Introduction
Bladder cancer can present in different pathological
stages. Approximately 80% of all bladder cancers initially present
as non-muscle-invasive bladder carcinoma (NMIBC) (1). Transurethral resection of bladder
tumor (TURBT) is known as the gold standard therapeutic method for
NMIBC; however, the recurrence rate ranges between 40 and 80%
regardless of complete resection (2). The risk of recurrence and progression
of NMIBC can be predicted and calculated for each patient using the
risk score suggested by the European Organization for Research and
Treatment of Cancer (3).
It is thought that just within a few hours after
TURBT, the free-floating tumor cells become firmly integrated to
nearby structures and are covered by extracellular matrix (4). Reportedly, one of the mechanisms of
early NMIBC recurrence after TURBT might be the dissemination of
free-floating tumor cells during surgery, with the subsequent
implantation of these cells after TURBT (5).
As NMIBC may recur and progress to muscle-invasive
cancer after initial treatment (1),
there is a need for efficient therapeutic strategies to decrease
possible recurrence and/or progression. An immediate single
instillation of chemotherapy (SIC) after TURBT is broadly
recognized as an effective preventive measure for intravesical
recurrence (IVR) in patients with NMIBC. This measure is especially
effective among those with low- or intermediate-risk NMIBC and with
low-grade Ta NMIBC according to the European Association of Urology
(EAU) and American Urological Association (AUA) guidelines,
respectively (4,6). Nevertheless, many urologists still
hesitate to apply SIC to patients with NMIBC because the procedure
is costly, may involve special postoperative care, and could result
in unexpected lower urinary tract symptoms, including micturition
pain, irritability reactions, and extravasation of intravesical
chemotherapy agents (7,8).
Conversely, continuous saline bladder irrigation
(CSBI) is another therapeutic and inhibitory option for IVR. Onishi
et al (9) have hypothesized
that CSBI after TURBT remove floating tumor cells and prevent tumor
cells from implanting on the bladder wall. They have concluded that
CSBI after TURBT may be a feasible prophylactic and therapeutic
option for patients with low- to intermediate-risk NMIBC (9). In a previous retrospective study,
Onishi et al (10) have
shown that CSBI after TURBT has a preventive effect on IVR of
NMIBC.
Urologists have been frequently performing CSBI
immediately after SIC at our institution. The objective is to
prevent catheter obstruction or genitourinary infection. In the
present study, we evaluated whether the combined treatment of CSBI
with concomitant SIC after TURBT has an inhibitory effect on IVR in
patients with NMIBC.
Patients and methods
Patients
We performed a retrospective review of the medical
records of 253 patients who underwent TURBT between January 2010
and February 2018. Patients were clinically diagnosed with NMIBC,
and the diagnosis was histologically confirmed as urothelial
carcinoma with or without other tumor cell types at our
institution. Processing of resected specimens was performed
according to standard pathological procedures. The pathological
staging of the primary tumor (pT) was determined according to the
American Joint Committee on Cancer TNM Classification (11), whereas tumor grading was determined
according to the 2004 WHO classification of urothelial tumors
(12). Patients were followed up
for at least 3 months postoperatively at our institution.
Our institutional ethics committee approved the
study protocol (ID 2734) on June 14, 2017. An opt-out approach on
the web page of the National Defense Medical College was used
rather than collecting written informed consent from all
participants. A total of 198 men and 55 women with a median age of
74 years (range, 33-98 years) were included in the present study.
The median follow-up period after TURBT was 32.9 months (range,
3.1-98.6 months).
Doxorubicin (DXR) was administered to all patients
immediately after TURBT, and all patients underwent either adjuvant
intravesical chemotherapy or immunotherapy. Patients received DXR
by three methods of administration: A single instillation of DXR
(60 mg in 30-40 ml saline) in 34 patients (group A); CSBI with DXR
80 mg (80 mg in 1 liter saline) in 40 patients (group B); and
overnight CSBI after a single instillation of DXR in 179 patients
(group C). The difference between groups B and C was that patients
in group B were treated with continuous irrigation of the bladder
with saline including DXR (80 mg in 1 liter saline), whereas those
in group C were treated with continuous bladder irrigation with
saline after a single instillation of chemotherapy (SIC).
Additional pathological and clinical data are shown in Table I.
 | Table IClinicopathological characteristics of
the enrolled patients. |
Table I
Clinicopathological characteristics of
the enrolled patients.
| Parameters | Immediate
instillation alone, n (%) (n=34) | Saline irrigation
including doxorubicin, n (%) (n=40) | Immediate
instillation plus saline irrigation, n (%) (n=179) | P-value |
|---|
| Age, years
(range) | 74.5 (46-92) | 71.5 (42-89) | 74 (33-98) | 0.343 |
| Sex | | | | 0.146 |
|
Men | 22 (64.7) | 32 (80.0) | 144 (80.4) | |
|
Women | 12 (35.3) | 8 (20.0) | 35 (19.6) | |
| Urine cytology | | | | 0.003 |
|
≥3b | 11 (33.3) | 14 (35.0) | 103 (58.2) | |
|
≤3a | 22 (66.7) | 26 (65.0) | 74 (41.8) | |
| Smoking history | | | | 0.146 |
|
Positive | 18 (52.9) | 30 (79.0) | 115 (66.9) | |
|
Negative | 16 (47.1) | 8 (21.0) | 57 (33.1) | |
| History of UTUC | | | | 0.339 |
|
Positive | 3 (8.8) | 6 (15.0) | 13 (7.3) | |
|
Negative | 31 (91.2) | 34 (85.0) | 166 (92.7) | |
| First or recurrent
tumor | | | | 0.003 |
|
Recurrent | 12 (35.3) | 17 (42.5) | 33 (18.4) | |
|
First | 22 (64.7) | 23 (57.5) | 146 (81.6) | |
| Solitary or multiple
tumors | | | | 0.784 |
|
Multiple | 22 (64.7) | 27 (67.5) | 126 (70.4) | |
|
Solitary | 12 (35.3) | 13 (32.5) | 53 (29.6) | |
| Histology | | | | 0.317 |
|
UC and other
subtypes | 1 (2.9) | 4 (10.0) | 18 (10.1) | |
|
UC
alone | 33 (97.1) | 36 (90.0) | 161 (89.9) | |
| pT status | | | | 0.036 |
|
pTis | 6 (17.7) | 0 (0.0) | 16 (8.9) | |
|
pT1 | 11 (32.3) | 18 (45.0) | 67 (37.4) | |
|
pTa | 17 (50.0) | 22 (55.0) | 96 (53.7) | |
| Tumor grade | | | | 0.205 |
|
High or
G3 | 23 (67.7) | 23 (57.5) | 129 (72.1) | |
|
PUNLMP/low | 11 (32.3) | 17 (42.5) | 50 (27.9) | |
| CIS | | | | 0.011 |
|
Positive | 10 (29.4) | 5 (12.5) | 63 (35.2) | |
|
Negative | 24 (70.6) | 35 (87.5) | 116 (64.8) | |
| Adjuvant therapy | | | | 0.010 |
|
BCG | 23 (67.7) | 15 (37.5) | 111 (62.0) | |
|
Chemotherapeutic
drugs | 11 (32.3) | 25 (62.5) | 68 (38.0) | |
Statistical analysis
Fisher's exact probability test and Kruskal-Wallis
test were used to evaluate significant differences in
clinicopathological factors among patients in groups A, B and C.
IVR-free survival curves were constructed using the Kaplan-Meier
method, and the statistical differences among the groups were
evaluated using the log-rank test. Additionally, univariate and
multivariate analysis was performed using Cox's proportional
hazards model before propensity score matching. Nearest-neighbor
propensity score matching was conducted using multiple logistic
regression analysis. For this, we designated group A and B or group
A and C as dependent variables, and all covariates shown in tables
as explanatory variables. Further, the difference in IVR-free
survival rates was also compared using the log-rank test after
adjusting for significant differences in several covariates between
the groups by nearest-neighbor propensity score matching. Fisher's
exact probability test and Mann-Whitney U test were used to
evaluate significant differences in clinicopathological factors
between the matched groups A and B and matched groups A and C.
Statistical analyses were performed with JMP Pro 11 (SAS
Institute). A P-value <0.05 was considered statistically
significant.
Results
Intravesical recurrence (IVR)-free
survival time among groups and independent factors for shortened
time to IVR
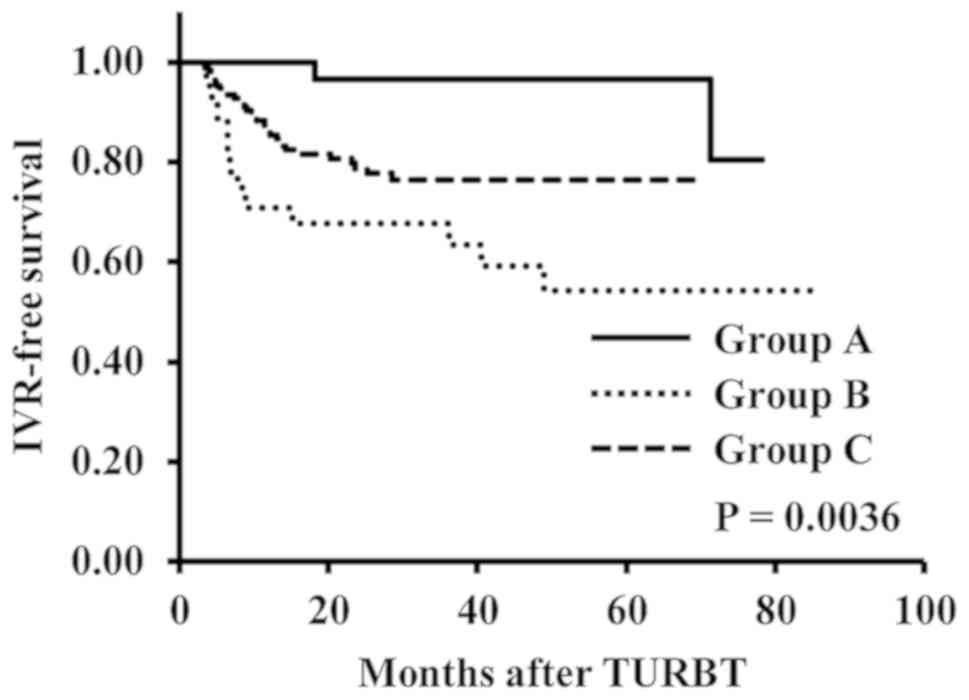
Prior to propensity score matching, we found that
time to IVR was significantly longer in the group A than in the
groups B and C (P=0.0036) (Fig. 1).
Additionally, a multivariate analysis using Cox's proportional
hazards model showed that CSBI (groups B and C) was a significant
independent factor for shorter time to IVR [group A to B: Hazard
ratio (HR), 8.905; 95% confidence interval (CI), 2.450-57.106;
P<0.001, and group A to C: HR, 4.193; 95% CI, 1.236-26.212;
P=0.018] (Table II). Several other
factors, including positive urine cytology, tumor history,
pathological tumor stage, presence of CIS, and adjuvant therapeutic
drugs, significantly differed among the three groups (P=0.003,
P=0.003, P=0.036, P=0.011 and P=0.010, respectively) (Table I).
 | Table IIUnivariate and multivariate analyses
of independent factors for IVR-free survival. |
Table II
Univariate and multivariate analyses
of independent factors for IVR-free survival.
| | Univariate | Multivariate |
|---|
| Pathological
measurements | Hazard ratio | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (<74 or ≥74
years) | 1.030 | 0.063 | 1.030 | 0.996-1.065 | 0.082 |
| Sex (male or
female) | 1.020 | 0.953 | | | |
| Smoking history
(positive or negative) | 0.995 | 0.987 | | | |
| History of UTUC
(positive or negative) | 0.642 | 0.457 | | | |
| Urine cytology
(positive or negative) | 1.957 | 0.021 | 2.106 | 1.150-3.963 | 0.016 |
| Tumor history
(recurrent or primary) | 1.792 | 0.061 | 1.855 | 0.947-3.522 | 0.071 |
| Tumor multiplicity
(multiple or single) | 1.393 | 0.294 | | | |
| Histology (UC with
others or UC alone) | 0.958 | 0.934 | | | |
| Pathological T
stage (T1 or Ta or Tis) | 1.411 | 0.279 | | | |
| Tumor grade
(G3/high or PUNLMP/low) | 1.752 | 0.096 | | | |
| Carcinoma in
situ (positive or negative) | 0.923 | 0.799 | | | |
| Adjuvant therapy
(BCG or chemotherapeutic drug) | 1.112 | 0.717 | | | |
| Immediate
instillation method (A or B or C) | 8.424 | 0.002 | 8.905 | 2.450-57.106 | <0.001 |
Nearest-neighbor propensity score
matching
We calculated the predicted probability as a
propensity score using multiple logistic regression analysis. By
using nearest-neighbor matching, we matched 18 pairs between groups
A and B and 33 pairs between groups A and C. Notably, we did not
find significant differences in any covariates between the matched
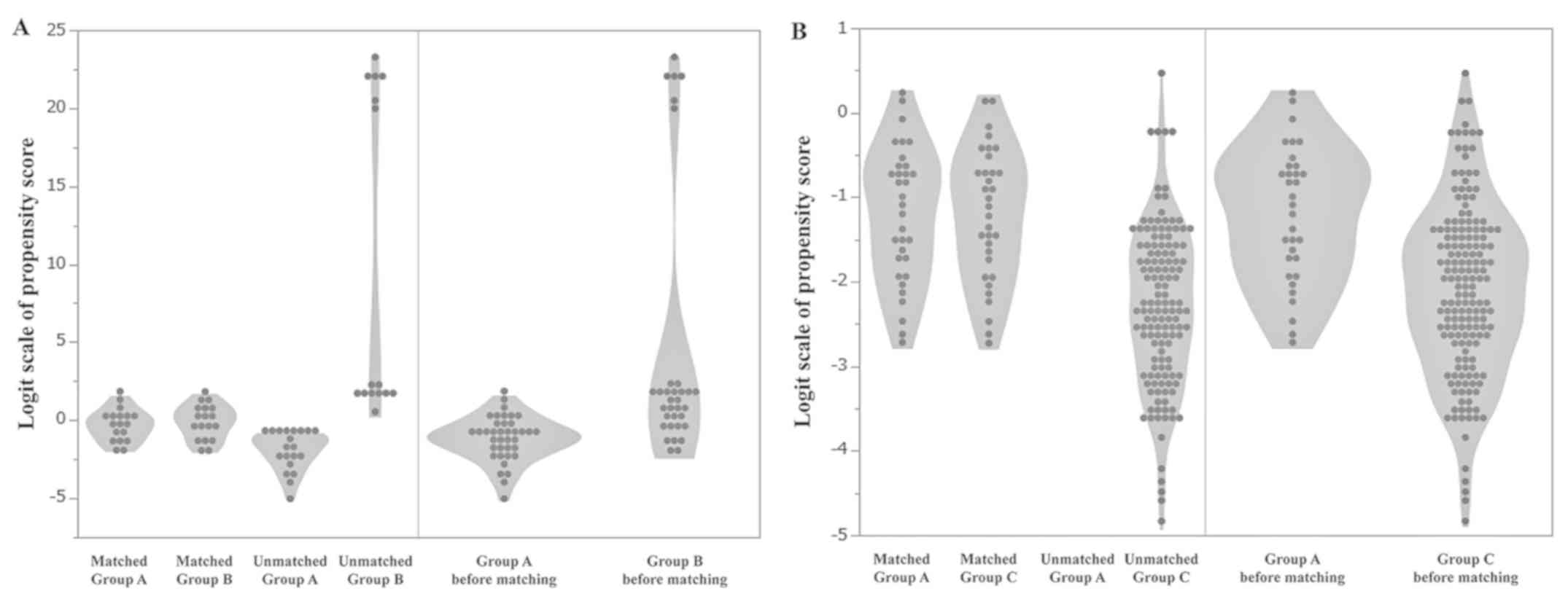
groups A and B and matched groups A and C (Tables III and IV). There was not any statistical
difference in the factors on the violin plots between matched
groups A and B (Fig. 2A) or between
matched groups A and C (Fig. 2B).
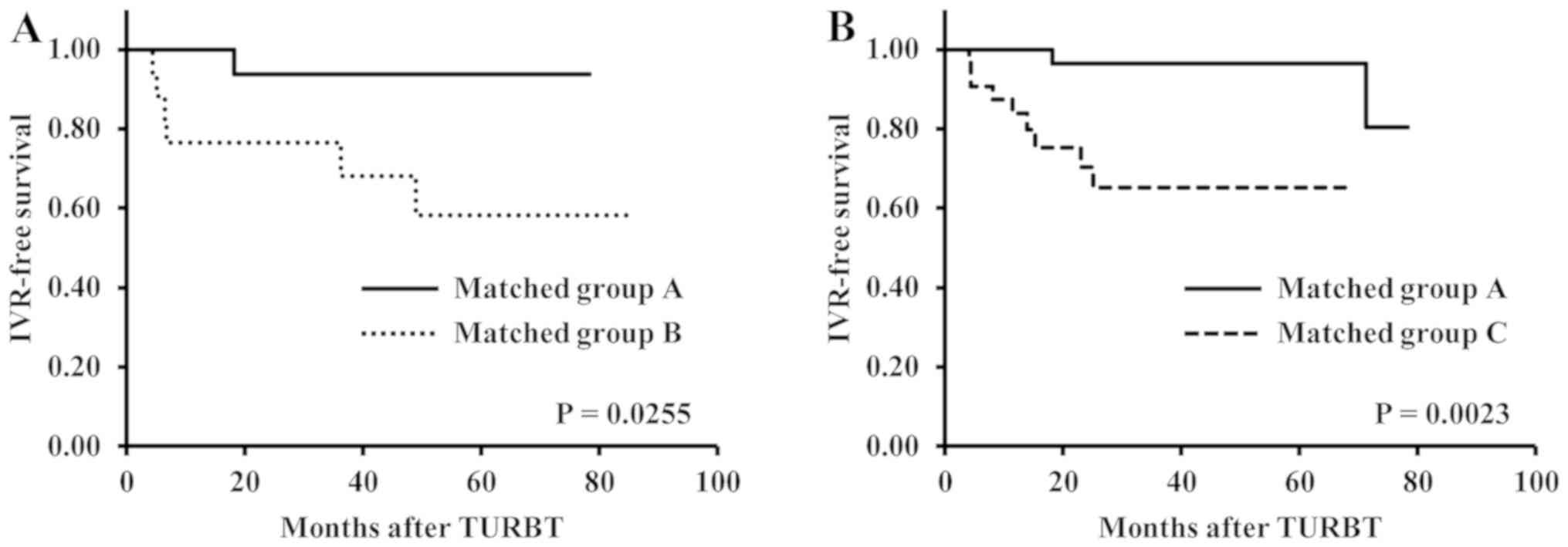
We did observe that time to IVR was significantly longer in matched
group A than in matched groups B and C (P=0.0255 and P=0.0023,
respectively) (Fig. 3A and B). In addition, the number of patients in
each group was decreased to diminish a significant difference in
each factor between the groups using nearest neighbor matching.
That is why the patients were censored at different times (Fig. 3A and B). The hazard ratio was 7.72 in the pairs
matched between the groups A and B and 12.49 in the pairs matched
between the groups A and C using Cox's proportional hazards model
(data not shown).
 | Table IIICharacteristics of patients matched
on propensity score. |
Table III
Characteristics of patients matched
on propensity score.
| Parameters | Immediate
instillation alone, n (%) (n=18) | Saline irrigation
including DXR, n (%) (n=18) | P-value |
|---|
| Age, years
(range) | 71 (46-92) | 75 (42-82) | 0.787 |
| Sex | | | 0.479 |
|
Male | 11 (61.1) | 13 (72.2) | |
|
Female | 7 (38.9) | 5 (27.8) | |
| Urine cytology | | | >0.999 |
|
≥3b | 13 (72.2) | 13 (72.2) | |
|
≤3a | 5 (27.8) | 5 (27.8) | |
| Smoking
history | | | 0.479 |
|
Positive | 11 (61.1) | 13 (72.2) | |
|
Negative | 7 (38.9) | 5 (27.8) | |
| History of
UTUC | | | 0.543 |
|
Positive | 2 (11.1) | 1 (5.6) | |
|
Negative | 16 (88.9) | 17 (94.4) | |
| First or recurrent
tumor | | | 0.717 |
|
Recurrent | 5 (27.8) | 6 (33.3) | |
|
First | 13 (72.2) | 12 (66.7) | |
| Solitary or
multiple tumors | | | 0.729 |
|
Multiple | 12 (66.7) | 11 (61.1) | |
|
Solitary | 6 (33.3) | 7 (38.9) | |
| Histology | | | 0.543 |
|
UC and other
subtypes | 1 (5.6) | 2 (11.1) | |
|
UC
alone | 17 (94.4) | 16 (88.9) | |
| pT status | | | >0.999 |
|
pTis | 0 (0.0) | 0 (0.0) | |
|
pT1 | 8 (44.4) | 8 (44.4) | |
|
pTa | 10 (55.6) | 10 (55.6) | |
| Tumor grade | | | 0.735 |
|
High or
G3 | 11 (61.1) | 10 (55.6) | |
|
PUNLMP/low | 7 (38.9) | 8 (44.4) | |
| CIS | | | >0.999 |
|
Positive | 2 (11.1) | 2 (11.1) | |
|
Negative | 16 (88.9) | 16 (88.9) | |
| Adjuvant
therapy | | | 0.738 |
|
BCG | 10 (55.6) | 9 (50.0) | |
|
Chemotherapeutic
drugs | 8 (44.4) | 9 (50.0) | |
 | Table IVCharacteristics of patients matched
on propensity score. |
Table IV
Characteristics of patients matched
on propensity score.
| Parameters | Immediate
instillation alone, n (%) (n=33) | Immediate
instillation plus saline irrigation, n (%) (n=33) | P-value |
|---|
| Age (range) | 75 (46-92) | 74 (50-85) | 0.724 |
| Sex | | | >0.999 |
|
Male | 21 (63.6) | 21 (63.6) | |
|
Female | 12 (36.4) | 12 (36.4) | |
| Urine cytology | | | 0.609 |
|
≥3b | 11 (33.3) | 13 (39.4) | |
|
≤3a | 22 (66.7) | 20 (60.6) | |
| Smoking
history | | | >0.999 |
|
Positive | 17 (51.5) | 17 (51.5) | |
|
Negative | 16 (48.5) | 16 (48.5) | |
| History of
UTUC | | | 0.641 |
|
Positive | 3 (9.1) | 2 (6.1) | |
|
Negative | 30 (90.9) | 31 (93.9) | |
| First or recurrent
tumor | | | 0.609 |
|
Recurrent | 11 (33.3) | 13 (39.4) | |
|
First | 22 (66.7) | 20 (60.6) | |
| Solitary or
multiple tumors | | | 0.796 |
|
Multiple | 21 (63.6) | 22 (66.7) | |
|
Solitary | 12 (36.4) | 11 (33.3) | |
| Histology | | | 0.236 |
|
UC and other
subtypes | 1 (3.0) | 0 (0.0) | |
|
UC
alone | 32 (97.0) | 33 (100.0) | |
| pT status | | | 0.784 |
|
pTis | 6 (18.2) | 8 (24.2) | |
|
pT1 | 11 (33.3) | 9 (27.3) | |
|
pTa | 16 (48.5) | 16 (48.5) | |
| Tumor grade | | | 0.792 |
|
High or
G3 | 22 (66.7) | 23 (69.7) | |
|
PUNLMP/low | 11 (33.3) | 10 (30.3) | |
| CIS | | | 0.601 |
|
Positive | 10 (30.3) | 12 (36.4) | |
|
Negative | 23 (69.7) | 21 (63.6) | |
| Adjuvant
therapy | | | 0.609 |
|
BCG | 22 (66.7) | 20 (60.6) | |
|
Chemotherapeutic
drugs | 11 (33.3) | 13 (39.4) | |
Discussion
In the multivariate analysis using Cox's
proportional hazards model without propensity score matching,
patients in the present study treated with SIC alone (group A)
showed a significantly higher IVR-free survival rate than those
treated with CSBI including DXR (group B), and SIC plus CSBI (group
C). Even after matching using the nearest-neighbor propensity
score, patients of matched group A had a significantly higher
IVR-free survival rate than those of matched groups B and C.
Actually, no difference was observed in the time to intravesical
recurrence between groups B and group C before and after propensity
score matching. However, significant differences in some factors
were observed between these two groups, as shown in Table I; thus, we speculated that we would
need to adjust the patients' background using nearest neighbor
matching.
As high IVR rates are not uncommon after TURBT in
patients with NMIBC, preventive treatments for IVR are required.
Gudjónsson et al (13)
showed that SIC with epirubicin after TURBT had an inhibitory
effect on disease recurrence in patients with NMIBC at low to
intermediate risk. Moreover, Sylvester et al (14) performed the first meta-analysis of
SIC and noted that SIC clearly lead to a reduction in IVR compared
to TURBT alone in patients with NMIBC. The same group recently
reported that a SIC after TURBT reduced the risk of disease
recurrence, with a decrease in the 5-year recurrence rate from 58.8
to 44.8% (15). Therefore, EAU as
well as AUA guidelines have currently recommended performing SIC
immediately after TURBT in patients with NMIBC (4,6).
Despite these recommendations and the benefits of SIC for patients
with NMIBC shown in previous randomized controlled trials and a
meta-analysis of SIC (8,16), the use of SIC after TURBT remains
under discussion. In fact, a study in European countries found that
SIC after TURBT was performed in only 33-43% of patients with NMIBC
in that setting (17).
In contrast, earlier reports suggested that CSBI had
a greater preventive effect on IVR compared to SIC (9,10,18,19).
One report showed that there were no significant differences in the
median time to first recurrence between patients treated with CSBI
and those who underwent immediate SIC with mitomycin C (9). Another study revealed that CBSI with
sterile water after TURBT might have the same preventive effect on
IVR as an immediate single dose of intravesical mitomycin C in
patients with NMIBC (18).
Our findings have some clinical implications. First,
although several studies reported no significant difference in time
to IVR between patients treated with CSBI alone and those treated
with SIC (9,10,18),
our results suggest that CSBI can weaken the inhibitory effect of
SIC on IVR if it is performed after SIC. Second, irrigation with
saline and DXR, which included a low concentration of DXR, did not
exert an anticancer effect, meaning that CSBI alone could not have
a preventive effect on IVR. We speculated that intravesical
irrigation should have the possibility of washing tumor cells out
of the bladder; however, it is possible that the urine flow
following SIC could lead to higher IVR-free survival rates.
This study has some potential limitations. First,
our sample size was relatively small, particularly in groups A and
B. A larger number of patients treated with SIC alone, CSBI after
SIC, and CSBI with DXR would have yielded more robust results than
those obtained, even after applying nearest-neighbor propensity
score matching. Second, although CSBI alone has been reported to
show a preventive effect on IVR (9,10), we
could not clarify the reason why CSBI after SIC did not show an
inhibitory effect on IVR in this study. Third, the effect of the
second TUR was not evaluated in the present study because the
number of patients who underwent a second TUR was relatively
small.
We found a higher IVR rate in patients with NMIBC
treated with CSBI irrespective of whether they received concomitant
SIC or CSBI with DXR, compared with patients treated with a SIC
alone. To the best of our knowledge, ours is the first paper
concluding that SIC alone can provide a higher IVR-free survival
rate than CSBI with DXR or CSBI with SIC. Further prospective
studies having a larger number of patients with NIMBC should be
conducted to confirm the abovementioned finding and thus to
validate SIC alone as an IVR prevention method.
Acknowledgements
Not applicable.
Funding
The present study was funded in part by a research
grant from National Defense Medical College (grant no. 21096).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KK, ST, AS, JA, AH and KI were involved in the
conception and design of the study. KK collected and analyzed the
data, and drafted the manuscript. KK and KI reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of National Defense Medical College (approval no. 2734).
All procedures were conducted in accordance with the 1964
Declaration of Helsinki and its later amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sylvester RJ: Natural history, recurrence,
and progression in superficial bladder cancer.
ScientificWorldJournal. 6:2617–2625. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Witjes JA: Topic issue on new treatments
in bladder cancer. World J Urol. 27:285–287. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sylvester RJ, Van Der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–475. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Babjuk M, Böhle A, Burger M, Compérat E,
Kaasinen E, Palou J, Rouprêt M, van Rhijn BWG, Shariat SF,
Sylvester R, et al: EAU Guidelines on Non-Muscle-invasive
Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol.
71:447–461. 2017.doi: 10.1016/j.eururo.2016.05.041. PubMed/NCBI View Article : Google Scholar
|
|
5
|
Soloway MS and Masters S: Urothelial
susceptibility to tumor cell implantation influence of
cauterization. Cancer. 46:1158–1163. 1980.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang SS, Bochner BH, Chou R, Dreicer R,
Kamat AM, Lerner SP, Lotan Y, Meeks JJ, Michalski JM, Morgan TM, et
al: Treatment of non-metastatic muscle-invasive bladder cancer:
AUA/ASCO/ASTRO/SUO Guideline. J Urol. 198:552–559. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Koya MP, Simon MA and Soloway MS:
Complications of intravesical therapy for urothelial cancer of the
bladder. J Urol. 175:2004–2010. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cookson MS, Chang SS, Oefelein MG,
Gallagher JR, Schwartz B and Heap K: National practice patterns for
immediate postoperative instillation of chemotherapy in nonmuscle
invasive bladder cancer. J Urol. 187:1571–1576. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Onishi T, Sugino Y, Shibahara T, Masui S,
Yabana T and Sasaki T: Randomized controlled study of the efficacy
and safety of continuous saline bladder irrigation after
transurethral resection for the treatment of non-muscle-invasive
bladder cancer. BJU Int. 119:276–282. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Onishi T, Sasaki T, Hoshina A and Yabana
T: Continuous saline bladder irrigation after transurethral
resection is a prophylactic treatment choice for non-muscle
invasive bladder tumor. Anticancer Res. 31:1471–1474.
2011.PubMed/NCBI
|
|
11
|
Sobin L, Gospodarowicz M and Wittekind C
(eds): TNM Classification of Malignant Tumours, 7th edition.
Wiley-Blackwell, Hoboken, NJ, p336, 2009.
|
|
12
|
John E, Guido S, Jonathan E and Isabell S:
Pathology and Genetics: Tumours of the Urinary System and Male
Genital System. IARC WHO Classification of Tumours, 2004.
|
|
13
|
Gudjónsson S, Adell L, Merdasa F, Olsson
R, Larsson B, Davidsson T, Richthoff J, Hagberg G, Grabe M, Bendahl
PO, et al: Should all patients with non-muscle-invasive bladder
cancer receive early intravesical chemotherapy after transurethral
resection? the results of a prospective randomised multicentre
study. Eur Urol. 55:773–780. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sylvester RJ, Oosterlinck W and Van Der
Meijden AP: A single immediate postoperative instillation of
chemotherapy decreases the risk of recurrence in patients with
stage Ta T1 bladder cancer: A meta-analysis of published results of
randomized clinical trials. J Urol. 171:2186–2190. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sylvester RJ, Oosterlinck W, Holmang S,
Sydes MR, Birtle A, Gudjonsson S, De Nunzio C, Okamura K, Kaasinen
E, Solsona E, et al: Systematic review and individual patient data
meta-analysis of randomized trials comparing a single immediate
instillation of chemotherapy after transurethral resection with
transurethral resection alone in patients with Stage pTa-pT1
Urothelial carcinoma of the Bladder: Which Patients Benefit from
the Instillation? Eur Urol. 69:231–244. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chamie K, Saigal CS, Lai J, Hanley JM,
Setodji CM, Konety BR and Litwin MS: Urologic Diseases in America
Project: Compliance with guidelines for patients with bladder
cancer: Variation in the delivery of care. Cancer. 117:5392–5401.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palou-Redorta J, Rouprêt M, Gallagher JR,
Heap K, Corbell C and Schwartz B: The use of immediate
postoperative instillations of intravesical chemotherapy after
TURBT of NMIBC among European countries. World J Urol. 32:525–530.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bijalwan P, Pooleri G and Thomas A:
Comparison of sterile water irrigation versus intravesical
mitomycin C in preventing recurrence of nonmuscle invasive bladder
cancer after transurethral resection. Indian J Urol. 33:144–148.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Do J, Jeh SU, Hwa JS, Hwa JS, Hyun JS, Seo
DH, Kam SC, Choi JH and Choi SM: MP08-20 overnight continuous
saline irrigation after transurethral resection for superficial
bladder cancer is helpful in prevention of early recurrence. J
Urol. 199(e104)2018.
|