine can be reached at a lower concentration with
a longer exposure time.
Bupivacaine is a common medication for pain control
that can be used directly at the surgical site, with peripheral
nerve blocks, and with epidural/spinal analgesia (28,29). A
continuous infusion can also be performed using patient controlled
analgesia or a continuous pump (30,31).
During the biopsy of sarcoma, seeding of the biopsy tract can occur
(32). Infiltrating the biopsy
tract with bupivacaine could decrease the risk of contamination
during the biopsy. Still, care should be taken not to develop
separate planes in the tissue where viable tumor cells could
extravasate. After resection, a catheter with a continuous pump of
bupivacaine could be used. In theory, this would bathe the
resection area in bupivacaine and hopefully decrease the risk of
local recurrence.
A limitation of this study is that it was conducted
in vitro, and therefore does not account for the dilutional
effects of bleeding or the absorption and clearance of bupivacaine
that would occur in vivo. The latter issue may be mitigated
with the use of a continuous infusion pump, which would continually
bathe the resected tumor bed as noted above. As with all in
vitro studies, the findings cannot be extrapolated to in
vivo situations. However, the controlled nature of in
vitro studies allows for a reproducible and quantitative means
of assessing cell viability. Using flow cytometry, we were able to
show a dose- and time-dependent cytotoxic effect of bupivacaine on
all of the sarcoma tumors that were analyzed.
A significant advantage to the use of bupivacaine is
that it is cost-effective, commonly used and has an established
side-effect profile. This would allow the medication to be used in
clinical trials without the need for the development of a new
medication. It also provides a method of pain control for the
patient and has been used to perform opioid-free anesthesia in
patients with breast cancer undergoing modified radical mastectomy
(33,34). Similarly, bupivacaine is commonly
used for pain control in orthopaedic surgery to decrease opioid
consumption after surgery (35).
Pain due to cancer is a significant problem and decreasing opioid
use in this patient population can have multiple benefits while
decreasing the complications associated with opioid use (36-38).
One concern regarding the use of bupivacaine is the
risk of systemic toxicity (39).
Inappropriate dosing or intravascular injection can result in
complications such as cardiac arrhythmia and arrest. In the current
study, bupivacaine caused cell death in vitro with an
IC50 between 0.04 and 0.05%, whereas 0.25 and 0.5%
bupivacaine are the typical concentrations used in the clinical
setting. The results are promising that the effects noted in this
study could occur in vivo at clinically appropriate doses.
Another consideration would be to administer bupivacaine while
utilizing isolated limb perfusion (40). Although the patient would still have
to be monitored for both local and systemic toxicity, this may
provide an option to treat sarcoma locally by directing the
medication through the blood supply.
In this study, we used tumor cells which were
harvested directly from patients as well as a commercial cell line.
The heterogeneity of the resected sarcoma is present in harvested
cells in addition to separate genetic factors for each patient.
Still, this raises the question of whether the treatment effect
only occurred due to these factors. The advantage of using the cell
line in this study would be that the treatment effect is
reproducible as the individual patient factors are absent. Sarcomas
are a heterogeneous group of malignancies with over 100 subtypes
described (41). This heterogeneity
is further observed in the different subtypes of sarcoma which can
lead to resistance to chemotherapy and a worse prognosis (42). The fact that each different subtype
of sarcoma in this study was responsive to treatment with
bupivacaine is promising. This demonstrates that not only the
heterogeneity of the different subtypes as well as the
heterogeneity of each individual patient was able to be effectively
treated.
Future experiments might compare tumor cell
viability on a larger number of sarcoma subtypes and with
additional formulations of bupivacaine, including 0.75% and
liposomal bupivacaine. Other LAs, such as lidocaine may also be
considered. If lidocaine also demonstrated toxicity, other
treatments, such as intravenous regional anesthesia with a Bier
block could also be considered to deliver the medication through
the circulation (43). Determining
the mechanism of action of bupivacaine would also be useful as this
may provide an opportunity to develop targeted therapies. An
increased understanding of the biomarkers involved would be
important as the mechanism causing cell death may differ between
the sarcoma subtypes. Testing the treatment in vivo in an
animal model would also determine whether the in vitro
findings are translatable.
In conclusion, these findings have potential
clinical relevance in the management of patients with sarcoma.
Consideration should be given to using bupivacaine while performing
biopsies to possibly eliminate contamination of the biopsy tract,
and as an adjuvant treatment after tumor resection. Further studies
are warranted to determine if these effects are demonstrated in
vivo.
Acknowledgements
The authors would like to thank Mrs. Elisabeth
Clarke, Clinical Research Coordinator, Department of Orthopaedic
Surgery, Loma Linda University Medical Center, for her support of
this research.
Funding
This study was funded by the Loma Linda University
Cancer Center, School of Medicine Dean's Office and Department of
Orthopaedic Surgery through the Grants to Promote Collaborative and
Translational Research program (GCAT 2016; grant no. 681163).
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
LMZ and SM conceived and designed this study. SM,
HRM and SO performed the testing on the cells and analyzed the
data. LMZ, SM, WLF, NLW, and TGS acquired the cells used for the
data in this study, interpreted the data, and drafted and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Loma Linda University
Institutional Review Board (IRB #58238) in accordance to the
provisions of the Declaration of Helsinki, and informed, written
consent was obtained from all patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nystrom LM, Reimer NB, Reith JD, Dang L,
Zlotecki RA, Scarborough MT and Gibbs CP Jr: Multidisciplinary
management of soft tissue sarcoma. Sci World J.
2013(852462)2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arndt CA, Rose PS, Folpe AL and Laack NN:
Common musculoskeletal tumors of childhood and adolescence. Mayo
Clin Proc. 87:475–487. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bacci G, Ferrari S, Longhi A, Donati D,
Manfrini M, Giacomini S, Briccoli A, Forni C and Galletti S:
Nonmetastatic osteosarcoma of the extremity with pathologic
fracture at presentation: Local and systemic control by amputation
or limb salvage after preoperative chemotherapy. Acta Orthop Scand.
74:449–454. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pobirci DD, Bogdan F, Pobirci O, Petcu CA
and Roşca E: Study of malignant fibrous histiocytoma: Clinical,
statistic and histopatological interrelation. Rom J Morphol
Embryol. 52:385–388. 2011.PubMed/NCBI
|
|
5
|
Chen S, Fritchie K, Wei S, Ali N, Curless
K, Shen T, Brini AT, Latif F, Sumathi V, Siegal GP and Cheng L:
Diagnostic utility of IDH1/2 mutations to distinguish
dedifferentiated chondrosarcoma from undifferentiated pleomorphic
sarcoma of bone. Hum Pathol. 65:239–246. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jeon DG, Song WS, Kong CB, Kim JR and Lee
SY: MFH of bone and osteosarcoma show similar survival and
chemosensitivity. Clin Orthop Relat Res. 469:584–590.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Natarajan MV, Mohanlal P and Bose JC: Limb
salvage surgery complimented by customised mega prostheses for
malignant fibrous histiocytomas of bone. J Orthop Surg (Hong Kong).
15:352–356. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun J, Zhang RM and Zheng YX: En bloc
resection and prosthesis implantation to treat malignant fibrous
histiocytoma of the humerus. Adv Clin Exp Med. 26:781–787.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rajwanshi A, Srinivas R and Upasana G:
Malignant small round cell tumors. J Cytol. 26:1–10.
2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eilber FC and Dry SM: Diagnosis and
management of synovial sarcoma. J Surg Oncol. 97:314–320.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sultan I, Rodriguez-Galindo C, Saab R,
Yasir S, Casanova M and Ferrari A: Comparing children and adults
with synovial sarcoma in the surveillance, epidemiology, and end
results program, 1983 to 2005: An analysis of 1268 patients.
Cancer. 115:3537–3547. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Krieg AH, Hefti F, Speth BM, Jundt G,
Guillou L, Exner UG, von Hochstetter AR, Cserhati MD, Fuchs B,
Mouhsine E, et al: Synovial sarcomas usually metastasize after
>5 years: A multicenter retrospective analysis with minimum
follow-up of 10 years for survivors. Ann Oncol. 22:458–467.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Deshmukh R, Mankin HJ and Singer S:
Synovial sarcoma: The importance of size and location for survival.
Clin Orthop Relat Res. 419:155–161. 2004.PubMed/NCBI
|
|
14
|
Paice JA and Ferrell B: The management of
cancer pain. CA Cancer J Clin. 61:157–182. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Borgeat A and Aguirre J: Update on local
anesthetics. Curr Opin Anaesthesiol. 23:466–471. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC
and Cheng SP: Local anesthetics induce apoptosis in human thyroid
cancer cells through the mitogen-activated protein kinase pathway.
PLoS One. 9(e89563)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chang YC, Liu CL, Chen MJ, Hsu YW, Chen
SN, Lin CH, Chen CM, Yang FM and Hu MC: Local anesthetics induce
apoptosis in human breast tumor cells. Anesth Analg. 118:116–124.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chapman GL, Zuckerman LM and Mirshahidi S:
The in vitro effects of bupivacaine on cartilage-forming tumor
cells. J Am Acad Orthop Surg. 27:e337–e345. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Exadaktylos AK, Buggy DJ, Moriarty DC,
Mascha E and Sessler DI: Can anesthetic technique for primary
breast cancer surgery affect recurrence or metastasis?
Anesthesiology. 105:660–664. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cummings KC, Xu F, Cummings LC and Cooper
GS: A comparison of epidural analgesia and traditional pain
management effects on survival and cancer recurrence after
colectomy: A population-based study. Anesthesiology. 116:797–806.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Biki B, Mascha E, Moriarty DC, Fitzpatrick
JM, Sessler DI and Buggy DJ: Anesthetic technique for radical
prostatectomy surgery affects cancer recurrence: A retrospective
analysis. Anesthesiology. 109:180–187. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tavare AN, Perry NJ, Benzonana LL, Takata
M and Ma D: Cancer recurrence after surgery: Direct and indirect
effects of anesthetic agents. Int J Cancer. 130:1237–1250.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aamri E and Basnawi A: Effects of
anesthesia & anesthetic techniques on cellular immunity. J
Anesth Crit Care Open Access. 7(00283)2017.
|
|
24
|
Vlenterie M, Litière S, Rizzo E, Marréaud
S, Judson I, Gelderblom H, Le Cesne A, Wardelmann E, Messiou C,
Gronchi A and van der Graaf TW: Outcome of chemotherapy in advanced
synovial sarcoma patients: Review of 15 clinical trials from the
European organisation for research and treatment of cancer soft
tissue and bone sarcoma group; setting a new landmark for studies
in this entity. Eur J Cancer. 58:62–72. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dan J, Gong X, Li D, Zhu G, Wang L and Li
F: Inhibition of gastric cancer by local anesthetic bupivacaine
through multiple mechanisms independent of sodium channel blockade.
Biomed Pharmacother. 103:823–828. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xuan W, Zhao H, Hankin J, Chen L, Yao S
and Ma D: Local anesthetic bupivacaine induced ovarian and prostate
cancer apoptotic cell death and underlying mechanisms in vitro. Sci
Rep. 6(26277)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vishwanatha S and Kalappa S: Continuous
femoral nerve blockade versus epidural analgesia for postoperative
pain relief in knee surgeries: A randomized controlled study.
Anesth Essays Res. 11:599–605. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tam KW, Chen SY, Huang TW, Lin CC, Su CM,
Li CL, Ho YS, Wang WY and Wu CH: Effect of wound infiltration with
ropivacaine or bupivacaine analgesia in breast cancer surgery: A
meta-analysis of randomized controlled trials. Int J Surg.
22:79–85. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dhanapal B, Sistla SC, Badhe AS, Ali SM,
Ravichandran NT and Galidevara I: Effectiveness of continuous wound
infusion of local anesthetics after abdominal surgeries. J Surg
Res. 212:94–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moslemi F, Rasooli S, Baybordi A and
Golzari SE: A comparison of patient controlled epidural analgesia
with intravenous patient controlled analgesia for postoperative
pain management after major gynecologic oncologic surgeries: A
randomized controlled clinical trial. Anesth Pain Med.
5(e29540)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Barrientos-Ruiz I, Ortiz-Cruz EJ,
Serrano-Montilla J, Bernabeu-Taboada D and Pozo-Kreilinger JJ: Are
biopsy tracts a concern for seeding and local recurrence in
sarcomas? Clin Orthop Relat Res. 475:511–518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tripathy S, Rath S, Agrawal S, Rao PB,
Panda A, Mishra TS and Nayak S: Opioid-Free anesthesia for breast
cancer surgery: An observational study. J Anaesthesiol Clin
Pharmacol. 34:35–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tripathy S, Mandal I, Rao PB, Panda A,
Mishra T and Kar M: Opioid-free anesthesia for breast cancer
surgery: A comparison of ultrasound guided paravertebral and
pectoral nerve blocks. A randomized controlled trial. J
Anaesthesiol Clin Pharmacol. 35:475–480. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shlaifer A, Sharfman ZT, Martin HD, Amar
E, Kazum E, Warschawski Y, Paret M, Brill S, Drexler M and Rath E:
Preemptive analgesia in hip arthroscopy: A randomized controlled
trial of preemptive periacetabular or intra-articular bupivacaine
in addition to postoperative intra-articular bupivacaine.
Arthroscopy. 33:118–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Manchikanti L, Manchikanti KN, Kaye AD,
Kaye AM and Hirsch JA: Challenges and concerns of persistent opioid
use in cancer patients. Expert Rev Anticancer Ther. 18:705–718.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pinkerton R and Hardy JR: Opioid addiction
and misuse in adult and adolescent patients with cancer. Intern Med
J. 47:632–636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Paice JA: Cancer pain management and the
opioid crisis in America: How to preserve hard-earned gains in
improving the quality of cancer pain management. Cancer.
124:2491–2497. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ok SH, Hong JM, Lee SH and Sohn JT: Lipid
emulsion for treating local anesthetic systemic toxicity. Int J Med
Sci. 15:713–722. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Neuwirth MG, Song Y, Sinnamon AJ, Fraker
DL, Zager JS and Karakousis GC: Isolated limb perfusion and
infusion for extremity soft tissue sarcoma: A contemporary
systematic review and meta-analysis. Ann Surg Oncol. 24:3803–3810.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bleloch JS, Ballim RD, Kimani S, Parkes J,
Panieri E, Willmer T and Prince S: Managing Sarcoma: Where have we
come from and where are we going? Ther Adv Med Oncol. 9:637–659.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lohberger B, Stuendl N, Leithner A, Rinner
B, Sauer S, Kashofer K and Liegl-Atzwanger B: Establishment of a
novel cellular model for myxofibrosarcoma heterogeneity. Sci Rep.
7(44700)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gale AL, Liberman SR, Berry S, Zavlin D
and Echo A: Fluorescent imaging evaluation of lidocaine
distribution following bier block in the upper extremity. Surg
Technol Int. 31:31–34. 2017.PubMed/NCBI
|