Introduction
Ameloblastic carcinoma (AC) is a very rare malignant
odontogenic tumor, with features of both ameloblastoma and
carcinoma. An approximate male-to-female ratio of AC is 2:1 and a
mandible-to-maxilla ratio is 1.7:1(1). The common symptoms of AC are pain,
swelling, and rapid growth. Clinically, AC is a typically
aggressive tumor with extensive local destruction. Lymph node
involvement and distant metastasis have also been reported
(2-6).
AC, first introduced as a distinct entity by Elzay in 1982
(1,2), is defined as a rare odontogenic
malignancy that combines the histological features of ameloblastoma
with cytological atypia regardless of metastasis, after
considerable debate, by the World Health Organization
classification of odontogenic tumors in 2005. It is classified into
primary- (de novo) and secondary-type (malignant
transformation of pre-existing ameloblastoma). Secondary-type AC is
further divided into two subtypes: Intraosseous and peripheral.
This classification was preserved with the 2017 update of the
classification.
No standard treatment has been established for this
rare tumor. Previous reports indicate complete surgical resection
with wide local excision and cervical lymph node dissection as a
commonly used approach (1-4,7);
however, aesthetic failure and dysfunction after surgery, such as
dysphagia, dysarthria, and nasopharyngeal closure dysfunction, are
severe. The treatment efficacy of systemic chemotherapy and/or
radiotherapy seemed poor and limited; local control rate is low and
physical strength may decline due to decreased bone marrow function
or difficulty in oral intake. These conservative therapies have
been used as an adjunctive therapy (1). Strojan et al reported that
cumulative dose of cisplatin in concurrent chemoradiation protocols
for head and neck squamous cell carcinoma (SCC) has a significant
positive correlation with survival (8). Among these non-surgical approaches,
intra-arterial infusion chemotherapy (IAIC) combined with
radiotherapy has been increasingly performed in recent years for
locally advanced head and neck cancers to avoid surgery. Several
studies using IAIC reported that the outcomes of this
organ-preserving approach were not inferior to those of surgery
(9-11).
Although AC is considered to be a radioresistant tumor, there are
some reports of good treatment results with X-rays, gamma knife,
and particle beam therapy, including proton beam therapy (PBT) and
carbon ion therapy (CIT) (12-16).
Particle beam therapy, which provides several advantages including
a rapid dose fall-off at the distal end and the possibility to
induce double strand DNA breaks leading to catastrophic damage to
cancer cells (17), is an effective
approach that provides high-dose irradiation to the tumor without
increasing toxicity to the normal tissue (18). Although the therapeutic effect of
particle beam therapy has been reported for non-SCC of the head and
neck (19), few reports
investigated its efficacy in AC (15,16).
Here we report a case of recurrent secondary-type AC treated by PBT
in combination with IAIC that resulted in a good long-term
course.
Case report
A 71-year-old man was referred to the Southern
Tohoku Proton Therapy Center in March 2009 with severe pain and
swelling of the right palatal gingiva. In October 2007, he was
diagnosed with ameloblastoma of the right maxilla based on the
histopathology of biopsy and underwent partial resection several
times owing to tumor recurrence. The patient was diagnosed with AC
in March 2009 based on pathological assessment after the fourth
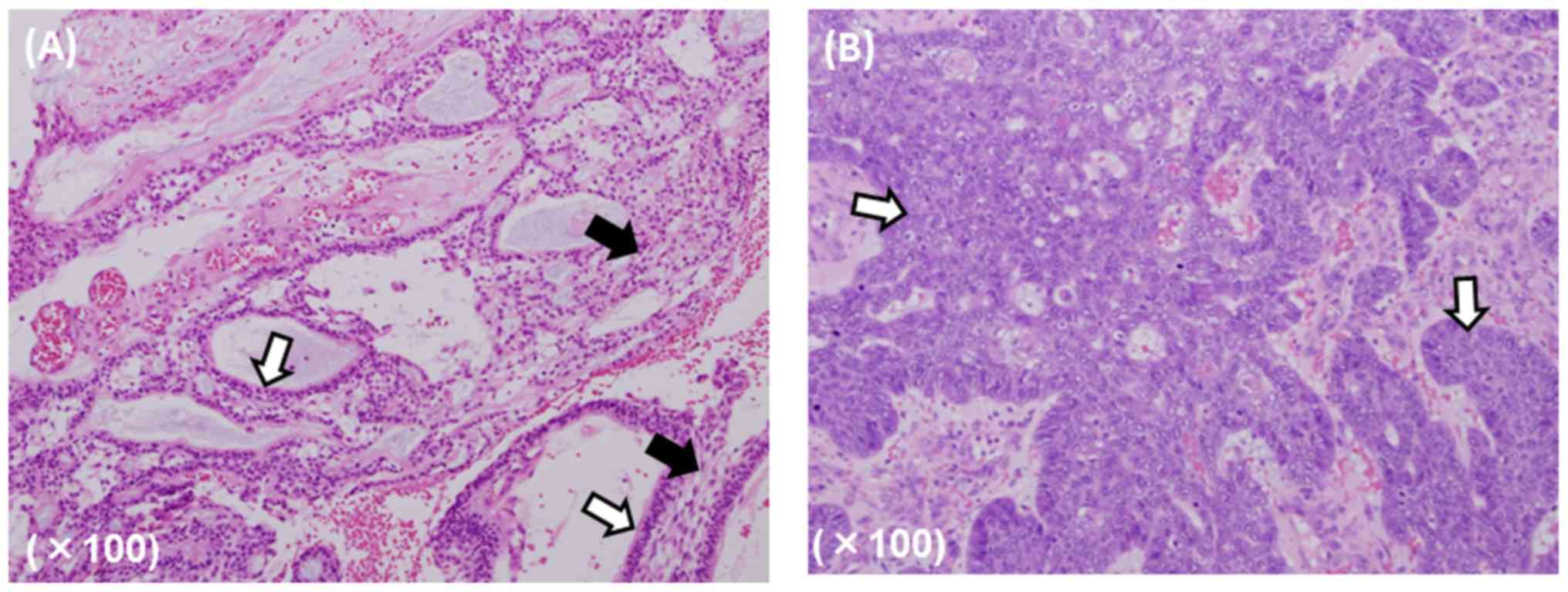
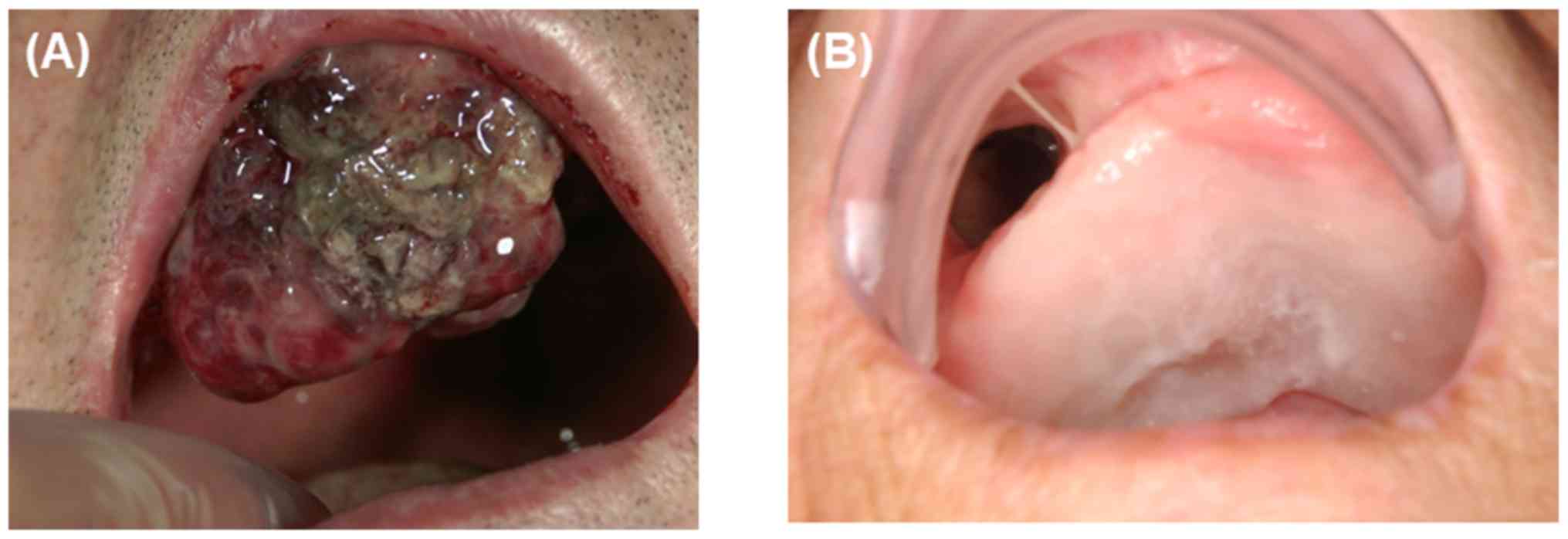
surgery (Fig. 1).
At the time of admission, his hard palate on the
right was swollen with bony expansion to the oral cavity, and the
tumor had invaded the right alveolar ridge. He had paresthesia of
the right face. Enhanced magnetic resonance imaging (MRI; Signa
HDx, GE Healthcare) revealed a large, heterogeneously enhanced mass
extending from the right maxillary sinus to the upper gingiva,
measuring ~50x70 mm in dimensions. The medial extension reached the
right nasal septum and the right ethmoid sinus. The tumor had
destroyed the floor of the right maxillary sinus, perforated the
anterior wall of the right maxillary sinus, and extended into the
surrounding soft tissue. 18F-fluorodeoxyglucose
(synthesized and used in our own facility) positron emission
tomography computed tomography (FDG-PET/CT; Discovery ST Elite, GE
Healthcare) showed high FDG concentration in the right maxillary
sinus (maximum standardized uptake value, 21.6). There were no
suspicious lymph nodes or remote metastases.
The growth speed of the tumor was extremely rapid,
and further surgical resection was deemed not to be sufficient for
possible tumor control. Therefore, the patient was treated using
PBT in combination with IAIC to the artery supplying the tumor.
Written informed consent was obtained from the patient. This
treatment was approved by the Ethics Committee of Southern Tohoku
Research Institute for Neuroscience (approval no. 338). This study
was conducted according to the principles of the Declaration of
Helsinki.
IAIC
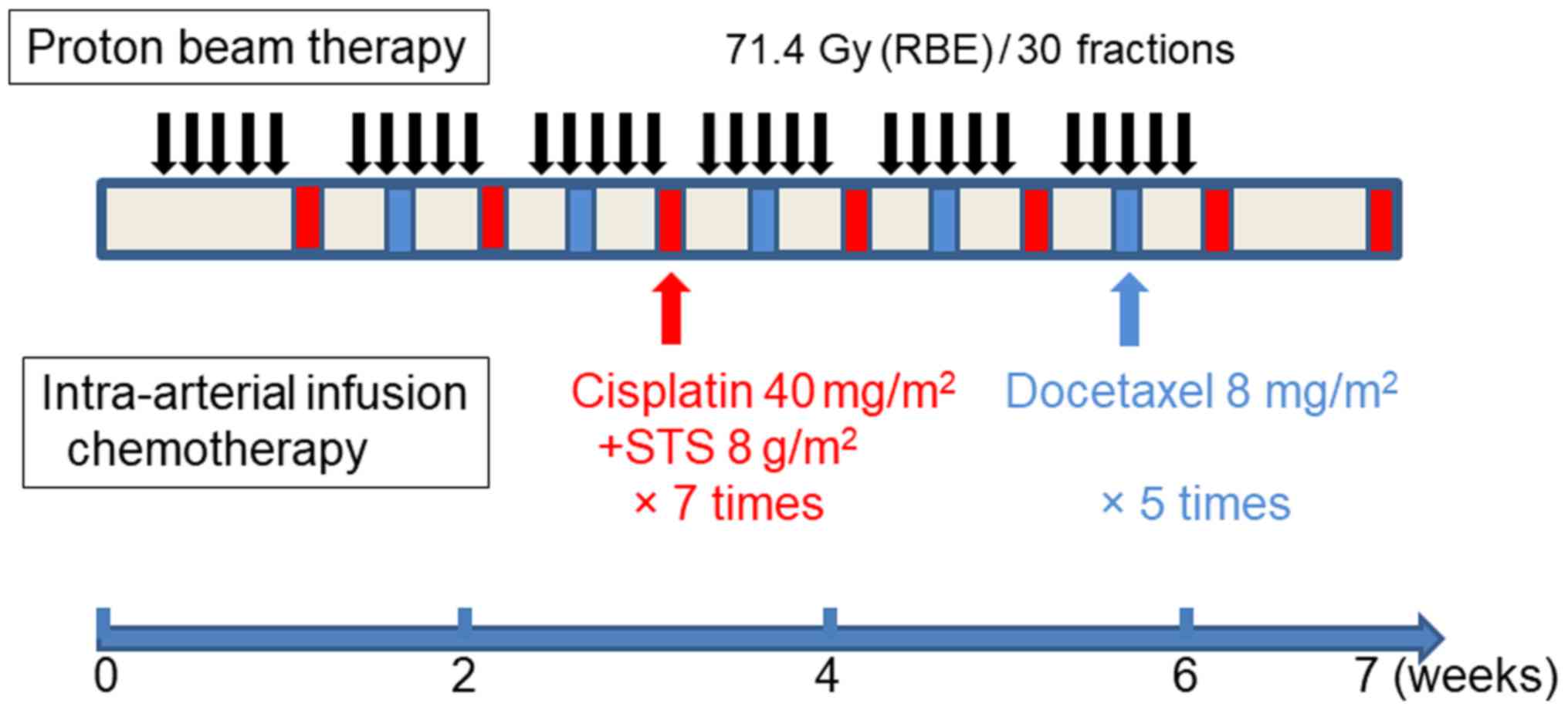
The treatment schedule is summarized in Fig. 2. The IAIC method described by Fuwa
et al (20) was followed.
Under local anesthesia (xylocaine injection 1% with epinephrine,
Aspen Japan), using fluoroscopy, a guide-wire (GT wire, 0.016 inch
diameter, Terumo Corp.) was inserted into the common carotid artery
from the superficial temporal artery (STA), and a thin catheter
(Anthron P-U catheter; tapering type, 5Fr in outer diameter, Toray,
Medical Corp.) was inserted from the STA into the external carotid
artery (ECA). As the main feeder of the tumor was the maxillary
artery (MA), the tip of the catheter was placed slightly to the
central side of the branching section of the MA. The tumor was
beyond the median line of the hard palate; therefore, two catheters
were inserted bilaterally. After determination of a stable position
for the catheter by digital subtraction angiography using a
contrast medium (Iopamirn 300), to confirm that the target area was
covered, blue dye (Indigocarmine, Daiichi Sankyo) was slowly
injected, and MRI was performed with slow injection of a low-dose
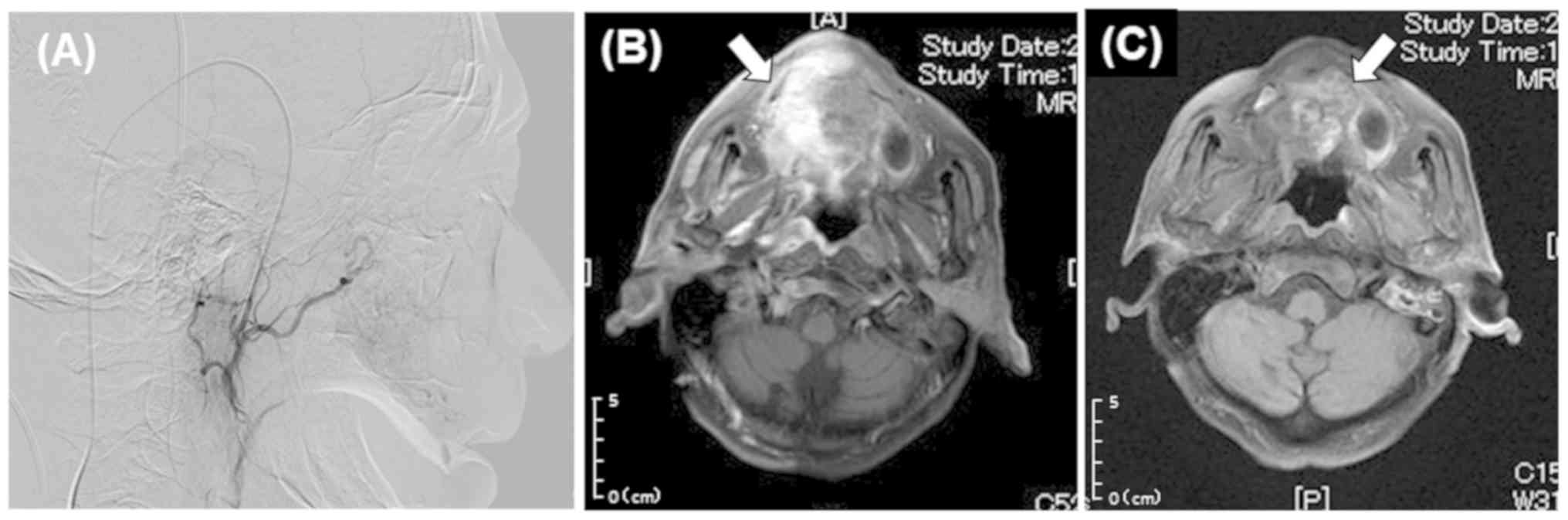
contrast medium (Gadovist IV, Bayer) via a catheter (21). IAIC was performed after confirming
good perfusion (Fig. 3). Based on
their reported effect, cisplatin (Maruko, Yakult) in combination
with docetaxel (Docetaxel, ELMED) was used (22). Briefly, once a week, 40
mg/m2 cisplatin was infused over 5 h via a catheter, and
8 mg/m2 docetaxel was infused over 2 h. During arterial
cisplatin infusion, 8 g/m2 sodium thiosulphate (Detoxol,
Nichi-Iko Pharmaceutical Co. Ltd.) as a neutralizing agent for
cisplatin was infused intravenously over 7 h. Cisplatin was
administered seven times on both sides, for a total dose of 500 mg.
Arterial docetaxel infusion was repeated five times for a total
dose of 60 mg in the right (affected) side and four times for a
total dose of 40 mg in the opposing side. A 5-hydroxytryptamin 3
receptor antagonist and corticosteroids were administered to
minimize nausea and vomiting before intra-arterial infusion.
PBT
The patient was positioned and immobilized with a
thermoplastic head mask to ensure high target repositioning
accuracy. CT images with 1-mm scan thickness were obtained using a
16-slice large-bore helical CT scanner (Aquilion LB; Canon).
Diagnostic MRI scans with 3-mm thickness were combined with
planning CT images for target delineation. A three-dimensional
treatment planning system (Xio-M, Elekta; and Hitachi) was used for
PBT planning. Gross tumor volume (GTV) 1 was outlined on CT images,
and clinical target volume (CTV) 1 was defined as GTV1 with a 4-mm
margin in all directions, while avoiding critical organs at risk
(brain stem, spinal cord, optic nerves, optic chiasma, and mandible
bone). CTV1 was expanded by 3 mm in all directions to create
planning target volume (PTV) 1 with the aim to compensate for setup
uncertainty. Because of the presence of penumbra and range of the
radiation, the following beam-specific margins were set: Proximal,
distal, and lateral margins as well as the smearing margin as a
margin for bolus (23). The
planning CT/MRI images for the boost plan were captured after 15
episodes of irradiation, and GTV2 was outlined. CTV2 was defined by
adding a 3-mm margin around GTV2 and modified to exclude organs at
risk. PTV2 was created in the same way as that for PTV1. Two
portals of 150-MeV noncoplanar beams were arranged at optimal
angles to avoid excess-dose exposure to the normal tissue. Doses
were calculated based on a pencil-beam algorithm. A spread-out
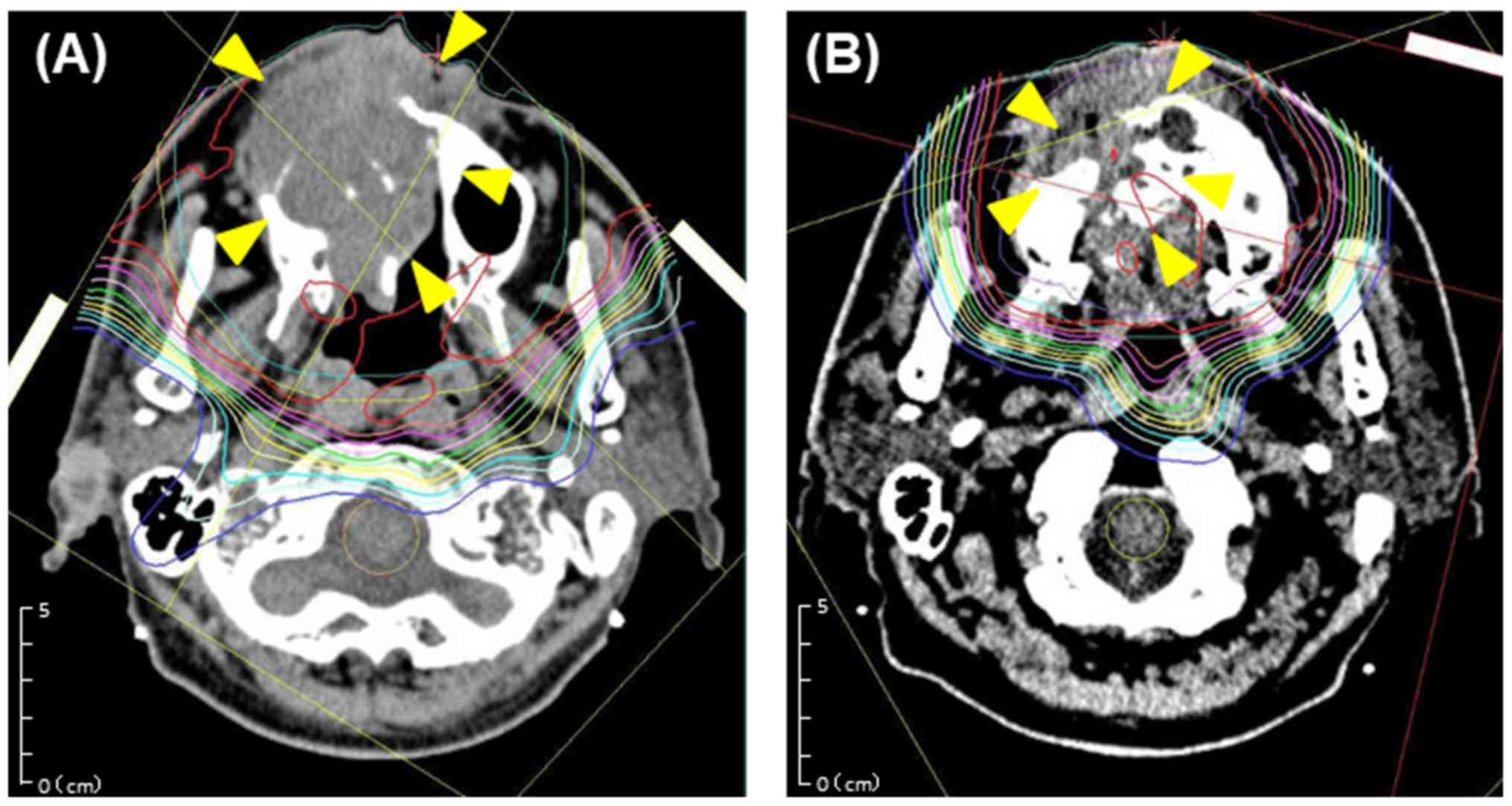
Bragg peak was tuned to the extent that was possible until PTV was
exposed to a 90% isodose of the prescribed dose (Fig. 4). The PBT system (Hitachi) at our
institution used a synchrotron and a passive scattering method in
which a proton beam passed a bar ridge filter, a range shifter, and
a bolus before entering the patient. A multileaf collimator, which
could be formed into an irregular field shape, was used. Daily
X-ray images were used for precise positioning. The patient was
prescribed a dose of 45.0 Gy relative biological effectiveness
(RBE) in 18 fractions to PTV1 (five fractions per week) as well as
a dose of 26.4 Gy (RBE) in 12 fractions to PTV2 for the boost. The
total irradiation dose was 71.4 Gy (RBE) in 30 fractions.
Follow-up and outcomes
The treatment response was evaluated 3 months after
treatment completion by contrast-enhanced MRI and clinical
examination. Additional follow-up examination using CT, MRI or
FDG-PET/CT was performed every 2-4 months for the first two years
and every 4-6 months thereafter. The response was evaluated
according to the Response Evaluation Criteria in Solid Tumors
guidelines version 1.1, and the Common Terminology Criteria for
Adverse Events version 4.0 was used to evaluate adverse effects
(24). The patient experienced
grade 3 mucositis, dermatitis, and neutropenia as early adverse
events, but there was no grade 4 or higher toxicities.
Additionally, as late adverse events, the patient developed grade 1
skin atrophy and hardening of the soft tissue on the right cheek
after treatment and an oral maxillary fistula at the site of tumor
three years after treatment. Furthermore, the patient experienced
grade 2 right optic nerve disorder at four years after treatment
and grade 2 radiation retinopathy of the right eye at six years
after treatment. The patient achieved complete response 3 months
after treatment based on the clinical assessment. The patient did
not experience recurrence or distant metastases following treatment
and died from another cause 94 months after the conclusion of
treatment for AC (Figs. 5 and
6).
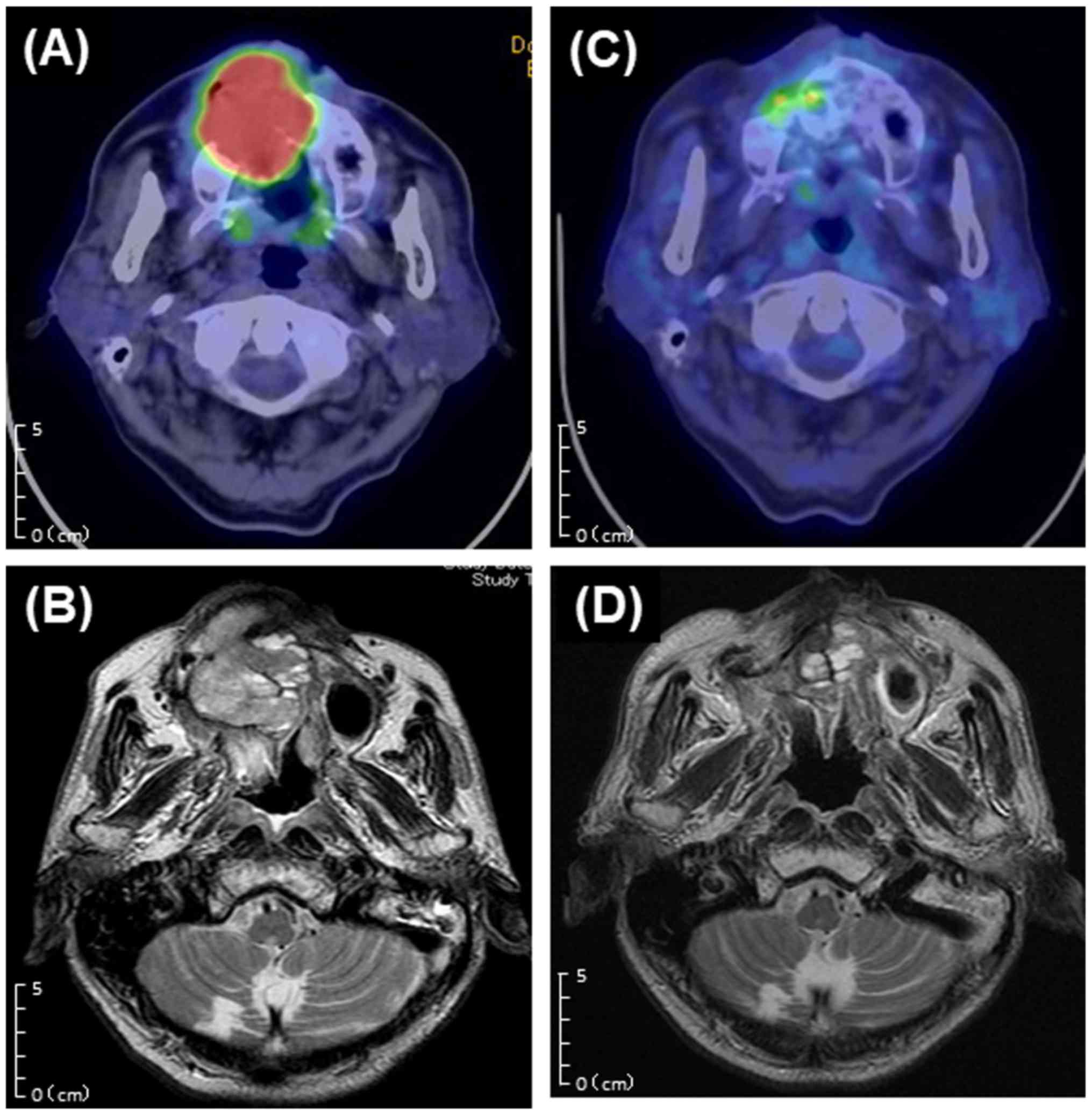 | Figure 5Comparison of before and after
treatment. (A) Before treatment (FDG-PET/CT, axial,
SUVmax=21.6). (B) Before treatment (T2-weighted MRI,
axial); MRI shows a large heterogeneous mass occupying the right
maxillary sinus and extending from the nasal cavity to sphenoid
sinus. (C) After 3 months of treatment (FDG-PET/CT, axial,
SUVmax=6.3). (D) After 6 months of treatment
(T2-weighted MRI, axial). CT, computed tomography; FDG-PET,
18F-fluorodeoxyglucose positron emission tomography;
SUVmax, maximum standardized uptake value. MRI, magnetic
resonance imaging. |
Discussion
Surgical resection is generally performed as an
approach for recurrent AC. Chemotherapy and radiotherapy are not
efficient and considered as treatment options for inoperable cases,
with poor prognostic outcomes (7).
Yoon et al reported that 5-year overall survival of 72.9%
for AC and recurrence rate after surgical resection was reported as
28.3%, which was 92.3% in patients treated with conservative
therapy using chemotherapy and radiotherapy (1). There are over 150 reports of patients
treated for AC, with radiotherapy utilized in approximately
one-third of the cases, especially in recent years (25-29).
In majority of the cases, the therapeutic effects of conventional
radiotherapy were inadequate, and high-dose radiation led to severe
chronic disorders such as osteoradionecrosis (2).
To date, a few studies have reported radical
radiation therapy for AC (12-14).
However, it may not be a desirable treatment from the view of
therapeutic effect because AC is resistant to X-ray therapy. PBT is
a type of particle therapy, similar to CIT. In comparison with
conventional radiotherapy, particle beams are characterized by
their unique Bragg peak and can deliver high-dose radiation to the
tumor while sparing normal tissues (18). Additionally, compared with
conventional X-ray therapy, a higher antitumor effect by direct
impairment of cancer cell DNA is expected (17). Importantly, the therapeutic effect
of PBT was reported in non-SCC (15,16,19).
One difference between proton beams and carbon ion beams is their
RBE; the RBE of protons is approximately 1.1, whereas that of
carbon ions is approximately 3.0. However, no significant
difference in the therapeutic effect between PBT and CIT was
reported (30). To date, only two
case reports of particle therapy for AC was published, including
one patient treated by CIT (15)
and one patient treated with PBT (16); however, AC appeared to have arisen
de novo in both cases. Most ACs appear to be de novo;
however, few cases of secondary-type AC arising from pre-existing
ameloblastoma were reported. The current case was diagnosed with
secondary-type peripheral AC, which recurred shortly after surgery,
advanced extensively, and exhibited high malignancy.
Recent studies assessing IAIC for locally advanced
head and neck cancers to avoid surgery reported excellent treatment
outcomes. Fuwa et al reported good clinical outcomes of
treatment with weekly IAIC and radiotherapy in a series of 92
patients with head and neck cancer, including 84 patients with oral
SCC (20). Mitsudo et al
assessed treatment results with daily IAIC and conventional
radiotherapy in a series of 112 cases with stage III and IV oral
SCC and reported that the 5-year local control rate and over-all
survival rate were 79.3 and 71.3%, respectively (11). Our previous study in which we used
PBT and IAIC in T4 SCC of the maxillary gingiva, the 3-year local
control and overall survival rates were 69 and 59%, respectively
(31). In the current case,
treatment of aggressive AC with a combination of IAIC and PBT
achieved a good therapeutic effect. To the best of our knowledge,
this is the first report of a good long-term course of 94 months
with radical treatment using chemoradiotherapy for postoperative
recurrent secondary-type AC. During the follow-up period, the
patient had skin atrophy and non-infectious fistula in the right
cheek. The cheek fistula had no effect on food and conversation,
and the patient did not wish for reconstructive surgery. Although
the tumor was close to the right optic nerve, visual acuity was
maintained for several years. However, the patient suffered from
ischemic syndrome of the right eye and developed grade 2 optic
nerve disorder four years after treatment. Blood flow disturbance
after radiation therapy have been reported in the past (32,33),
and are considered as adverse event of the treatment. It indicates
that additional effort to avoid risk organ may be necessary to
reduce adverse events with this therapeutic approach. In this
study, a passive scattering method, which is difficult to apply for
complicated cases, was used to deliver the proton beam. In the
future, intensity-modulated proton beam therapy using a pencil bam
scanning system can be used to reduce late adverse events.
Although case series studies with large samples are
necessary for further elucidation of this treatment approach, the
current case illustrates the good long-term outcome of recurrent
secondary-type AC treated with PBT and IAIC. The beneficial
therapeutic effect and organ preservation were achieved without any
severe late adverse events, suggesting that PBT in combination with
IAIC might be an effective treatment option for inoperable, locally
advanced AC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data used and/or analyzed during this published
article are available from the corresponding author on reasonable
request.
Authors' contributions
KT and NF contributed to the study concept and
clinical study design. KT and TN collected data. KT wrote the
initial draft of the manuscript. KM and MM conducted the literature
search. HS performed histopathological examinations. TN and TK
prepared the treatment plans of the case and carried out follow-up.
AT evaluated the patient's radiographs. TK established the
patient's setup preparation and verifications. KT, AT, NF, TK and
HS evaluated the patients and participated in the therapy. KT, KM,
NF, and MM prepared the final manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This treatment method was approved by The Ethics
Committee of Southern Tohoku Research Institute for Neuroscience.
Written consent was obtained from the patient at our
institution.
Patient consent for publication
The reported case has been approved by the patient
for academic use only.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoon HJ, Hong SP, Lee JI, Lee SS and Hong
SD: Ameloblastic carcinoma: An analysis of 6 cases with review of
the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
108:904–913. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Benlyazid A, Lacroix-Triki M, Aziza R,
Gomez-Brouchet A, Guichard M and Sarini J: Ameloblastic carcinoma
of the maxilla: Case report and review of the literature. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 104:e17–e24.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kumaran PS, Anuradha V, Gokkulakrishnan S,
Thambiah L, Jagadish AK and Satheesh G: Ameloblastic carcinoma: A
case series. J Pharm Bioallied Sci. 6 (Suppl 1):S208–S211.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fomete B, Adebayo ET, Ayuba GI and Okeke
UA: Ameloblastic carcinoma of the maxilla: A report of two cases
and a review of the literature. J Korean Assoc Oral Maxillofac
Surg. 42:43–46. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Uzawa N, Suzuki M, Miura C, Tomomatsu N,
Izumo T and Harada K: Primary ameloblastic carcinoma of the
maxilla: A case report and literature review. Oncol Lett.
9:459–467. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goldenberg D, Sciubba J, Koch W and Tufano
RP: Malignant odontogenic tumors: A 22-year experience.
Laryngoscope. 114:1770–1774. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Datta R, Winston JS, Diaz-Reyes G, Loree
TR, Myers L, Kuriakose MA, Rigual NR and Hicks WL Jr: Ameloblastic
carcinoma: Report of an aggressive case with multiple bony
metastases. Am J Otolaryngol. 24:64–69. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Strojan P, Vermorken JB, Beitler JJ, Saba
NF, Haigentz M Jr, Bossi P, Worden FP, Langendijk JA, Eisbruch A,
Mendenhall WM, et al: Cumulative cisplatin dose in concurrent
chemoradiotherapy for head and neck cancer: A systematic review.
Head Neck. 38:e2151–e2158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fuwa N, Ito Y, Matsumoto A, Kamata M,
Kodaira T, Furutani K, Sasaoka M, Kimura Y and Morita K: A
combination therapy of continuous superselective intraarterial
carboplatin infusion and radiation therapy for locally advanced
head and neck carcinoma. Phase I study. Cancer. 89:2099–2105.
2000.PubMed/NCBI
|
|
10
|
Robbins KT, Storniolo AM, Kerber C,
Vicario D, Seagren S, Shea M, Hanchett C, Los G and Howell SB:
Phase I study of highly selective supradose cisplatin infusions for
advanced head and neck cancer. J Clin Oncol. 12:2113–2120.
1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mitsudo K, Koizumi T, Iida M, Iwai T,
Nakashima H, Oguri S, Kioi M, Hirota M, Koike I, Hata M and Tohnai
I: Retrograde superselective intra-arterial chemotherapy and daily
concurrent radiotherapy for stage III and IV oral cancer: Analysis
of therapeutic results in 112 cases. Radiother Oncol. 111:306–310.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Perera E, Lindquist C, Hughes C and Thomas
S: The use of Gamma Knife stereotactic radiosurgery in the
treatment of ameloblastic carcinoma. Int J Oral Maxillofac Surg.
42:934–938. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Koca T, Başaran H, Arslan D, Sezen D,
Cerkeşli ZA, Kılınç O, Karaca S, Başsorgun CI, Okay HO and Demirci
M: Prominent response with helical tomotherapy in recurrent
ameloblastic carcinoma of maxillary sinus: A case report. Radiat
Oncol. 9(157)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aoki T, Akiba T, Kondo Y, Sasaki M,
Kajiwara H and Ota Y: The use of radiation therapy in the
definitive management of ameloblastic carcinoma: A case report.
Oral Surg Oral Med Oral Pathol Oral Radiol. 127:e56–e60.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jensen AD, Ecker S, Ellerbrock M,
Nikoghosyan A, Debus J and Münter MW: Carbon ion therapy for
ameloblastic carcinoma. Radiat Oncol. 6(13)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yamagata K, Ishikawa H, Saito T and Bukawa
H: Proton beam therapy for ameloblastic carcinoma of the maxilla:
Report of a rare case. J Oral Maxillofac Surg. 77:227.e1–227.e5.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fokas E, Kraft G, An H and
Engenhart-Cabillic R: Ion beam radiobiology and cancer: Time to
update ourselves. Biochim Biophys Acta. 1796:216–229.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Urie MM, Sisterson JM, Koehler AM, Goitein
M and Zoesman J: Proton beam penumbra: Effects of separation
between patient and beam modifying devices. Med Phys. 13:734–741.
1986.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Patel SH, Wang Z, Wong WW, Murad MH,
Buckey CR, Mohammed K, Alahdab F, Altayar O, Nabhan M, Schild SE
and Foote RL: Charged particle therapy versus photon therapy for
paranasal sinus and nasal cavity malignant diseases: A systematic
review and meta-analysis. Lancet Oncol. 15:1027–1038.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fuwa N, Kodaira T, Furutani K, Tachibana H
and Nakamuta T: A new method of selective intra-arterial infusion
therapy via the superficial temporal artery for head and neck
cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
105:78378–78379. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nakamura T, Fuwa N, Takayama K, Inokuchi
H, Tomoda T, Takada A, Makita C, Shiomi M, Yokouchi J and Watanabe
K: Phase I study of weekly docetaxel and cisplatin arterial
infusion for recurrent head and neck cancer. Head Neck.
34:1634–1639. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yabuuchi H, Kuroiwa T, Tajima T, Tomita K,
Ochiai N and Kawamoto K: Efficacy of intra-arterial infusion
therapy using a combination of cisplatin and docetaxel for
recurrent head and neck cancers compared with cisplatin alone. Clin
Oncol (R Coll Radiol). 15:467–472. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Torres MA, Chang EL, Mahajan A, Lege DG,
Riley BA, Zhang X, Lii M, Kornguth DG, Pelloski CE and Woo SY:
Optimal treatment planning for skull base chordoma: Photons,
protons, or a combination of both? Int J Radiat Oncol Biol Phys.
74:1033–1039. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0. Published May 28, 2009; Revised version
4.03 June 14, 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
Accessed July 12, 2019.
|
|
25
|
Saluja TS and Hosalkar R: Reconnoitre
ameloblastic carcinoma: A prognostic update. Oral Oncol.
77:118–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Simko EJ, Brannon RB and Eibling DE:
Ameloblastic carcinoma of the mandible. Head Neck. 20:654–659.
1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Infante-Cossio P, Hernandez-Guisado JM,
Fernandez-Machin P, Garcia-Perla A, Rollon-Mayordomo A and
Gutierrez-Perez JL: Ameloblastic carcinoma of the maxilla: A report
of 3 cases. J Craniomaxillofac Surg. 26:159–162. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Philip M, Morris CG, Werning JW and
Mendenhall WM: Radiotherapy in the treatment of ameloblastoma and
ameloblastic carcinoma. J HK Coll Radiol. 8:157–161. 2005.
|
|
29
|
Atkinson CH, Harwood AR and Cummings BJ:
Ameloblastoma of the jaw: A reappraisal of the role of megavoltage
irradiation. Cancer. 53:869–873. 1984.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Demizu Y, Fujii O, Terashima K, Mima M,
Hashimoto N, Niwa Y, Akagi T, Daimon T, Murakami M and Fuwa N:
Particle therapy for mucosal melanoma of the head and neck. A
single-institution retrospective comparison of proton and carbon
ion therapy. Strahlenther Onkol. 190:186–191. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Endo H, Takayama K, Mitsudo K, Nakamura T,
Seto I, Yamaguchi H, Ono T, Suzuki M, Azami Y, Wada H, et al:
Proton beam therapy in combination with intra-arterial infusion
chemotherapy for T4 squamous cell carcinoma of the maxillary
gingiva. Cancers (Basel). 10(333)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang Y, Luo D, Peng W, Huang F and Peng Y:
Ocular ischemic syndrome secondary to carotid artery occlusion as a
late complication of radiotherapy of nasopharyngeal carcinoma. J
Neuroophthalmol. 30:315–320. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gupta A, Dhawahir-Scala F, Smith A, Young
L and Charles S: Radiation retinopathy: Case report and review. BMC
Ophthalmol. 7(6)2007.PubMed/NCBI View Article : Google Scholar
|