|
1
|
Zarrabi K and Wu S: Current and emerging
therapeutic targets for metastatic renal cell carcinoma. Curr Oncol
Rep. 20(41)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chang AJ, Zhao L, Zhu Z, Boulanger K, Xiao
H, Wakefield MR, Bai Q and Fang Y: The past, present and future of
immunotherapy for metastatic renal cell carcinoma. Anticancer Res.
39:2683–2687. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu N, Weng S, Wang J, Chen J, Yu L, Fang
X and Yuan Y: Preclinical rationale and clinical efficacy of
antiangiogenic therapy and immune checkpoint blockade combination
therapy in urogenital tumors. J Cancer Res Clin Oncol.
145:3021–3036. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Almeer RS, Soliman D, Kassab RB, AlBasher
GI, Alarifi S, Alkahtani S, Ali D, Metwally D and Abdel Moneim AE:
Royal jelly abrogates cadmium-induced oxidative challenge in mouse
testes: Involvement of the Nrf2 pathway. Int J Mol Sci.
19(3979)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Petelin A, Kenig S, Kopinč R, Deželak M,
Černelič Bizjak M and Jenko Pražnikar Z: Effects of royal jelly
administration on lipid profile, satiety, inflammation, and
antioxidant capacity in asymptomatic overweight adults. Evid Based
Complement Alternat Med. 2019(4969720)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miyata Y and Sakai H: Anti-Cancer and
protective effects of royal jelly for therapy-induced toxicities in
malignancies. Int J Mol Sci. 19(3270)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Münstedt K and Männle H: Using bee
products for the prevention and treatment of oral mucositis induced
by cancer treatment. Molecules. 24(3023)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
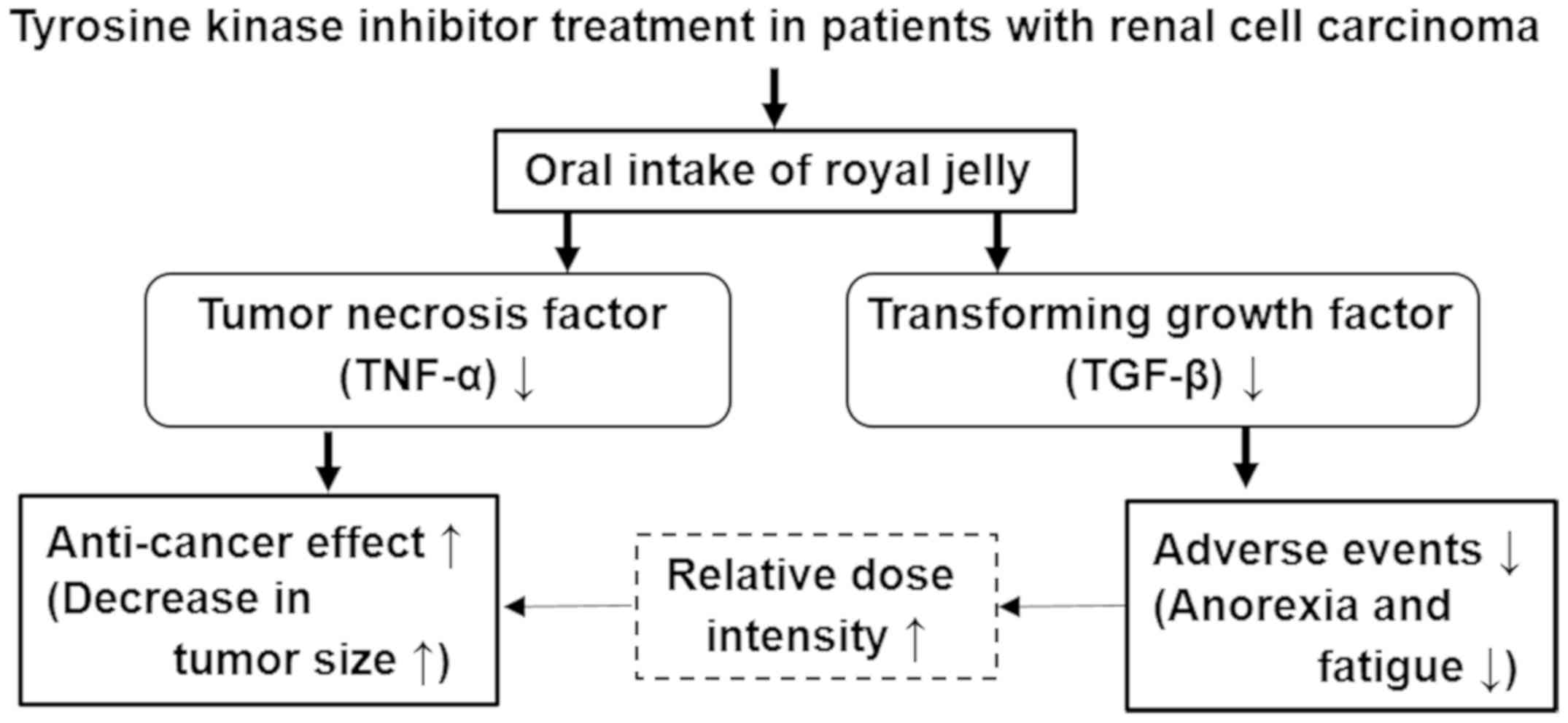
Araki K, Miyata Y, Ohba K, Nakamura Y,
Matsuo T, Mochizuki Y and Sakai H: Oral intake of royal jelly has
protective effects against tyrosine kinase inhibitor-induced
toxicity in patients with renal cell carcinoma: A randomized,
double-blinded, placebo-controlled trial. Medicines (Basel).
6(2)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nakaya M, Onda H, Sasaki K, Yukiyoshi A,
Tachibana H and Yamada K: Effect of royal jelly on bisphenol
A-induced proliferation of human breast cancer cells. Biosci
Biotechnol Biochem. 71:253–255. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gismondi A, Trionfera E, Canuti L, Di
Marco G and Canini A: Royal jelly lipophilic fraction induces
antiproliferative effects on SH-SY5Y human neuroblastoma cells.
Oncol Rep. 38:1833–1844. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang S, Shao Q, Geng H and Su S: The
effect of royal jelly on the growth of breast cancer in mice. Oncol
Lett. 14:7615–7621. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang YC, Chou WM, Widowati DA, Lin IP and
Peng CC: 10-hydroxy-2-decenoic acid of royal jelly exhibits
bactericide and anti-inflammatory activity in human colon cancer
cells. BMC Complement Altern Med. 18(202)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang X and Shapiro DJ: The immune system
and inflammation in breast cancer. Mol Cell Endocrinol.
382:673–682. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sethi G, Sung B and Aggarwal BB: TNF: A
master switch for inflammation to cancer. Front Biosci.
13:5094–5107. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
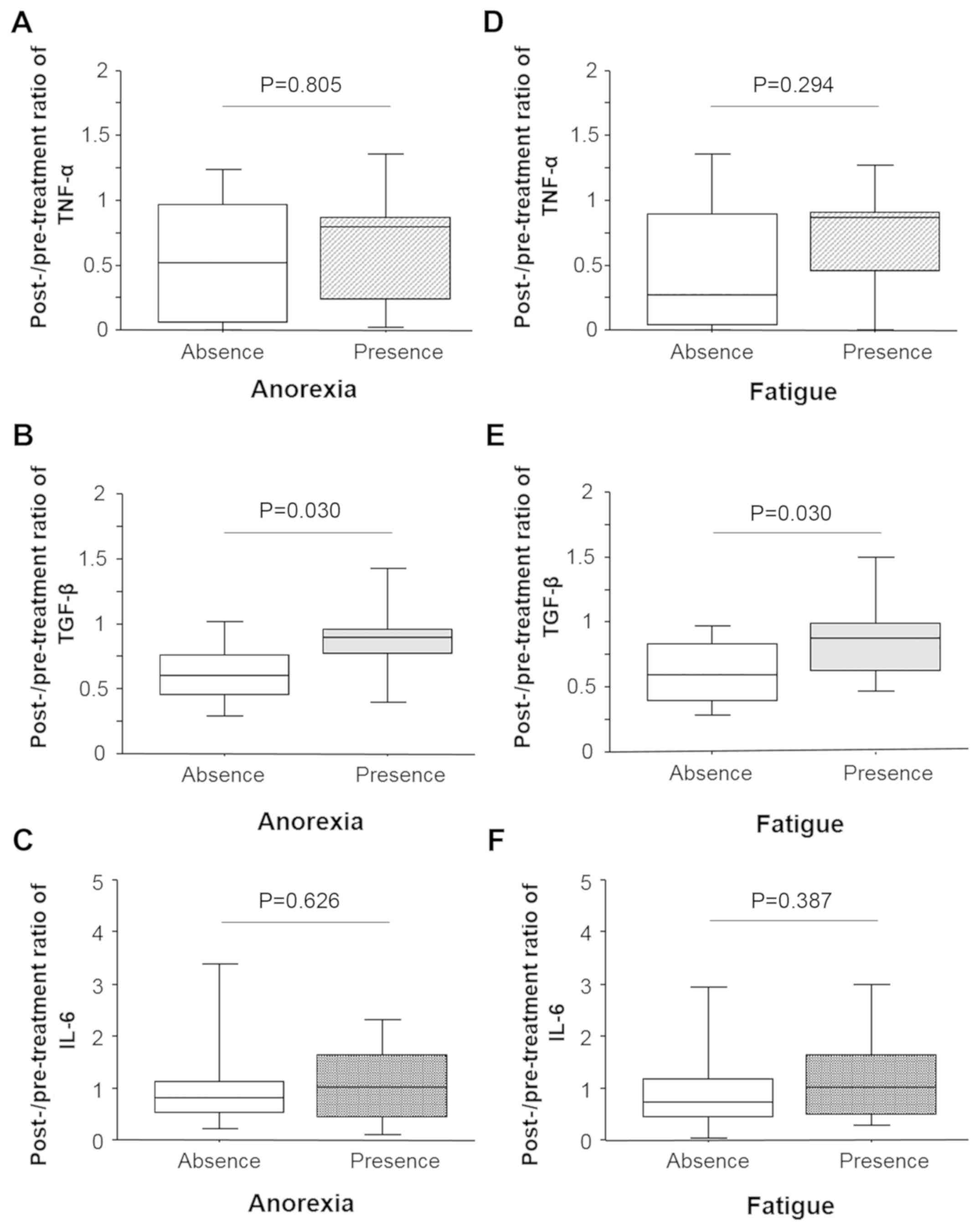
Blundell S, Ray KK, Buckland M and White
PD: Chronic fatigue syndrome and circulating cytokines: A
systematic review. Brain Behav Immun. 50:186–195. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Greco SH, Tomkötter L, Vahle AK, Rokosh R,
Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A,
et al: TGF-β blockade reduces mortality and metabolic changes in a
validated murine model of pancreatic cancer cachexia. PLoS One.
10(e0132786)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mikami S, Mizuno R, Kosaka T, Saya H, Oya
M and Okada Y: Expression of TNF-α and CD44 is implicated in poor
prognosis, cancer cell invasion, metastasis and resistance to the
sunitinib treatment in clear cell renal cell carcinomas. Int J
Cancer. 136:1504–1514. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nguyen CB, Kumar S, Zucknick M, Kristensen
VN, Gjerstad J, Nilsen H and Wyller VB: Associations between
clinical symptoms, plasma norepinephrine and deregulated immune
gene networks in subgroups of adolescent with chronic fatigue
syndrome. Brain Behav Immun. 76:82–96. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Strawbridge R, Sartor ML, Scott F and
Cleare AJ: Inflammatory proteins are altered in chronic fatigue
syndrome-A systematic review and meta-analysis. Neurosci Biobehav
Rev. 107:69–83. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Q, Tu H, Zhu M, Liang D, Ye Y, Chang
DW, Long Y and Wu X: Circulating obesity-driven biomarkers are
associated with risk of clear cell renal cell carcinoma: A
two-stage, case-control study. Carcinogenesis. 40:1191–1197.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun L, Gao Z, Luo L, Tan H and Zhang G:
Estrogen affects cell growth and IGF-1 receptor expression in renal
cell carcinoma. Onco Targets Ther. 11:5873–5878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kohno K, Okamoto I, Sano O, Arai N, Iwaki
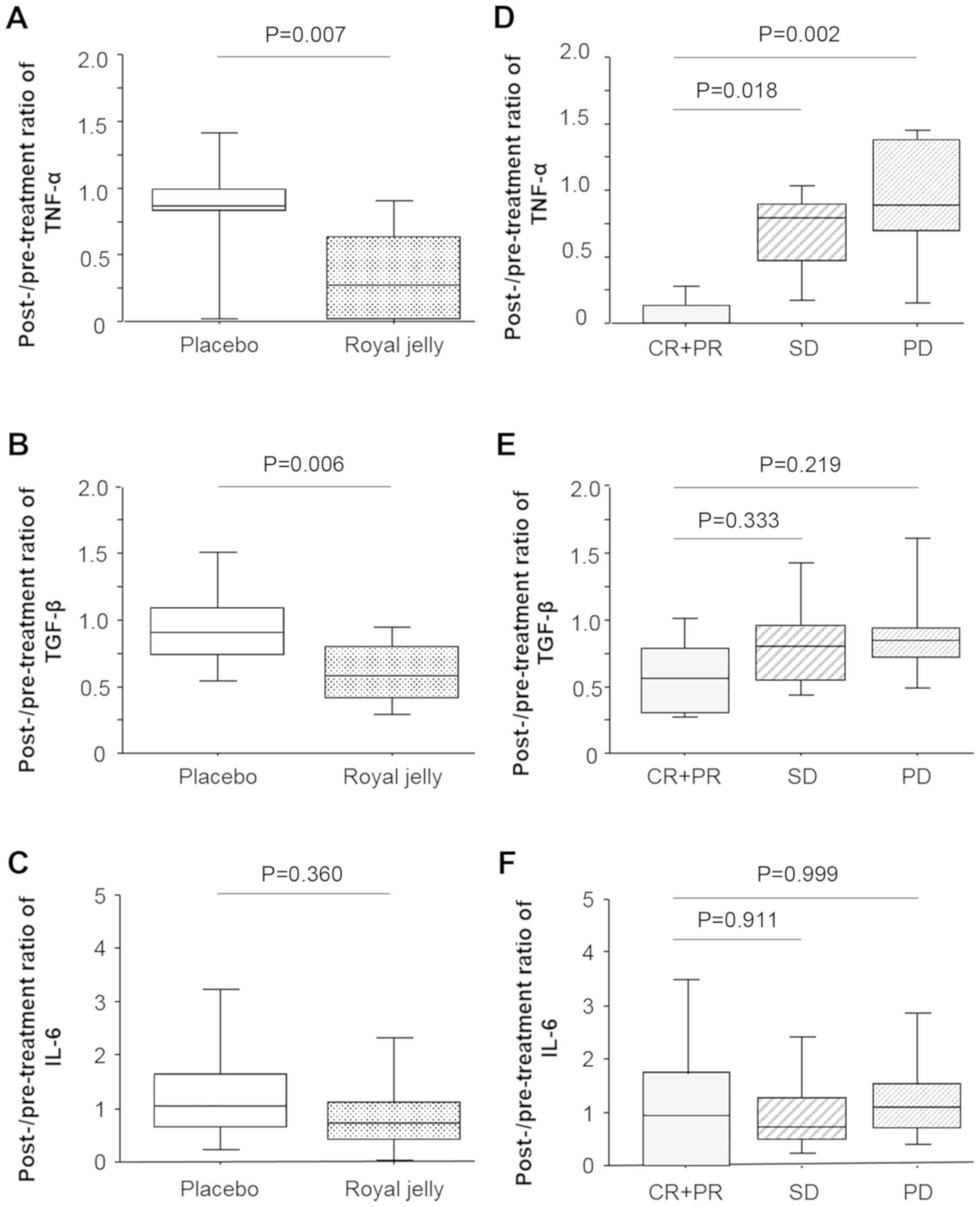
K, Ikeda M and Kurimoto M: Royal jelly inhibits the production of
proinflammatory cytokines by activated macrophages. Biosci
Biotechnol Biochem. 68:138–145. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugiyama T, Takahashi K, Tokoro S, Gotou
T, Neri P and Mori H: Inhibitory effect of
10-hydroxy-trans-2-decenoic acid on LPS-induced IL-6 production via
reducing IκB-ζ expression. Innate Immun. 18:429–437.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zargar HR, Hemmati AA, Ghafourian M, Arzi
A, Rezaie A and Javad-Moosavi SA: Long-term treatment with royal
jelly improves bleomycin-induced pulmonary fibrosis in rats. Can J
Physiol Pharmacol. 95:23–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miyata Y, Matsuo T, Sagara Y, Ohba K,
Ohyama K and Sakai H: A mini-review of reactive oxygen species in
urological cancer: Correlation with NADPH oxidases, angiogenesis,
and apoptosis. Int J Mol Sci. 18(2214)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takahashi K, Sugiyama T, Tokoro S, Neri P
and Mori H: Inhibition of interferon-γ-induced nitric oxide
production by 10-hydroxy-trans-2-decenoic acid through inhibition
of interferon regulatory factor-8 induction. Cell Immunol.
273:73–78. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abu-Serie MM and Habashy NH: Two purified
proteins from royal jelly with in vitro dual anti-hepatic damage
potency: Major royal jelly protein 2 and its novel isoform X1. Int
J Biol Macromol. 128:782–795. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun KH, Sun GH, Wu YC, Ko BJ, Hsu HT and
Wu ST: TNF-α augments CXCR2 and CXCR3 to promote progression of
renal cell carcinoma. J Cell Mol Med. 20:2020–2028. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shin SY, Kim CG, Jung YJ, Jung Y, Jung H,
Im J, Lim Y and Lee YH: Euphorbia humifusa willd exerts inhibition
of breast cancer cell invasion and metastasis through inhibition of
TNFα-induced MMP-9 expression. BMC Complement Altern Med.
16(413)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Deng Y, Long L, Wang K, Zhou J, Zeng L, He
L and Gong Q: Icariside, II, a broad-spectrum anti-cancer agent,
reverses beta-amyloid-induced cognitive impairment through reducing
inflammation and apoptosis in rats. Front Pharmacol.
8(39)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Harrison ML, Obermueller E, Maisey NR,
Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, et
al: Tumor necrosis factor alpha as a new target for renal cell
carcinoma: Two sequential phase II trials of infliximab at standard
and high dose. J Clin Oncol. 25:4542–4549. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kawashima A, Uemura M, Kato T, Ujike T,
Nagahara A, Fujita K, Imamura R, Yamanaka Y, Tomiyama E, Tanigawa
G, et al: Results of weekday-on and weekend-off administration
schedule of sunitinib therapy for advanced renal cell carcinoma.
Int J Clin Oncol. 24:78–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Iwamoto K, Ishihara H, Takagi T, Kondo T,
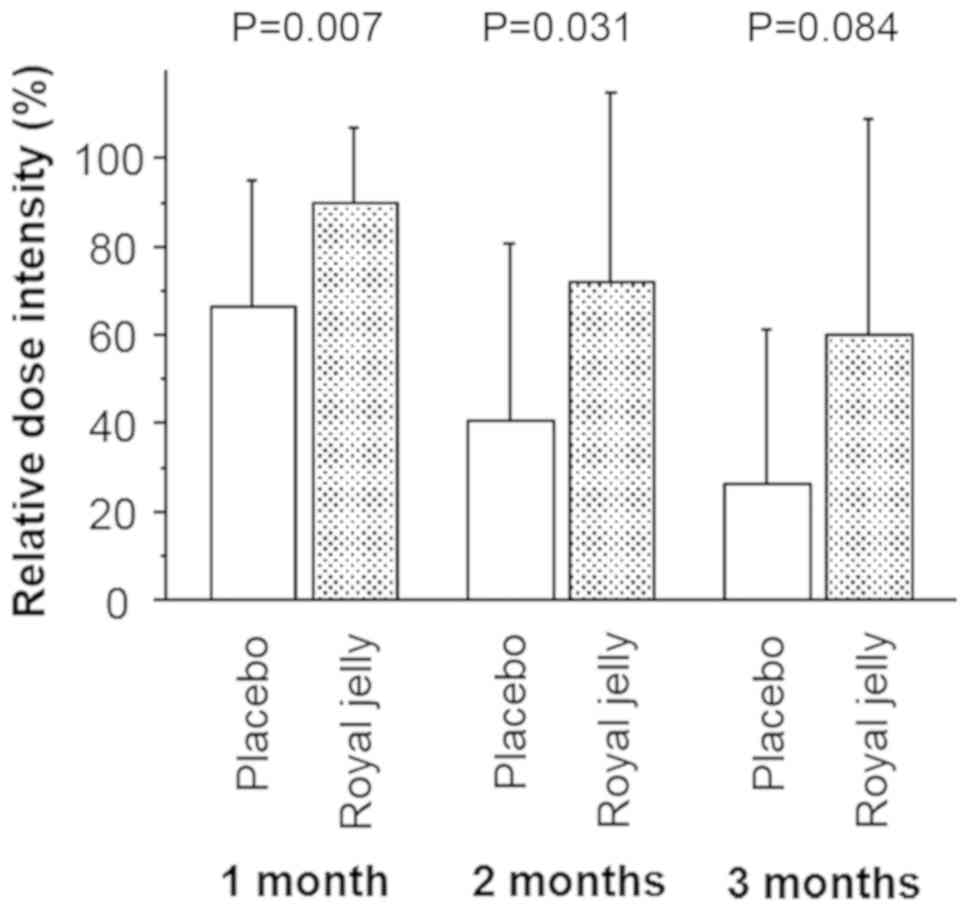
Yoshida K, Iizuka J and Tanabe K: Evaluation of relative dose
intensity during the early phase of first-line sunitinib treatment
using a 2-week-on/1-week-off regimen for metastatic renal cell
carcinoma. Med Oncol. 35(78)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ohba K, Miyata Y, Yasuda T, Asai A,
Mitsunari K, Matsuo T, Mochizuki Y, Matsunaga N and Sakai H:
Efficacy and safety of sunitinib alternate day regimen in patients
with metastatic renal cell carcinoma in Japan: Comparison with
standard 4/2 schedule. Asia Pac J Clin Oncol. 14:153–158.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee L, Ito T, Igarashi H, Miki M, Fujimori
N, Kawabe K, Jensen RT and Ogawa Y: Dose and schedule modification
are required for long-term continuation of sunitinib in Japanese
patients with advanced pancreatic neuroendocrine tumors. Cancer
Chemother Pharmacol. 81:163–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zugmaier G, Paik S, Wilding G, Knabbe C,
Bano M, Lupu R, Deschauer B, Simpson S, Dickson RB and Lippman M:
Transforming growth factor beta 1 induces cachexia and systemic
fibrosis without an antitumor effect in nude mice. Cancer Res.
51:3590–3594. 1991.PubMed/NCBI
|