Introduction
At the initial diagnosis, approximately 75% of
patients with urothelial carcinoma of the bladder have non-muscle
invasive disease, which is generally treated by transurethral
resection of the bladder tumor (TURBT) either alone or in
combination with intravesical instillation therapy (1). However, it has been well documented
that non-muscle invasive bladder cancer (NMIBC) is characterized by
diverse biological and clinical features (2). Indeed, the prognosis of NMIBC patients
is generally favorable, with survival rates >80% at 5 years
after the initial TURBT. However, recurrence rates of NMIBC
patients range from 50-70 and 10-15% of these patients develop
muscle invasive disease (3-5).
Accordingly, it is very important to perform detailed assessment of
oncological outcomes of NMIBC patients with a high risk of disease
recurrence and progression.
To date, multiple risk factors for NMIBC, including
tumor size, number of tumors, grade, stage, prior recurrent status
and concomitant carcinoma in situ (CIS), have been identified
(6). Furthermore, several systems
have been developed by incorporating such prognostic
characteristics in order to facilitate risk prediction and guide
therapeutic strategies for each NMIBC patient, such as the European
Organization for Research and Treatment of Cancer (EORTC) and the
Spanish Urological Club for Oncological Treatment (CUETO) scoring
systems (6-8).
However, although these systems have been proposed, it remains
difficult to timely provide optimal treatment to NMIBC patients,
particularly high-risk patients (9,10). For
example, Xylinas et al analyzed the significance of the
EORTC and CUETO systems based on the data from 4689 patients with
NMIBC, and found that these systems provide low positive predictive
values for disease recurrence and progression, resulting in the
overestimation of the progression risk in high-risk patients
(10).
Considering these findings, to precisely
characterize the prognostic status in high-risk NMIBC patients
treated in routine clinical practice, we retrospectively evaluated
the oncological outcomes in a total of 186 consecutive Japanese
patients with high-risk NMIBC who underwent TURBT at our
institution, and investigated the prognostic impact of several
clinicopathological parameters in these patients.
Patients and methods
Ethics approval
The design of the present study was approved by the
research Ethics Committee of our institution (No. 14-290). The need
to obtain informed consent from the included patients was waived
because of its retrospective design; however, an opportunity for
opt-out regarding this study was provided through the web site at
our institution. In this study, a standard linkable
de-identification of data was conducted by masking personal
identifiers for privacy protection.
Patients
After excluding patients with an observation period
<1 month after TURBT, this study included a total of 186
consecutive patients who underwent TURBT between January 2007 and
June 2017 at our institution. They were pathologically diagnosed
with NMIBC, and were classified as having high-risk disease based
on the European Urological Association (EAU) guidelines (4). All clinicopathological data used in
this study were obtained from the medical records for each patient.
In this series, recurrence-free survival (RFS), progression-free
survival (PFS) and overall survival (OS) were defined as the length
of time from performed TURBT to pathologically diagnosed
intravesical recurrence, that to diagnosed muscle invasive disease
or upper tract urothelial carcinoma and that to death as a result
of any cause, respectively.
Evaluation
All patients included in this study had
cystoscopically confirmed tumors and/or positive findings on
urinary cytology. In patients with intravesical lesions suggesting
pure CIS alone, random bladder biopsy targeting the trigone,
posterior wall, bilateral lateral walls, dome, anterior wall,
prostatic urethra in men and/or suspicious regions, was performed,
whereas the remaining patients with evidently visible tumors
underwent complete TURBT. All pathological specimens were processed
according to standard pathological procedures. Tumors were staged
according to the 2002 American Joint Committee on Cancer TNM system
and graded according to the 2004 World Health
Organization/International Society of Urologic Pathology
classification.
Treatment
As a rule, the second TUR was conducted for patients
who were diagnosed with pT1 or high-grade tumor at our institution;
however, omitting the second TUR was permitted after considering
the clinical features of each patient such age and performance
status. In addition, although the protocol for adjuvant
intravesical instillation therapy was not strictly standardized,
BCG therapy was generally performed for patients based on the
physician's preference, patient tolerability and
clinicopathological characteristics as previously reported
(11). Follow-up of patients after
TURBT of NMIBC was carried out as follows: Cystoscopy and urinary
cytology were performed every 3-6 months for 2 years after TUR, and
then every 6 months from 3 to 5 years. Upon detection of visible
tumors or hyperemic mucosa by cystoscopy and/or positive findings
on urinary cytology, transurethral biopsy of the abnormal region
and/or TURBT of the tumor were performed.
Statistical analysis
All statistical analyses were performed using EZR
software (Saitama Medical Center, Jichi Medical University,
ver.1.36), and P<0.05 were considered significant. The RFS, PFS
and OS rates were calculated by the Kaplan-Meier method. The
prognostic significance of certain factors was assessed by
univariate and multivariate analyses using the Cox proportional
hazards regression model. In this study, the factors that reached
P<0.1 in univariate analysis were included in multivariate
analysis with backward stepwise selection.
Results
Patient characteristics
The characteristics of 186 patients included in this
study are summarized in Table I. In
this series, 47 (25.3%) of the 186 patients underwent the second
TUR following the initial TURBT, of whom 17 (36.2%) were diagnosed
as having residual urothelial carcinoma, including 1 (2.1%) with
muscle invasive disease. In addition, intravesical BCG therapy was
performed for 108 patients (58.1%), consisting of 96 (88.9%) and 12
(11.1%) receiving induction therapy alone and induction therapy
followed by maintenance therapy, respectively.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | No of patients |
|---|
| Mean age (range)
(years) | 73 (36-93) |
| Sex | |
|
Male | 155 |
|
Female | 31 |
| Number of tumors | |
|
Solitary | 82 |
|
Multiple | 104 |
| Tumor diameter | |
|
<3
cm | 152 |
|
≥3 cm | 34 |
| Recurrence
status | |
|
Primary | 157 |
|
Recurrence | 29 |
| Tumor grade | |
|
Low-grade | 19 |
|
High-grade | 167 |
| T category | |
|
pTa | 76 |
|
pT1 | 91 |
|
pTis | 19 |
| Concomitant CIS | |
|
Positive | 43 |
|
Negative | 143 |
| Second TUR | |
|
Yes | 47 |
|
No | 139 |
| BCG therapy | |
|
Yes | 108 |
|
No | 78 |
Prognosis
During the observation period in this study (median,
39.6 months), disease recurrence, disease progression and overall
deaths occurred in 54 (29.0%), 14 (7.5%) and 19 (10.2%) patients,
respectively. The RFS, PFS and OS rates in the 186 patients were as
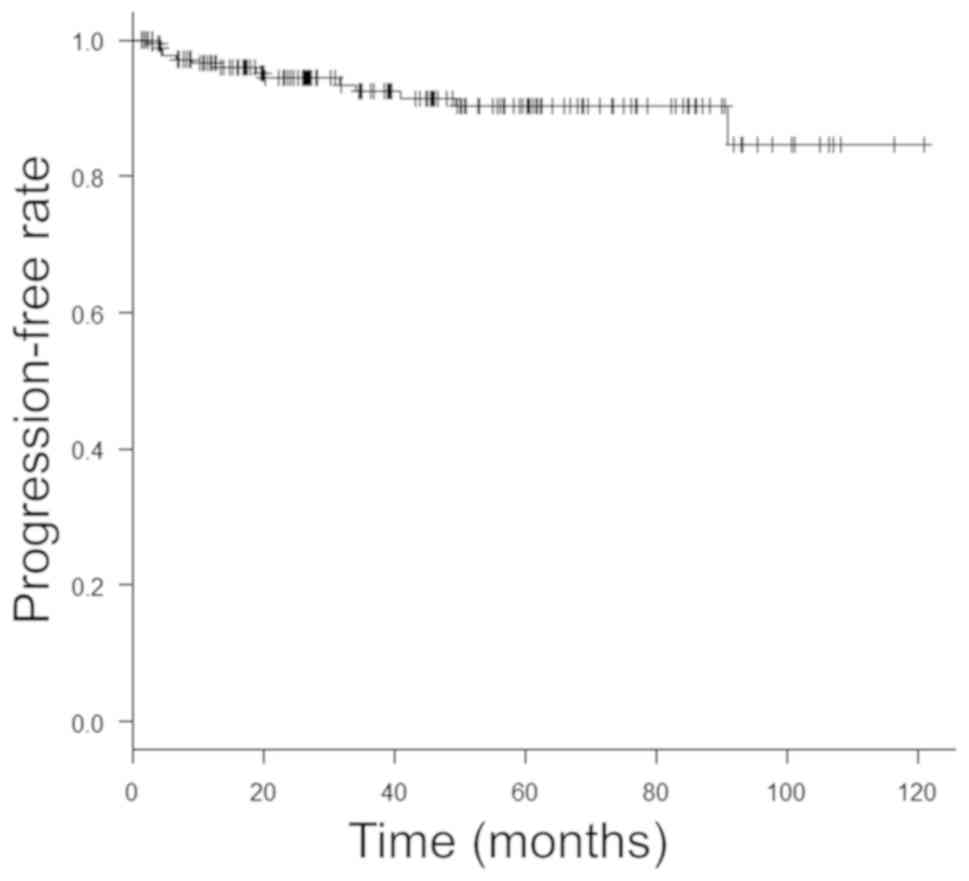
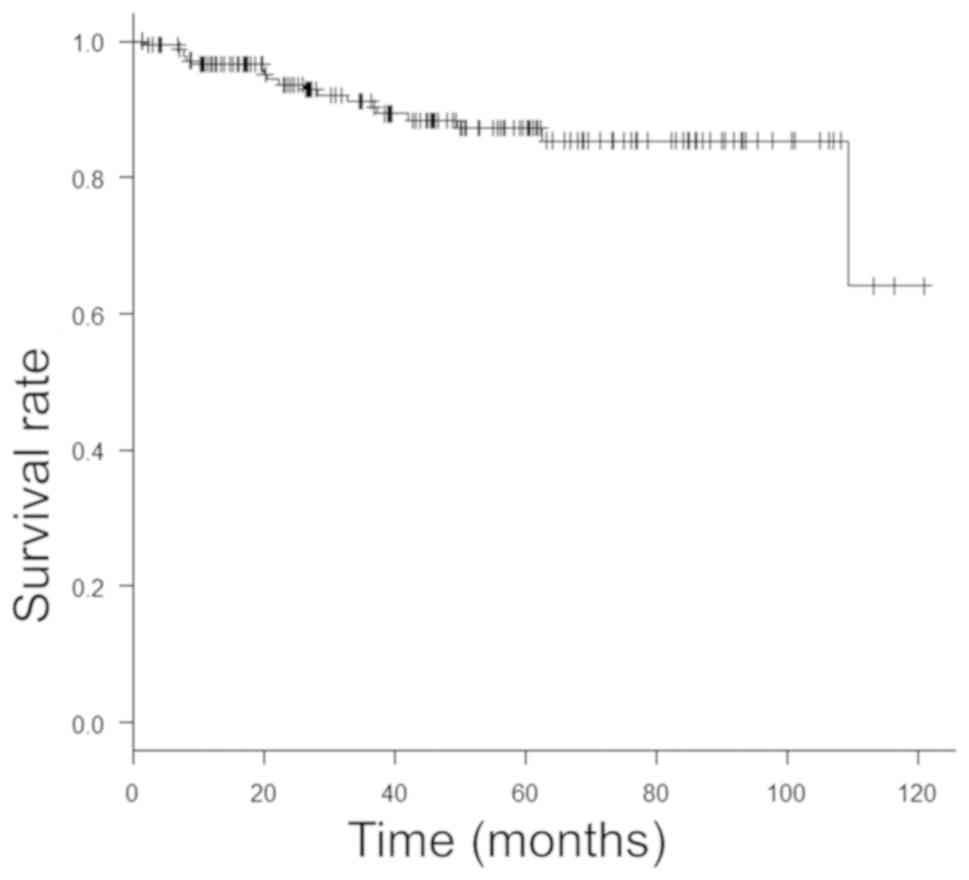
follows: 78.3, 96.6 and 96.6%, respectively, at 1 year, 68.9, 92.5
and 91.1%, respectively, at 3 years, and 66.6, 90.2 and 87.2%,
respectively, at 5 years (Figs. 1,
2 and 3).
We then assessed the effects of several parameters,
including age, sex, tumor multiplicity, tumor size, prior recurrent
status, tumor grade, pathological T category, concomitant CIS,
second TUR and intravesical BCG therapy, on the RFS, PFS and OS
(Tables II, III and IV, respectively). Univariate analyses
identified significant factors for oncological outcomes in the 186
patients as follows: BCG therapy for RFS (P=0.022), none for PFS,
and age for OS (P=0.014). Furthermore, the following factors were
demonstrated to be independently associated with the oncological
outcomes in multivariate analyses: Tumor multiplicity and BCG
therapy for RFS (P=0.018 and P=0.008, respectively), tumor
multiplicity and recurrence status for PFS (P=0.043 and P=0.029,
respectively), and age and tumor multiplicity for OS (P=0.008 and
P=0.041, respectively).
 | Table IIPrognostic factors for recurrence-free
survival. |
Table II
Prognostic factors for recurrence-free
survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years (<70
vs. ≥70) | 1.33 (0.76-2.35) | 0.31 | - | - |
| Sex (male vs.
female) | 1.43 (0.64-3.16) | 0.38 | - | - |
| Number of tumors
(solitary vs. multiple) | 1.76
(1.00-3.10) | 0.051 | 2.00
(1.12-3.59) | 0.018 |
| Tumor diameter
(<3 vs. ≥3 cm) | 1.80
(0.99-3.26) | 0.053 | - | - |
| Recurrence status
(primary vs. recurrence) | 1.00
(0.49-2.05) | 1.00 | - | - |
| Tumor grade
(low-grade vs. high-grade) | 0.59
(0.27-1.31) | 0.20 | - | - |
| T category (pTa,
pTis vs. pT1) | 1.38
(0.81-2.37) | 0.23 | - | - |
| Concomitant CIS
(yes vs. no) | 0.62
(0.31-1.23) | 0.17 | - | - |
| Second TUR (yes vs.
no) | 0.79
(0.41-1.50) | 0.46 | - | - |
| BCG therapy (yes
vs. no) | 0.54
(0.31-0.92) | 0.022 | 0.48
(0.28-0.82) | 0.008 |
 | Table IIIPrognostic factors for
progression-free survival. |
Table III
Prognostic factors for
progression-free survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years (<70
vs. ≥70) | 0.80
(0.28-2.24) | 0.67 | - | - |
| Sex (male vs.
female) | 0.97
(0.22-4.37) | 0.97 | - | - |
| Number of tumors
(solitary vs. multiple) | 3.19
(0.89-11.5) | 0.076 | 3.78
(1.04-13.8) | 0.043 |
| Tumor diameter
(<3 vs. ≥3 cm) | 0.29
(0.038-2.22) | 0.23 | - | - |
| Recurrence status
(primary vs. recurrence) | 0.28
(0.92-8.41) | 0.071 | 3.46
(1.14-10.5) | 0.029 |
| Tumor grade
(low-grade vs. high-grade) | 1.21
(0.16-9.35) | 0.85 | - | - |
| T category (pTa,
pTis vs. pT1) | 2.63
(0.82-8.41) | 0.10 | - | - |
| Concomitant CIS
(yes vs. no) | 0.79
(0.22-2.84) | 0.72 | - | - |
| Second TUR (yes vs.
no) | 0.73
(0.20-2.64) | 0.64 | - | - |
| BCG therapy (yes
vs. no) | 1.03
(0.34-3.08) | 0.96 | - | - |
 | Table IVPrognostic factors for overall
survival. |
Table IV
Prognostic factors for overall
survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years (<70
vs. ≥70) | 4.78
(1.37-16.7) | 0.014 | 5.64
(1.56-20.4) | 0.008 |
| Sex (male vs.
female) | 1.39
(0.32-6.05) | 0.66 | - | - |
| Number of tumors
(solitary vs. multiple) | 2.46
(0.88-6.83) | 0.085 | 2.96
(1.05-8.40) | 0.041 |
| Tumor diameter
(<3 vs. ≥3 cm) | 0.21
(0.027-1.54) | 0.12 | - | - |
| Recurrence status
(primary vs. recurrence) | 1.32
(0.44-3.99) | 0.62 | - | - |
| Tumor grade
(low-grade vs. high-grade) | 1.83
(0.24-13.9) | 0.56 | - | - |
| T category (pTa,
pTis vs. pT1) | 1.48
(0.59-3.71) | 0.40 | - | - |
| Concomitant CIS
(yes vs. no) | 1.29
(0.49-3.42) | 0.61 | - | - |
| Second TUR (yes vs.
no) | 0.52
(0.15-1.81) | 0.31 | - | - |
| BCG therapy (yes
vs. no) | 1.21
(0.46-3.19) | 0.70 | - | - |
Discussion
Factors reflecting prognostic outcomes can be highly
beneficial when counseling cancer patients to determine their
therapeutic options and follow-up schedules. This is especially
true for patients with NMIBC, because they exhibit highly
heterogeneous clinical courses with varying oncological outcomes,
particularly those classified into the high-risk group (2-5).
Although several major clinical guidelines clarified optimal
therapeutic strategies for high-risk NMIBC patients, the
oncological outcomes of these patients have not been well
documented due to complicated therapeutic strategies (4). Accordingly, it is difficult to
properly provide highly standardized treatments under strict
criteria in clinical practice. In this study, therefore, we
retrospectively analyzed the oncological outcomes of a total of 186
consecutive Japanese patients with high-risk NMIBC in order to
investigate the impact of several clinicopathological parameters on
these outcomes and to precisely clarify the therapeutic status of
high-risk NMIBC.
To date, various factors predicting disease
recurrence and progression have been reported for NMIBC to enable
satisfactory clinical decision-making based on an objective risk
stratification system (6).
Moreover, several models have been developed using multiple
prognostic parameters to further individualize therapeutic
strategies for NMIBC patients (6-8).
Of these, the EORTC model integrated in the EAU guidelines may
currently be the most commonly used (7). Thus, we used this system to identify
186 high-risk NMIBC patients treated at our institution. It was
recommended to perform the second TUR followed by intensive
adjuvant treatment, including intravesical BCG therapy and
cystectomy, after initial TURBT for patients with high-risk NMIBC
in the present major clinical guidelines (4,9).
However, only 25.3 and 58.1% of the 186 patients included in this
study underwent the second TUR and BCG therapy, respectively, due
to advanced age, unfavorable performance status, rejection of
additional therapies and intolerance of BCG therapy. This suggested
insufficient treatment for high-risk NMIBC patients at our
institution; however, similar to this series, there have been
several previous studies showing that a considerable proportion of
NMIBC patients are not optimally managed according to current
clinical guidelines (12-16).
For example, a retrospective study including 23 centers in Western
countries reported that the second TUR was carried out for only 38%
of patients with T1G3 NMIBC (12),
whereas SEER-Medicare data revealed that only 29% of patients with
high-grade disease received BCG therapy (13).
The oncological outcomes in this series were
comparatively favorable, with 5-year RFS, PFS and OS rates of 66.6,
90.2 and 87.2%, respectively, and the proportion of patients with
residual tumor during the second TUR was 36.2%. These findings are
comparable with those in previous studies (8,12,17-21).
For example, Kluth et al analyzed the prognostic outcomes in
892 patients with T1 high-grade NMIBC, and reported 5-year RFS, PFS
and OS rates of 54, 85 and 78%, respectively (17). Similarly, Kikuchi et al
reported 1-, 3- and 5-year RFS rates of 77.0, 61.3 and 52.8%,
respectively, in 3237 Japanese patients with Ta or T1 bladder
cancer (20). Furthermore, the
proportion of patients diagnosed with residual tumor during the
second TUR ranged between 33 and 44% (20-22).
Considering the low proportion of patients in this series treated
in accordance with the current guidelines, the oncological outcomes
were not inferior to those in previous report. Therefore, more
satisfactory oncological outcomes may be expected for patients
stratified into the high-risk group by appropriately providing the
guideline-recommended standard treatments.
There have been several studies investigating
prognostic factors in NMIBC patients with unfavorable
characteristics (12,16-24).
For example, Gontero et al identified age, tumor size and
concomitant CIS as independent predictors of disease progression in
T1G3 NMIBC patients initially treated by intravesical BCG
instillation (12), whereas
Branchereau et al identified lymphovascular invasion as a
predictor of OS in patients with high-grade stage pT1 bladder
cancer (24). To date, however,
there is limited information regarding specific prognostic factors
for high-risk NMIBC patients. Thus, it is of interest to explore
useful parameters closely associated with oncological outcomes in
high-risk NMIBC patients. In this series, multivariate analyses
identified the following independent factors for poor oncological
outcomes: Multiple tumors and absence of BCG therapy for RFS,
multiple tumors and recurrent tumors for PFS, and elderly age and
multiple tumors for OS. Considering the common effects of tumor
multiplicity on the RFS, PFS and OS, high-risk NMIBC patients with
multiple tumors should be treated more aggressively and followed
more closely.
There were several limitations in this study. First,
although the sample size was large for a cohort consisting of
high-risk NMIBC patients alone, this was a retrospective study with
a comparatively short observation period, and conducted in routine
clinical practice without strict criteria regarding therapeutic
strategy and follow-up schedule. Therefore, it will be of interest
to perform further assessments by dividing patients with follow-up
period >5 years according to whether treatments were performed
based on current recommendations or not. Second, several recent
studies demonstrated poor discrimination of disease recurrence and
progression by currently accepted risk classification systems,
including the EORTC risk tables, with an overestimation of these
risks in high-risk patients in particular (10,25).
Thus, this point should be considered when interpreting the
outcomes of this study. Lastly, as suggested by a number of
previous studies (26-31),
conventional clinicopathological parameters alone may be
insufficient to precisely predict the oncological outcomes,
suggesting the promising role of molecular biomarkers, such as
Ki-67, cell cycle-associated proteins and neutrophil to lymphocyte
ratio, for this objective.
In conclusion, we retrospectively investigated the
oncological outcomes of a total of 186 Japanese patients with
high-risk NMIBC. The 5-year RFS, PFS and OS were 66.6, 90.2 and
87.2%, respectively, and the following factors were found to be
independently associated with the oncological outcomes: Tumor
multiplicity and introduction of BCG therapy for RFS, tumor
multiplicity and recurrence status for PFS, and age and tumor
multiplicity for OS. These findings suggest that favorable outcomes
were achieved for the 186 patients; however, a more aggressive
treatment schedule should be considered for patients with
independent prognostic factors, especially those with multiple
tumors.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM, HM and AO conceived the study and wrote the
manuscript. TS and TI acquired and analyzed the general data of
patients. DM and KT reviewed, collected data and assisted prognosis
analysis. HM supervised the study, revised the manuscript and gave
final approval of the version to be published. All authors have
read and approved the final version of the manuscript..
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Hamamatsu University School of Medicine (Shizuoka,
Japan; approval no. 14-290). Informed consent was waived due to the
retrospective design of the present study. An opportunity for
opt-out regarding the current study was provided through the
institutional website.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no completing
interests.
References
|
1
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
et al: Bladder cancer: Epidemiology, staging and grading, and
diagnosis. Urology. 66 (6 Suppl 1):S4–S34. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Audenet F, Attalla K and Sfakianos JP: The
evolution of bladder cancer genomics: What have we learned and how
can we use it? Urol Oncol. 36:313–320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van den Bosch S and Alfred Witjes J:
Long-term cancer-specific survival in patients with high-risk,
non-muscle-invasive bladder cancer and tumour progression: A
systematic review. Eur Urol. 60:493–500. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martin-Doyle W, Leow JJ, Orsola A, Chang
SL and Bellmunt J: Improving selection criteria for early
cystectomy in high-grade t1 bladder cancer: A meta-analysis of
15,215 patients. J Clin Oncol. 33:643–650. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kluth LA, Black PC, Bochner BH, Catto J,
Lerner SP, Stenzl A, Stenzl A, Sylvester R, Vickers AJ, Xylinas E
and Shariat SF: Prognostic and prediction tools in bladder cancer:
A comprehensive review of the literature. Eur Urol. 68:238–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–477. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fernandez-Gomez J, Madero R, Solsona E,
Unda M, Martinez-Piñeiro L, Gonzalez M, Portillo J, Ojea A, Pertusa
C, Rodriguez-Molina J, et al: Predicting nonmuscle invasive bladder
cancer recurrence and progression in patients treated with bacillus
Calmette-Guerin: The CUETO scoring model. J Urol. 182:2195–2203.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Orsola A, Palou J and Solsona E: High-risk
nonmuscle invasive bladder cancer. Hematol Oncol Clin North Am.
29:227–236, viii. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xylinas E, Kent M, Kluth L, Pycha A,
Comploj E, Svatek RS, Lotan Y, Trinh QD, Karakiewicz PI, Holmang S,
et al: Accuracy of the EORTC risk tables and of the CUETO scoring
model to predict outcomes in non-muscle-invasive urothelial
carcinoma of the bladder. Br J Cancer. 109:1460–1466.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hinotsu S, Akaza H, Naito S, Ozono S,
Sumiyoshi Y, Noguchi S, Yamaguchi A, Nagamori S, Terai A, Nasu Y,
et al: Maintenance therapy with bacillus Calmette-Guérin Connaught
strain clearly prolongs recurrence-free survival following
transurethral resection of bladder tumour for non-muscle-invasive
bladder cancer. BJU Int. 108:187–195. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gontero P, Sylvester R, Pisano F, Joniau
S, Vander Eeckt K, Serretta V, Larré S, Di Stasi S, Van Rhijn B,
Witjes AJ, et al: Prognostic factors and risk groups in T1G3
non-muscle-invasive bladder cancer patients initially treated with
Bacillus Calmette-Guérin: Results of a retrospective multicenter
study of 2451 patients. Eur Urol. 67:74–82. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Patschan O, Holmäng S, Hosseini A,
Liedberg F, Ljungberg B, Malmström PU, Rosell J and Jahnson S: Use
of bacillus Calmette-Guérin in stage T1 bladder cancer: Long-term
observation of a population-based cohort. Scand J Urol. 49:127–132.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Spencer BA, McBride RB, Hershman DL, Buono
D, Herr HW, Benson MC, Gupta-Mohile S and Neugut AI: Adjuvant
intravesical bacillus Calmette-Guérin therapy and survival among
elderly patients with non-muscle-invasive bladder cancer. J Oncol
Pract. 9:92–98. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Witjes JA, Palou J, Soloway M, Lamm D,
Kamat AM, Brausi M, Persad R, Buckley R, Colombel M and Böhle A:
Current clinical practice gaps in the treatment of intermediate-
and high-risk non-muscle-invasive bladder cancer (NMIBC) with
emphasis on the use of bacillus Calmette-Guérin (BCG): Results of
an international individual patient data survey (IPDS). BJU Int.
112:742–750. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lenis AT, Donin NM, Litwin MS, Saigal CS,
Lai J, Hanley JM, Konety BR and Chamie K: Urologic Diseases in
America Project: Association between number of endoscopic
resections and utilization of bacillus Calmette-Guérin therapy for
patients with high-grade, non-muscle-invasive bladder cancer. Clin
Genitourin Cancer. 15:e25–e31. 2017.
|
|
17
|
Kluth LA, Xylinas E, Crivelli JJ, Passoni
N, Comploj E, Pycha A, Chrystal J, Sun M, Karakiewicz PI, Gontero
P, et al: Obesity is associated with worse outcomes in patients
with T1 high grade urothelial carcinoma of the bladder. J Urol.
190:480–486. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chamie K, Ballon-Landa E, Daskivich TJ,
Bassett JC, Lai J, Hanley JM, Konety BR, Litwin MS and Saigal CS:
Urologic Diseases in America Project: Treatment and survival in
patients with recurrent high-risk non-muscle-invasive bladder
cancer. Urol Oncol. 33:20.e9–20.e17. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baltacı S, Bozlu M, Yıldırım A, Gökçe Mİ,
Tinay İ, Aslan G, Can C, Türkeri L, Kuyumcuoğlu U and Mungan A:
Significance of the interval between first and second transurethral
resection on recurrence and progression rates in patients with
high-risk non-muscle-invasive bladder cancer treated with
maintenance intravesical bacillus Calmette-Guérin. BJU Int.
116:721–726. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kikuchi E, Fujimoto H, Mizutani Y, Okajima
E, Koga H, Hinotsu S, Shinohara N, Oya M and Miki T: Cancer
Registration Committee of the Japanese Urological Association:
Clinical outcome of tumor recurrence for Ta, T1 non-muscle invasive
bladder cancer from the data on registered bladder cancer patients
in Japan: 1999-2001 report from the Japanese urological
association. Int J Urol. 16:279–286. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Grimm MO, Steinhoff C, Simon X,
Spiegelhalder P, Ackermann R and Vogeli TA: Effect of routine
repeat transurethral resection for superficial bladder cancer: A
long-term observational study. J Urol. 170:433–437. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jahnson S, Wiklund F, Duchek M, Mestad O,
Rintala E, Hellsten S and Malmström PU: Results of second-look
resection after primary resection of T1 tumour of the urinary
bladder. Scand J Urol Nephrol. 39:206–201. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lazica DA, Roth S, Brandt AS, Böttcher S,
Mathers MJ and Ubrig B: Second transurethral resection after Ta
high-grade bladder tumor: A 4.5-year period at a single university
center. Urol Int. 92:131–135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Branchereau J, Larue S, Vayleux B, Karam
G, Bouchot O and Rigaud J: Prognostic value of the lymphovascular
invasion in high-grade stage pT1 bladder cancer. Clin Genitourin
Cancer. 11:182–188. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hernández V, De La Peña E, Martin MD,
Blázquez C, Diaz FJ and Llorente C: External validation and
applicability of the EORTC risk tables for non-muscle-invasive
bladder cancer. World J Urol. 29:409–414. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ding W, Gou Y, Sun C, Xia G, Wang H, Chen
Z, Tan J, Xu K and Qiang D: Ki-67 is an independent indicator in
non-muscle invasive bladder cancer (NMIBC); combination of EORTC
risk scores and Ki-67 expression could improve the risk
stratification of NMIBC. Urol Oncol. 32:42.e13–e19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu B, Miyake H, Nishikawa M and Fujisawa
M: Expression profile of epithelial-mesenchymal transition markers
in non-muscle-invasive urothelial carcinoma of the bladder:
Correlation with intravesical recurrence following transurethral
resection. Urol Oncol. 33:110.e11–e18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Behnsawy HM, Miyake H, Abdalla MA, Sayed
MA, Ahmed Ael-F and Fujisawa M: Expression of cell cycle-associated
proteins in non-muscle-invasive bladder cancer: Correlation with
intravesical recurrence following transurethral resection. Urol
Oncol. 29:495–501. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yuk HD, Jeong CW, Kwak C, Kim HH and Ku
JH: Elevated neutrophil to lymphocyte ratio predicts poor prognosis
in non-muscle invasive bladder cancer patients: Initial
intravesical bacillus calmette-guerin treatment after transurethral
resection of bladder tumor setting. Front Oncol.
8(642)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Passoni N, Gayed B, Kapur P, Sagalowsky
AI, Shariat SF and Lotan Y: Cell-cycle markers do not improve
discrimination of EORTC and CUETO risk models in predicting
recurrence and progression of non-muscle-invasive high-grade
bladder cancer. Urol Oncol. 34:485.e7–485.e14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fahmy O, Khairul-Asri MG, Stenzl A and
Gakis G: Systemic anti-CTLA-4 and intravesical
Bacille-Calmette-Guerin therapy in non-muscle invasive bladder
cancer: Is there a rationale of synergism? Med Hypotheses.
92:57–58. 2016.PubMed/NCBI View Article : Google Scholar
|