Introduction
Renal cell carcinoma (RCC) is a commonly occurring
urological cancer that is characterized by a high metastatic
potential at diagnosis and after radical surgery. Although various
immunotherapies, including immune-checkpoint inhibitors, are used
as standard therapy for patients with metastatic RCC, molecular
targeted therapies, such as tyrosine kinase inhibitors (TKIs), are
efficacious therapeutic interventions (1). However, the increase in overall
survival of patients associated with these therapies are far from
satisfactory. Therefore, understanding the functioning of new
therapeutic agents is important and is a feat addressed by clinical
trials (2,3).
Royal jelly (RJ) is a milky secretion from the
hypopharyngeal and mandibular glands of worker bees and possesses
antimicrobial, anti-inflammatory, and antioxidant properties
(4,5). RJ is important in maintaining the
quality of life and suppresses adverse events in patients
undergoing anti-cancer therapies. RJ protects against organ
dysfunctions and discomfort caused by various cancer therapies
(6,7). We have previously reported that RJ
suppresses the adverse events caused by TKIs in patients with RCC
(8). Several in vivo and
in vitro studies have shown that RJ directly and indirectly
exhibits anti-cancer effects in various malignancies (9-12).
However, the detailed mechanisms employed by RJ in protecting
against cancer and adverse events caused by anti-cancer therapy
remains to be understand.
An important biological function of RJ is the
regulation of inflammation and immunity (4,5).
Interestingly, inflammation and immunity are important for
carcinogenesis and malignant invasiveness in multiple cancers
(13,14). Moreover, various pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α, tumor necrosis
factor (TGF)-β, and interleukin (IL)-6 correlate with malignant
transformation and occurrence of adverse events caused by
anti-cancer therapies in various types of malignancies (15-22).
Previous reports have shown that RJ regulates the synthesis of
these pro-inflammatory cytokines (23-25).
However, the correlation and mechanism employed by RJ in
stimulating anti-cancer effects and suppressing adverse events by
molecular targeted therapy in patients with RCC are yet to be
elucidated.
We have previously shown that oral intake of RJ
suppresses TKI-induced toxicity in patients with RCC in a
randomized, double-blinded, placebo-controlled study (8). In this study, we investigated how
orally administered RJ affects the anti-cancer effects induced by
TKIs in the same patient cohort. Moreover, we analyzed the
correlation between RJ-induced effects and changes in the serum
levels of TNF-α, TGF-β, and IL-6. Finally, we have demonstrated the
benefits of administering RJ to advanced RCC patients awaiting TKI
treatment in a preliminary clinical trial.
Materials and methods
Patients
Our study cohort consisted of 33 patients (23 males
and 10 females) with RCC awaiting TKI treatment at the Nagasaki
University Hospital (Nagasaki, China). The median (range) age at
the time of treatment was 68 (54-79) years. There were 16 and 17
patients with a performance status of 0 and 1, respectively. In our
study population, 27 and 24 patients were diagnosed with high grade
(Fuhrman grade 3 and 4) and high pT stage (pT3 and 4) cancer,
respectively. All the patients had lymph node and/or distant
metastasis. We used the clinicopathological features and
eligibility criteria as per our previous report (8).
Study design
In this study, we performed a randomized,
double-blind, placebo-controlled trial; patients were divided into
two groups using computer-generated random numbers (17 in the
placebo and 16 in the RJ group). Tumors were measured by computed
tomography within the 3 months of the beginning and end of
administering RJ or placebo. A group of patients was examined twice
during the course of the study to check for adverse events. Tumor
response was categorized based on the Response Evaluation Criteria
in Solid Tumor version 1.1 as complete response (CR), partial
response (PR), stable disease (SD), or progressive disease (PD)
(26). Toxicity was evaluated using
the Common Terminology Criteria for Adverse Events version 5.0 by
the National Cancer Institute. In this study, adverse events were
divided into two groups (absence or presence of and Grade 1-4)
regardless of severity owing to the relatively small cohort. Serum
levels of TNF-α, TGF-β, and IL-6 were quantified by enzyme-linked
immunosorbent assay (R&D systems, Inc.; MN) before and after 3
months of treatment.
Protocol
As shown in our previous report (8), the starting dose of sunitinib,
pazopanib, axitinib, and sunitinib was 50, 800, 10, and 800 mg/day,
respectively. Upon observing intolerable adverse events, the doses
were decreased to 25.0-37.5, 400-600, 5, and 400 mg/day,
respectively. TKI administration was stopped once continuous
intolerable adverse events were observed. Other molecular targeted
therapies, including TKIs and/or m-TOR inhibitors, were
administered as soon as possible in all the patients. Patients with
a rest period of over 30 days were excluded from this study. We did
not use immune check-point inhibitors owing to the lack of approval
for treatment of RCC at the time. Relative dose intensity (RDI) was
calculated as the ratio of ‘delivered’ to ‘planned’ dose intensity.
In short, 100% RDI means that the TKI was administered at the dose
mentioned in the original protocol.
RJ was procured from Yamada Agriculture Center Inc
(Okayama, Japan). RJ and placebo were prepared as capsules
containing 900 mg RJ and starch, respectively. They were similar in
taste, smell, size, shape, and color. Capsules were administered
orally four times per day (after breakfast, lunch, and dinner and
before bedtime) for three months.
The study protocol was approved by the Human Ethics
Review Committee of Nagasaki University Hospital (Nagasaki, Japan;
No. 15102604-2 and it was registered as UMIN000020152). All
experiments complied with the principles embodied in the
Declaration of Helsinki. All the patients provided written informed
consent to participate in all aspects of the study. This was a
double-blind study.
Statistical analysis
Results have been expressed as the mean and standard
deviation for data with normal distribution or median and
interquartile range for data with non-normal distribution.
Student's t-test and Mann-Whitney U test was used to compare the
continuous variables in data with normal and non-normal
distribution, respectively. Multiple comparisons of the data were
analyzed by Scheffé's method. ANOVA was performed prior to
Scheffe's method. Chi-square test was used for categorical
comparison of the data. All the statistical analyses were
two-sided, significance was set at P<0.05, and were performed on
a personal computer using the statistical package StatView for
Windows (version 5.0, Abacus Concept, Inc., Berkeley, CA).
Results
Patient background
As shown in our previous report (8), 21, 7, 4, and 1 patient(s) were treated
with sunitinib, pazopanib, axitinib, and sorafenib, respectively:
There was no significant difference between the patients in the
placebo and RJ-administered groups (P=0.539). Moreover, the
patients showed similar clinicopathological features including age
(P=0.101), sex (P=0.909), performance status (P=0.598), grade of
cancer (P=0.425), pT stage (P=0.201), and metastasis to the lymph
node (P=0.881) and distant organs (P=0.325).
Tumor response and adverse events
No patient exhibited CR within 2-3 months of
administering the TKI or placebo. One patient exhibited CR after
intake of RJ. The frequency of patients with PR in the RJ group
(50.0%) was twice as much as that in the placebo group (17.6%).
Compared to placebo-administered patients, no patient exhibited PD
in the RJ group. Finally, our data demonstrated that oral intake of
RJ improved the anti-cancer effects 3 months after TKI treatment
(P=0.037; Table I).
 | Table IThree month response. |
Table I
Three month response.
| Response | Total | Placebo | Royal jelly | P-value |
|---|
| | | | | 0.037 |
| Complete response;
N (%) | 1 (3.0) | 0 (0.0) | 1 (6.3) | |
| Partial
response | 11 (33.3) | 3 (17.6) | 8 (50.0) | |
| Stable disease | 16 (48.5) | 9 (52.9) | 7 (43.8) | |
| Progressive
disease | 5 (15.2) | 5 (29.4) | 0 (0.0) | |
Hypertension was among the most common adverse
events (n=23; 67.9%); fatigue (n=20; 60.6%), anorexia (n=18;
60.0%), and hand-foot syndrome (n=18; 60.0%) were the other common
adverse events. Blood tests revealed leukopenia, anemia, renal
dysfunction, liver dysfunction, and thyroid abnormality in 11
(36.7%), 9 (27.3%), 15 (45.5%), 7 (21.2%), and 14 patients (42.4%),
respectively. Among these adverse events, anorexia and fatigue in
patients in the RJ group was significantly lower compared to that
in the placebo group (P=0.009 and P<0.001, respectively;
Table II). Detailed information on
the adverse events caused by TKI treatment have been described
previously (8).
 | Table IIFrequency of anorexia and
fatigue. |
Table II
Frequency of anorexia and
fatigue.
| Factor | Placebo | Royal jelly | P-value |
|---|
| Anorexia | | | 0.009 |
|
Absence | 4 (23.5) | 11 (68.8) | |
|
Presence | 13 (76.5) | 5 (31.2) | |
| Fatigue | | | <0.001 |
|
Absence | 2 (11.8) | 11 (68.8) | |
|
Presence | 15 (88.2) | 5 (31.2) | |
Relative dose density
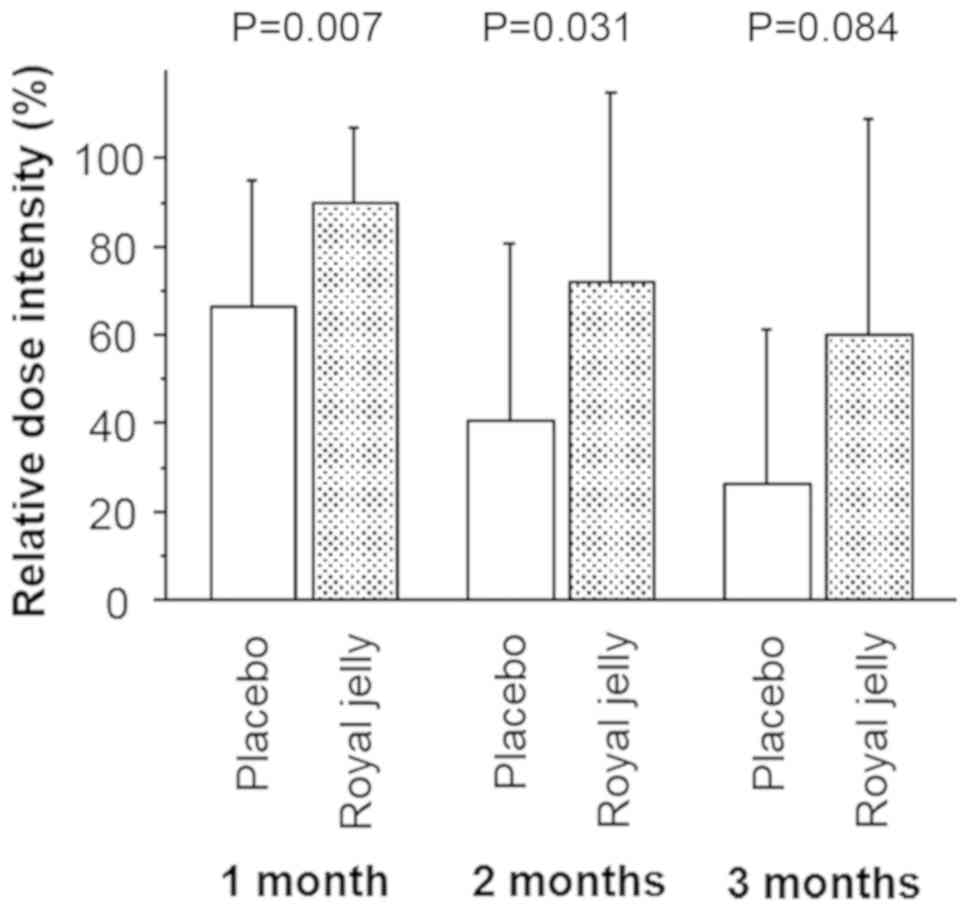
As shown in Fig. 1,
1-month RDI (mean/standard deviation; 88.6/21.2%) in the patients
in the RJ group was significantly higher (P=0.007) than that in
patients administered placebo (68.6/28.7%). After 2 months of
treatment, TKI treatment was discontinued and decreased in 15
(88.2%) and 8 patients (50.0%) in the RJ and placebo groups,
respectively; 2-month RDIs for the patients in the RJ and placebo
groups showed similar differences (71.9/43.2 and 40.4/40.1%,
respectively; P=0.031; Fig. 1). At
the end of the study period (3 months), the initial dose
administered was maintained in 7 (43.8%) and 2 patients (11.8%) in
the RJ and placebo groups, respectively. The 3-month RDI in
patients in the RJ group was slightly higher than that in the
placebo group; however, this difference was not significant
(P=0.114 and 0.084, respectively; Fig.
1).
Correlation between serum levels of
TNF-α, TGF-β, and IL-6 and tumor response
Prior to treatment with TKI, serum levels of TNF-α
in patients in the RJ group [mean/standard deviation and median
(interquartile range) were 5.90/5.46 and 5.02 (0.00-11.60)] was
higher than that in placebo-administered patients [2.37/3.49 and
2.37 (1.17-5.83)]. However, this difference was not significant
(P=0.378). Similarly, patients in the RJ and placebo groups showed
no significant difference in the serum levels of IL-6 [10.69/13.21
and 4.52 (1.62-18.20) versus 9.57/13.28 and 2.13 (1.55-11.97),
P=0.564] and TGF-β [537.14/226.18 and 532.00 (324.41-707.83) versus
600.77/309.34 and 543.78 (360.38-786.93), P=0.719].
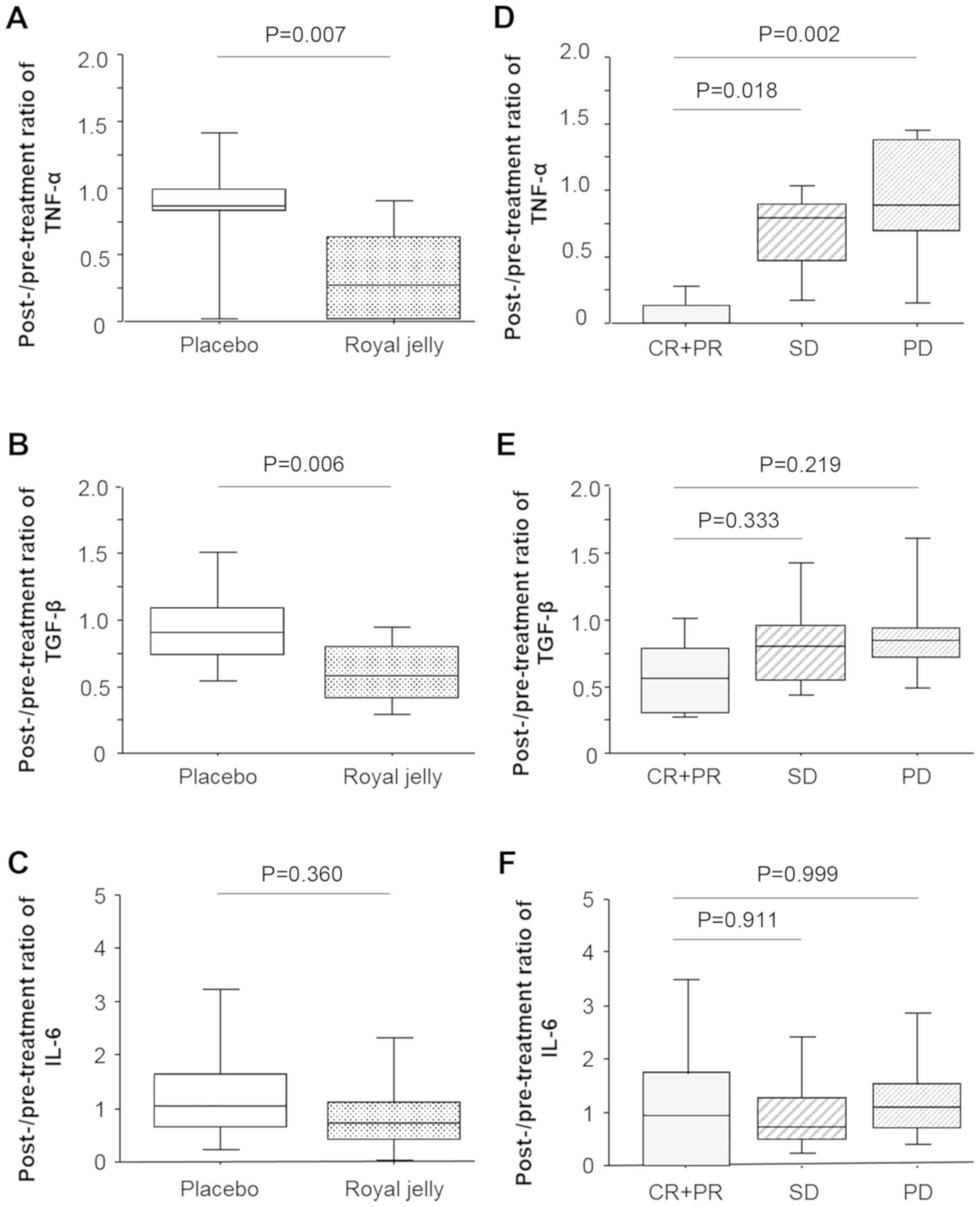
Post- and pre-treatment ratios of these variables in
patients in the RJ and placebo groups are shown in Fig. 2. The ratio of the levels of TNF-α in
patients of the RJ group was significantly lower than that in
patients in the placebo group (P=0.007; Fig. 2A). The serum levels of TGF-β showed
a similar trend (P=0.006; Fig. 2B).
However, there was no significant difference between the decreased
serum levels of IL-6 in the RJ and placebo groups (P=0.221;
Fig. 2C). Next, we determined the
correlation between the decreased variables and tumor response upon
TKI treatment by Scheffé's method (Fig.
2D-2F). Post- and pre-treatment ratios of the levels of TNF-α
in patients exhibiting PR was significantly lower than that in
patients exhibiting SD and PD (P=0.018 and 0.002, respectively;
Fig. 2D). Similar trends, albeit
non-significant, were observed for the levels of TGF-β between
patients exhibiting PR and SD or PD (P=0.333 and 0.219,
respectively; Fig. 2E). The
decrease in serum IL-6 did not correlate with tumor response
(Fig. 2F).
Correlation between serum levels of
TNF-α, TGF-β, and IL-6 and adverse events
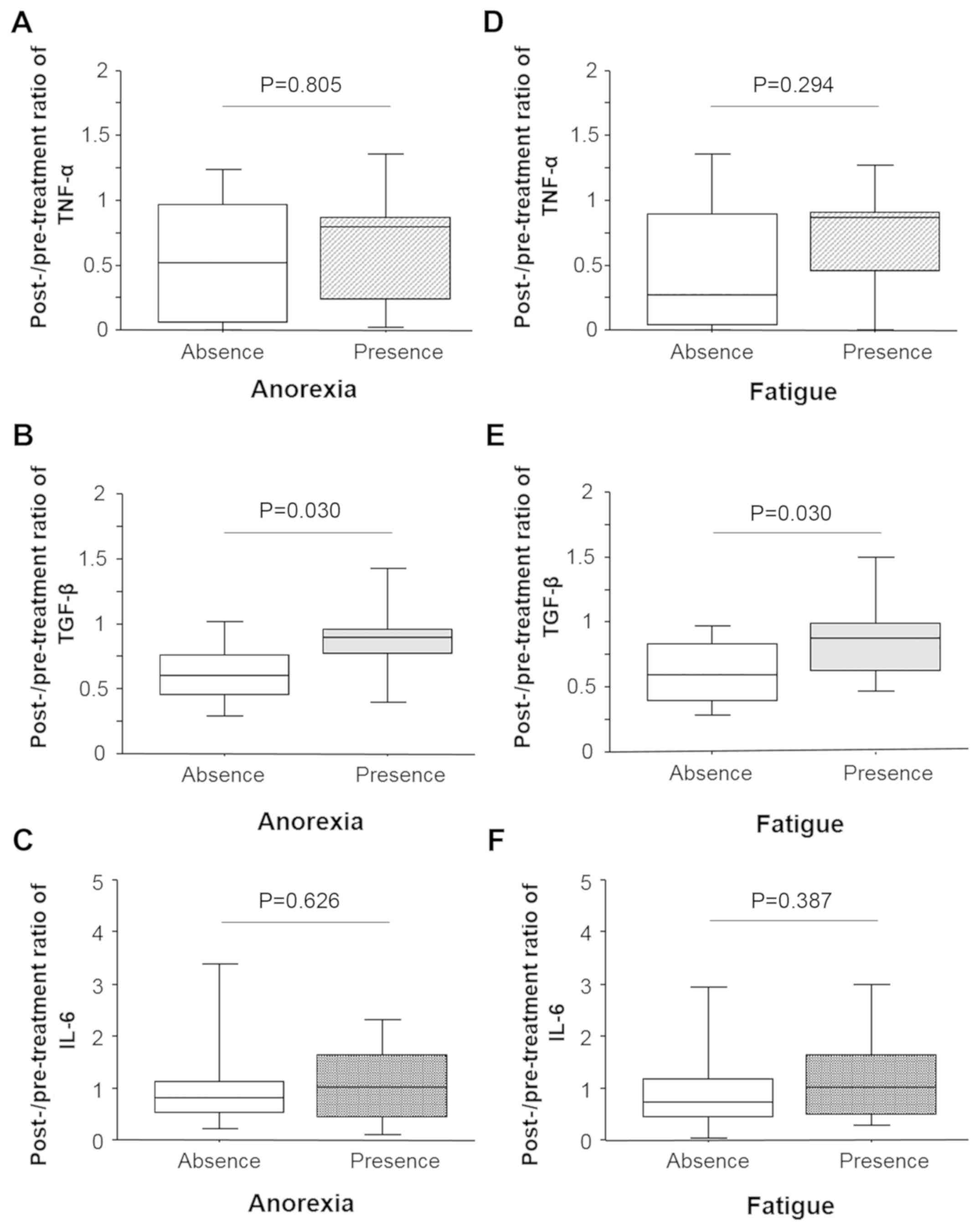
Post- and pre-treatment ratios of the serum levels
of TNF-α did not correlate with anorexia (Fig. 3A). The serum levels of TGF-β in
patients with anorexia was significantly higher (P=0.030) as
compared to those without anorexia (Fig. 3B). Similar to TNF-α, there was no
significant correlation between the post- and pre-treatment ratios
of the serum levels of IL-6 and anorexia (Fig. 3C). Similar results were observed for
patients with fatigue (Fig. 3D-F):
The post- and pre-treatment ratio of the serum levels of TGF-β
correlated with fatigue (P=0.030) whereas that of TNF-α and IL-6
did not.
There was no significant difference between the
post- and pre-treatment ratios of the serum levels of TGF-β, TNF-α,
and IL-6 and other adverse events like digestive symptoms (P=0.719,
0.743, and 0.439, respectively), hypertension (P=0.389, 0.089, and
0.938, respectively), and oral mucositis (P=0.338, 0.346, and
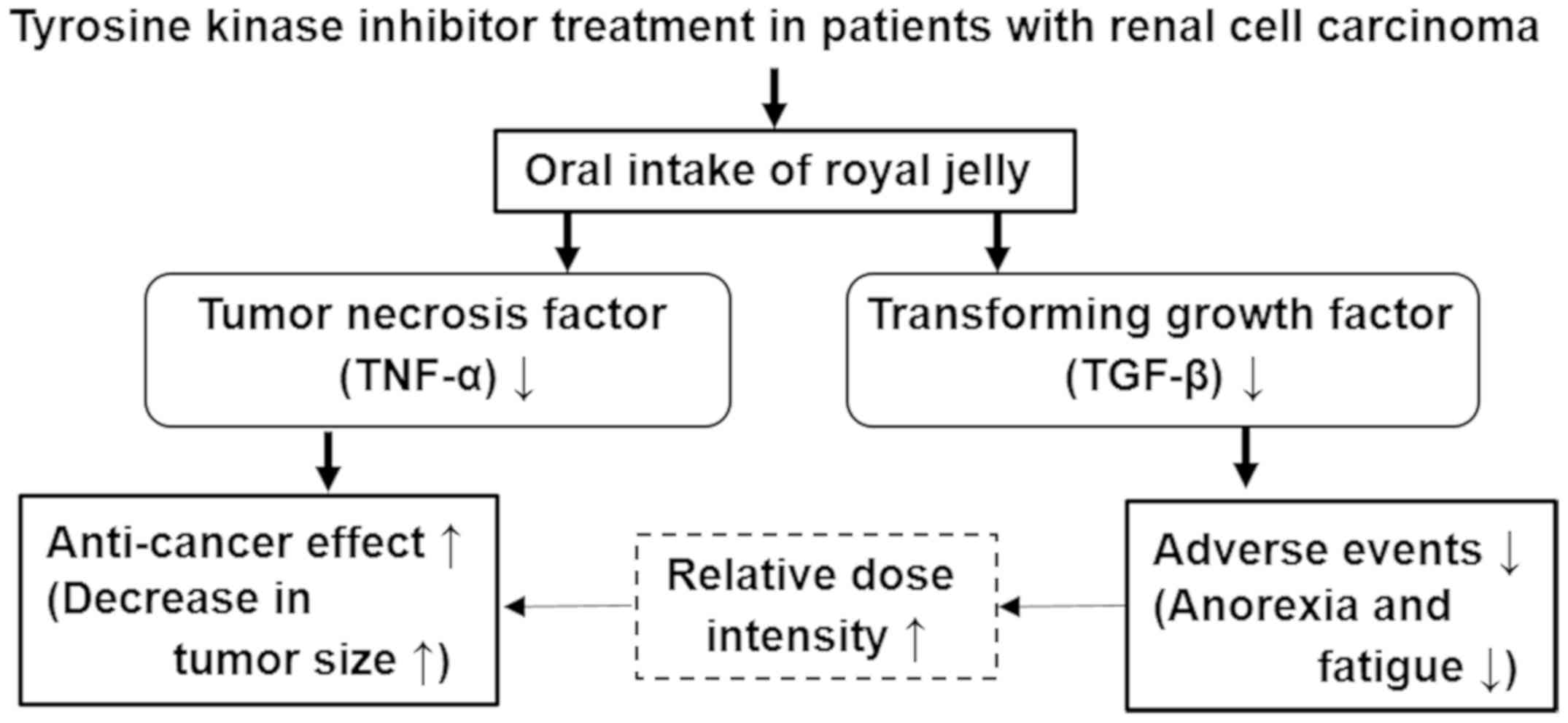
0.985, respectively). Finally, based on our results, a schematic
summarizing the main results is shown in Fig. 4.
Discussion
This study demonstrated that oral administration of
RJ improves tumor response in patients with advanced RCC undergoing
TKI treatment till 3 months of beginning the molecular targeted
therapy. To the best of our knowledge, this is the first study on
the anti-cancer effects of RJ in patients with RCC. Several in
vivo and in vitro studies have reported the anti-cancer
effects of RJ in malignancies (9,11). RJ
reduces proliferation of the MCF-7 breast cancer cells, especially
in estrogen-induced proliferating MCF-7 cells (9). The major fatty acid
10-hydroxy-2-decenoic acid (10-HAD) inhibits the WiDr colon cancer
cells upon treatment with RJ in a dose-dependent manner (12); the lipophilic fraction of RJ
possesses anti-proliferative activity against the SH-SY5Y human
neuroblastoma cells (10). Oral
administration of RJ inhibits the growth of the 4T1 mouse mammary
tumor cells in a mouse model of breast cancer (11). Thus, we speculated that RJ directly
affects proliferation of RCC cells.
Several reports have shown the indirect mechanisms
involved in the anti-cancer effects of RJ, such as changes in tumor
microenvironments and immune response in various cancers (11,12,27).
Our results showed that oral intake of RJ decreased the serum
levels of TNF-α; their levels in the serum in patients exhibiting
CR and PR was remarkably lower than those in patients exhibiting SD
or PD. In vitro studies have shown that RJ reduces TNF-α
synthesis from macrophage cells (24,28).
RJ inhibits TNF-α production in mouse peritoneal macrophages
stimulated by lipopolysaccharide and interferon-γ (23). Combining 10-HAD with RJ suppresses
the production of TNF-α in colon cancer cells (12). Serum levels of TNF-α in rats treated
with cyclophosphamide and RJ was significantly lower than those
with only cyclophosphamide treatment (29). Thus, our results of the decreased
serum levels of TNF-α upon RJ treatment was in accordance with that
of previous studies.
TNF-α is a major mediator of cancer-induced
inflammation in the tumor microenvironment and malignant
transformation by regulating various types of biomolecules and
chemokines (15). Several in
vivo and in vitro studies have demonstrated that TNF-α
levels correlate with risk of cancer, tumor growth, invasion, and
metastasis of RCC (18,21,30).
Furthermore, various anti-cancer agents suppress the malignant
transformation and tumor progression by interfering with
TNF-α-related functions in malignancies including RCC (22,31,32). A
clinical trial demonstrated that intravenously injecting
infliximab, an anti-TNF-α monoclonal antibody, decreases tumor size
and prognosis in RCC patients, suggesting that TNF-α is a putative
therapeutic target in such patients (33). Thus, decreasing TNF-α levels
suppresses malignant invasiveness and enhances anti-cancer effects
in RCC among other cancers. TNF-α is pivotal in the resistance
against sunitinib treatment in RCC (18). A high expression of CD44 correlated
with worse outcomes of sunitinib treatment in 25 patients with
metastatic RCC with TNF-α as one of the key modulators; CD44
expression induced by TNF-α contributes to acquiring resistance to
sunitinib treatment (18). Although
our study population was treated with various TKIs, we speculate
that decreasing TNF-α may be important in eliciting anti-cancer
effects in RCC patients. Also, TNF-α-related anti-cancer effects
could have been induced by oral intake of RJ in our cohort.
Unfortunately, there is no scope to discuss and conclude this
hypothesis since there is no known correlation between RJ, TNF-α,
and anti-cancer effects in patients with RCC. A study recently
showed that the synthesis of TNF-α is inhibited by 10-HAD in human
colon cancer cells (12). More
recently, it has been shown that purified proteins from RJ improved
the damage caused to necrotic hepatocytes by reducing the levels of
TNF-α; this phenomenon correlated with anti-cancer effects in the
HepG2 hepatocellular cancer cells (29).
RDI may have regulated the anti-cancer effects in
our study population. We observed that a percentage of patients
treated with the initial dose versus patients treated with RJ was
significantly higher as compared to those administered placebo. The
1-month and 2-month RDIs for patients in the RJ group were
significantly higher than those in the placebo groups. The dose
intensity correlated with anti-cancer effects induced by multiple
types of anti-cancer agents used for malignancies. Continuing
sunitinib therapy for more than one course and maintaining more
than 60% of 1-month RDI correlated with better outcome for
progression-free survival in patients with metastatic RCC (34). Similarly, more than 65% of two
course-RDI should be maintained for optimal therapeutic efficacy of
sunitinib treatment (35). Taken
together, higher intensities of TKIs upon oral administration of RJ
dictates better anti-cancer effects in patients with RCC. We also
speculated that suppression of TKIs induced toxicities by RJ is
important in maintaining the RDI and stimulated anti-cancer effects
in our study cohort. Several reports have shown that successfully
managing drug-induced toxicities leads to the long-term
continuation of sunitinib and positively correlates with
anti-cancer effects in various types of cancers including RCC
(36,37). We have previously demonstrated that
oral intake of RJ suppresses the frequency and severity of several
adverse events, such as anorexia and fatigue, in RCC patients
treated with TKIs (8); and this
study confirmed in this study used by different criterion. However,
the detailed mechanism employed by mediating these protective
effects remains to be studied.
To understand the protective effects mediated by RJ
against adverse events in TKI-treated patients, we focused on the
levels of TNF-α, TGF-β, and IL-6 in patients with advanced RCC. We
found that significant decrease in the serum levels of TGF-β level
upon oral intake of RJ significantly correlated with the
suppression of adverse events, such as fatigue and anorexia. TGF-β
promotes chronic fatigue syndrome and cancer-induced cachexia
including anorexia (16,20,38).
The serum/plasma levels of TGF-β increases in patients with chronic
fatigue syndrome and/or cancer-induced anorexia (16,19,20).
Furthermore, in a murine model of pancreatic cancer cachexia,
pharmacologic blockage of TGF-β improves cancer-related cachexia
including weight loss and skeletal muscle proteolysis (17). Inhibiting TGF-β did not affect
cancer cell proliferation and subcutaneous tumor growth (17). Although there is no clear
correlation between TGF-β and RJ in vivo, oral intake of RJ
reverses TGF-β levels in the bronchoalveolar lavage fluid induced
by bleomycin treatment in rats (25). These findings are in accordance with
our results that RJ-induced decrease in TGF-β levels correlates
with improved anorexia and fatigue but does not correlate with
tumor response after TKI treatment in RCC patients. RJ and 10-HDA
inhibit IL-6 production in macrophages (23,24);
however, there is little information on the influence of RJ on the
serum levels of IL-6 in cancer patients. Our results showed that
oral administration of RJ did not affect serum IL-6 levels in
patients with RCC. However, this is preliminary and more detailed
studies are necessary to conclude this.
Although this is the first clinical trial on the
oral administration of RJ in RCC patients and its effects on
anti-cancer activities and adverse events, this study has several
limitations. The most important is the small cohort of patients in
the placebo and RJ groups. Another limitation is the use of
multiple TKIs and patients with non-uniform characteristics.
Moreover, the RJ capsules were provided by a company that sell
supplements made from honey including RJ. Therefore, to avoid any
bias, we conducted this trial using a double-blind randomized
analysis; selection of administration, data collection, and
analysis were performed by a third party approved by our IRB.
Furthermore, RJ was administrated only for 3 months. We did not
observe any adverse events induced by RJ in our study cohort.
Therefore, we emphasize the need for detailed clinical trials with
longer period of treatment in the future to evaluate the treatment
strategies in patients with advanced RCC.
In conclusion, oral administration of RJ
strengthened anti-cancer effects of molecular targeted agents in
patients with metastatic RCC. Moreover, it suppressed the frequency
of anorexia and fatigue induced by TKI therapy. RJ was useful in
maintaining the RDI compared to patients with placebo. Furthermore,
RJ decreased the serum levels of TNF-α and TGF-β and such changes
were speculated to correlate with the enhanced tumor response and
suppressed adverse events.
Acknowledgements
The authors would like to thank Ms. Mitsuko Yoneda
(Nagasaki University Hospital) for providing technical support.
Funding
This study was supported by a Yamada Research grant
(grant no. 2015-20; to YMi).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the author on reasonable request.
Authors' contributions
YMi conceived and designed the experiments,
performed data analysis, and contributed to the writing of the
manuscript. KA performed the experiments and contributed to sample
collection and writing of the manuscript. KA, YN, TY, YMu and AO
performed the experiments and analyzed the data. KM, TM, KO and YMo
contributed to sample and clinical data collection. HS conceived
and designed the experiments and contributed to drafting and
revising the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The purpose of the present study was explained to
the participants, who all signed written consent forms prior to
participating in the study. This study design was approved by the
Institutional Review Board of Nagasaki University Hospital
(Nagasaki, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zarrabi K and Wu S: Current and emerging
therapeutic targets for metastatic renal cell carcinoma. Curr Oncol
Rep. 20(41)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chang AJ, Zhao L, Zhu Z, Boulanger K, Xiao
H, Wakefield MR, Bai Q and Fang Y: The past, present and future of
immunotherapy for metastatic renal cell carcinoma. Anticancer Res.
39:2683–2687. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu N, Weng S, Wang J, Chen J, Yu L, Fang
X and Yuan Y: Preclinical rationale and clinical efficacy of
antiangiogenic therapy and immune checkpoint blockade combination
therapy in urogenital tumors. J Cancer Res Clin Oncol.
145:3021–3036. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Almeer RS, Soliman D, Kassab RB, AlBasher
GI, Alarifi S, Alkahtani S, Ali D, Metwally D and Abdel Moneim AE:
Royal jelly abrogates cadmium-induced oxidative challenge in mouse
testes: Involvement of the Nrf2 pathway. Int J Mol Sci.
19(3979)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Petelin A, Kenig S, Kopinč R, Deželak M,
Černelič Bizjak M and Jenko Pražnikar Z: Effects of royal jelly
administration on lipid profile, satiety, inflammation, and
antioxidant capacity in asymptomatic overweight adults. Evid Based
Complement Alternat Med. 2019(4969720)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miyata Y and Sakai H: Anti-Cancer and
protective effects of royal jelly for therapy-induced toxicities in
malignancies. Int J Mol Sci. 19(3270)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Münstedt K and Männle H: Using bee
products for the prevention and treatment of oral mucositis induced
by cancer treatment. Molecules. 24(3023)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Araki K, Miyata Y, Ohba K, Nakamura Y,
Matsuo T, Mochizuki Y and Sakai H: Oral intake of royal jelly has
protective effects against tyrosine kinase inhibitor-induced
toxicity in patients with renal cell carcinoma: A randomized,
double-blinded, placebo-controlled trial. Medicines (Basel).
6(2)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nakaya M, Onda H, Sasaki K, Yukiyoshi A,
Tachibana H and Yamada K: Effect of royal jelly on bisphenol
A-induced proliferation of human breast cancer cells. Biosci
Biotechnol Biochem. 71:253–255. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gismondi A, Trionfera E, Canuti L, Di
Marco G and Canini A: Royal jelly lipophilic fraction induces
antiproliferative effects on SH-SY5Y human neuroblastoma cells.
Oncol Rep. 38:1833–1844. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang S, Shao Q, Geng H and Su S: The
effect of royal jelly on the growth of breast cancer in mice. Oncol
Lett. 14:7615–7621. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang YC, Chou WM, Widowati DA, Lin IP and
Peng CC: 10-hydroxy-2-decenoic acid of royal jelly exhibits
bactericide and anti-inflammatory activity in human colon cancer
cells. BMC Complement Altern Med. 18(202)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang X and Shapiro DJ: The immune system
and inflammation in breast cancer. Mol Cell Endocrinol.
382:673–682. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sethi G, Sung B and Aggarwal BB: TNF: A
master switch for inflammation to cancer. Front Biosci.
13:5094–5107. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Blundell S, Ray KK, Buckland M and White
PD: Chronic fatigue syndrome and circulating cytokines: A
systematic review. Brain Behav Immun. 50:186–195. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Greco SH, Tomkötter L, Vahle AK, Rokosh R,
Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A,
et al: TGF-β blockade reduces mortality and metabolic changes in a
validated murine model of pancreatic cancer cachexia. PLoS One.
10(e0132786)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mikami S, Mizuno R, Kosaka T, Saya H, Oya
M and Okada Y: Expression of TNF-α and CD44 is implicated in poor
prognosis, cancer cell invasion, metastasis and resistance to the
sunitinib treatment in clear cell renal cell carcinomas. Int J
Cancer. 136:1504–1514. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nguyen CB, Kumar S, Zucknick M, Kristensen
VN, Gjerstad J, Nilsen H and Wyller VB: Associations between
clinical symptoms, plasma norepinephrine and deregulated immune
gene networks in subgroups of adolescent with chronic fatigue
syndrome. Brain Behav Immun. 76:82–96. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Strawbridge R, Sartor ML, Scott F and
Cleare AJ: Inflammatory proteins are altered in chronic fatigue
syndrome-A systematic review and meta-analysis. Neurosci Biobehav
Rev. 107:69–83. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Q, Tu H, Zhu M, Liang D, Ye Y, Chang
DW, Long Y and Wu X: Circulating obesity-driven biomarkers are
associated with risk of clear cell renal cell carcinoma: A
two-stage, case-control study. Carcinogenesis. 40:1191–1197.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun L, Gao Z, Luo L, Tan H and Zhang G:
Estrogen affects cell growth and IGF-1 receptor expression in renal
cell carcinoma. Onco Targets Ther. 11:5873–5878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kohno K, Okamoto I, Sano O, Arai N, Iwaki
K, Ikeda M and Kurimoto M: Royal jelly inhibits the production of
proinflammatory cytokines by activated macrophages. Biosci
Biotechnol Biochem. 68:138–145. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugiyama T, Takahashi K, Tokoro S, Gotou
T, Neri P and Mori H: Inhibitory effect of
10-hydroxy-trans-2-decenoic acid on LPS-induced IL-6 production via
reducing IκB-ζ expression. Innate Immun. 18:429–437.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zargar HR, Hemmati AA, Ghafourian M, Arzi
A, Rezaie A and Javad-Moosavi SA: Long-term treatment with royal
jelly improves bleomycin-induced pulmonary fibrosis in rats. Can J
Physiol Pharmacol. 95:23–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miyata Y, Matsuo T, Sagara Y, Ohba K,
Ohyama K and Sakai H: A mini-review of reactive oxygen species in
urological cancer: Correlation with NADPH oxidases, angiogenesis,
and apoptosis. Int J Mol Sci. 18(2214)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takahashi K, Sugiyama T, Tokoro S, Neri P
and Mori H: Inhibition of interferon-γ-induced nitric oxide
production by 10-hydroxy-trans-2-decenoic acid through inhibition
of interferon regulatory factor-8 induction. Cell Immunol.
273:73–78. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abu-Serie MM and Habashy NH: Two purified
proteins from royal jelly with in vitro dual anti-hepatic damage
potency: Major royal jelly protein 2 and its novel isoform X1. Int
J Biol Macromol. 128:782–795. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun KH, Sun GH, Wu YC, Ko BJ, Hsu HT and
Wu ST: TNF-α augments CXCR2 and CXCR3 to promote progression of
renal cell carcinoma. J Cell Mol Med. 20:2020–2028. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shin SY, Kim CG, Jung YJ, Jung Y, Jung H,
Im J, Lim Y and Lee YH: Euphorbia humifusa willd exerts inhibition
of breast cancer cell invasion and metastasis through inhibition of
TNFα-induced MMP-9 expression. BMC Complement Altern Med.
16(413)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Deng Y, Long L, Wang K, Zhou J, Zeng L, He
L and Gong Q: Icariside, II, a broad-spectrum anti-cancer agent,
reverses beta-amyloid-induced cognitive impairment through reducing
inflammation and apoptosis in rats. Front Pharmacol.
8(39)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Harrison ML, Obermueller E, Maisey NR,
Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, et
al: Tumor necrosis factor alpha as a new target for renal cell
carcinoma: Two sequential phase II trials of infliximab at standard
and high dose. J Clin Oncol. 25:4542–4549. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kawashima A, Uemura M, Kato T, Ujike T,
Nagahara A, Fujita K, Imamura R, Yamanaka Y, Tomiyama E, Tanigawa
G, et al: Results of weekday-on and weekend-off administration
schedule of sunitinib therapy for advanced renal cell carcinoma.
Int J Clin Oncol. 24:78–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Iwamoto K, Ishihara H, Takagi T, Kondo T,
Yoshida K, Iizuka J and Tanabe K: Evaluation of relative dose
intensity during the early phase of first-line sunitinib treatment
using a 2-week-on/1-week-off regimen for metastatic renal cell
carcinoma. Med Oncol. 35(78)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ohba K, Miyata Y, Yasuda T, Asai A,
Mitsunari K, Matsuo T, Mochizuki Y, Matsunaga N and Sakai H:
Efficacy and safety of sunitinib alternate day regimen in patients
with metastatic renal cell carcinoma in Japan: Comparison with
standard 4/2 schedule. Asia Pac J Clin Oncol. 14:153–158.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee L, Ito T, Igarashi H, Miki M, Fujimori
N, Kawabe K, Jensen RT and Ogawa Y: Dose and schedule modification
are required for long-term continuation of sunitinib in Japanese
patients with advanced pancreatic neuroendocrine tumors. Cancer
Chemother Pharmacol. 81:163–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zugmaier G, Paik S, Wilding G, Knabbe C,
Bano M, Lupu R, Deschauer B, Simpson S, Dickson RB and Lippman M:
Transforming growth factor beta 1 induces cachexia and systemic
fibrosis without an antitumor effect in nude mice. Cancer Res.
51:3590–3594. 1991.PubMed/NCBI
|