Introduction
Intraductal lesions of the breast, include usual
ductal hyperplasia (UDH), atypical ductal hyperplasia (ADH), ductal
carcinoma in situ (DCIS), benign intraductal papilloma (IDP)
with or without atypia, and malignant papillary carcinoma (1). Intraductal lesions are often
associated with pathological nipple discharge (PND), with papilloma
being the most common cause (40-70%), followed by adenomatous or
papillary epithelial proliferation (14%) (2,3). One
to 23% of women with PND are diagnosed with invasive breast cancer
or ductal carcinoma in situ worldwide (4-6).
However, intraductal lesions may be asymptomatic and can be
detected through routine mammography screening. Sometimes they can
be found due to other symptoms such as palpable lump(s), or
associated micro-calcification (7).
Fiber-optic ductoscopy is important for the
diagnosis of patients with PND, and it is now becoming
indispensable (8,9). Women without PND are usually diagnosed
through ultrasound-guided core needle biopsy (CNB) (7,10).
However, it has been reported that physicians fail to obtain the
appropriate specimens from atypical or malignant lesions through
CNB, due to histological heterogeneity. It has been demonstrated
that CNB is not concordant with surgical excision due to high rates
of upgrading of precursor lesions into carcinoma (7,10-16).
For this reason, numerous authors have advocated surgical excision
of intraductal lesions, such as benign papilloma (with or without
atypia), but others conclude that CNB would remove all lesions
(17,18). Increasingly, the focus of studies
has been on patients with intraductal lesions without nipple
discharge than with nipple discharge. However, in none of those
studies have researchers drawn attention to the importance of PND.
There is no consensus on whether such patients should undergo
routine ductoscopy examination. Recent research has suggested that
precursor lesions of cancer include IDPs and ADH (10,19).
Therefore, it is important to identify intraductal lesions in women
without PND at an early stage.
The objective of the present study was to
retrospectively survey potential risk factors associated with
intraductal lesions (IDPs, ADH and DCIS) in patients without PND
and to provide recommendations for clinicians.
Materials and methods
Study design and patients'
information
The histopathology and imaging databases were
searched for patients who had been diagnosed with non- or
intraductal lesions after post-operative histopathological
examination in the 13 month-period from April 2016 to April 2017 at
the Department of Breast Surgery within China-Japan Union hospital
of Jilin University (Jilin, China). The patients presented in
outpatient because of routine physical examination or breast
palpable lump(s). The age range of the patients was 12-77 years of
age, with a median age of 40 years.
Lesions with mastitis and invasive carcinoma as
indicated through post-operative histopathological analysis and
patients with PND were excluded. Patients were divided into the
following study groups: Intraductal lesions (IDP, ADH and DCIS) and
control group: Non-intraductal lesions (fibroadenoma, adenosis,
cysts and lobular carcinoma in situ). In this study,
intraductal papilloma included both central papilloma and
peripheral papilloma. Intraductal papilloma with an atypical lesion
was defined as ADH. DCIS was categorized as pure DCIS and
intraductal papilloma with DCIS. As LCIS arises from the breast
lobular epithelium of the breast rather than the breast ductal
epithelium, so LCIS was placed under non-intraductal lesions in our
study (20,21). Once eligibility was established
based on histopathological diagnosis, we extracted data of clinical
variables, such as patient age, course of disease (year),
menopausal status, age at menarche, number of pregnancies and
abortions, non-menstrual breast pain; and imaging features, such as
tumor size, number, margin and shape of masses, distance from
nipple, masses with or without blood flow, as well as duct ectasia
indicated through ultrasound, and calcification indicated through
mammography. Breast Imaging Reporting and Data System (BI-RADS)
categories were measured using ultrasound.
The study was approved by the China-Japan Union
Hospital of Jilin University (included all content related to the
patient; project approval no. 201620218). Even though this was a
retrospective study, the hospital Ethics Committee evaluated it
carefully and suggested it could waive the informed consent
(including the 12-year-old patient's guardians), based on our
institutional policy of strict maintenance of anonymity.
Ultrasonography and pathology
assessment
All patients were evaluated using a Philips IU22A
Ultrasound Imaging system (line probe, probe frequency 9-15 MHZ)
and all ultrasonography examinations were performed by two
physicians with 5 years of experience in US diagnosis. Each
surgically resected specimen was fixed in formalin and embedded in
paraffin for histological analysis, which was performed by three
pathologists specialized in breast diagnosis. The pathologists were
blinded to the US reports. Diagnosis was made based on the 2012 WHO
classification of tumors of the breast (22).
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistics for Windows (version 20.0; IBM Corp., Armonk). First,
comparison of categorical data was conducted using the
χ2 test. Then, a multivariate logistic regression
analysis was used to determine risk factors that may be associated
with intraductal lesions without PND. P<0.05 was considered
statistically significant.
Results
Postoperative histopathology
findings
A total of 370 lesions in 255 patients without PND,
and with a postoperative histopathology diagnosis of intraductal
lesions (IDP or IDPs, ADH and DCIS) or non-intraductal lesions
(fibroadenoma, adenosis, cysts and lobular carcinoma in
situ), were included in the study. Of the 255 patients, 115
patients had bilateral lesions, while 140 patients had unilateral
lesions. ADH was found in 29 cases and DCIS was found in 16 cases.
The distribution of the histopathological diagnoses is summarized
in Table I.
 | Table ISummary of postoperative
histopathology findings. |
Table I
Summary of postoperative
histopathology findings.
| Group | No. |
|---|
| Study | |
|
Papilloma | 111 |
|
ADH | 29 |
|
DCIS | 16 |
|
Overall | 156 |
| Control | |
|
Fibroadenoma | 101 |
|
Cysts | 32 |
|
Fibroadenoma
and cysts | 6 |
|
Adenosis and
and cysts | 38 |
|
Adenosis | 37 |
|
Overall | 214 |
Univariate analyses (characteristics
of patients)
The clinicopathological parameters of the surgical
histopathological diagnosis data of 370 lesions were compared based
on the presence or absence of intraductal lesions (Table II). The average age of these
patients was 43 years (range, 12-77 years), with an average of 54
years in the study group, and 41 years in the control group. We
confirmed that there was only one 12-year old patient in the
present study, her information was not removed prior to the
statistical analysis, and this case did not affect the results. The
data in our study demonstrated that age was associated with
intraductal lesions (P<0.001). In addition, we found that age at
first menstruation was statistically different among patients of
each group (P=0.047). In terms of menopausal status, no significant
difference was found between the two groups (P=0.186). Regarding
the number of pregnancies and abortions, there was a statistically
significant difference between the two groups (P=0.009; P<0.001,
respectively). Similarly, there was a difference between the groups
with regard to the course of disease (P=0.033). We also evaluated
non-menstrual breast pain, and the difference was statistically
significant between the two groups (P=0.003) (Table II).
 | Table IIUnivariate analysis of
characteristics of intraductal lesions patients compared with
non-intraductal lesions patients. |
Table II
Univariate analysis of
characteristics of intraductal lesions patients compared with
non-intraductal lesions patients.
| Clinical
characteristics | Study group no.
(%) | Control group no.
(%) | χ2 | P-value |
|---|
| Age, years | | | | |
|
≤34 | 15 (16.7) | 75 (83.3) | | |
|
35-49 | 109 (49.8) | 110 (50.2) | | |
|
≥50 | 32 (52.5) | 29 (47.5) | 31.843 |
<0.001a |
| Course of disease,
years | | | | |
|
≤1 | 124 (45.4) | 149 (54.6) | | |
|
>1 | 32 (33.0) | 65 (67.0) | 4.536 | 0.033a |
| Age at
menarche | | | | |
|
10 | 0 (0.0) | 2 (100.0) | | |
|
11 | 0 (0.0) | 3 (100.0) | | |
|
12 | 5 (45.5) | 6 (54.5) | | |
|
13 | 25 (33.8) | 49 (66.2) | | |
|
14 | 49 (43.8) | 63 (56.2) | | |
|
15 | 42 (45.7) | 50 (54.3) | | |
|
16 | 14 (37.8) | 23 (62.2) | | |
|
17 | 11 (78.6) | 3 (21.4) | | |
|
18 | 0 (0.0) | 2 (100.0) | | |
|
19 | 2 (100.0) | 0 (0.0) | 17.114 | 0.047a |
| Menopausal
state | | | | |
|
Non-menopausal | 132 (40.9) | 191 (59.1) | | |
|
Menopausal | 24 (51.1) | 23 (48.9) | 1.749 | 0.186 |
| Number of
pregnancies | | | | |
|
0 | 11 (20.4) | 43 (79.6) | | |
|
≥1 | 145 (45.90) | 171 (54.1) | 12.313 |
<0.001a |
| Number of
abortions | | | | |
|
0 | 56 (34.6) | 106 (65.4) | | |
|
≥1 | 100 (48.1) | 108 (51.9) | 6.815 | 0.009a |
| Non-menstrual
breast pain | | | | |
|
No | 115 (38.5) | 184 (61.5) | | |
|
Yes | 41 (57.7) | 30 (42.3) | 8.75 | 0.003a |
Univariate analyses (characteristics
of imaging)
Table III shows
the imaging characteristics of intraductal lesions, compared with
non-intraductal lesions, in patients without PND. In terms of
breast duct ectasia, lesion size, and distance from nipple,
significant differences (all P<0.01) were found. In the 156
samples without PND that were confirmed positive for intraductal
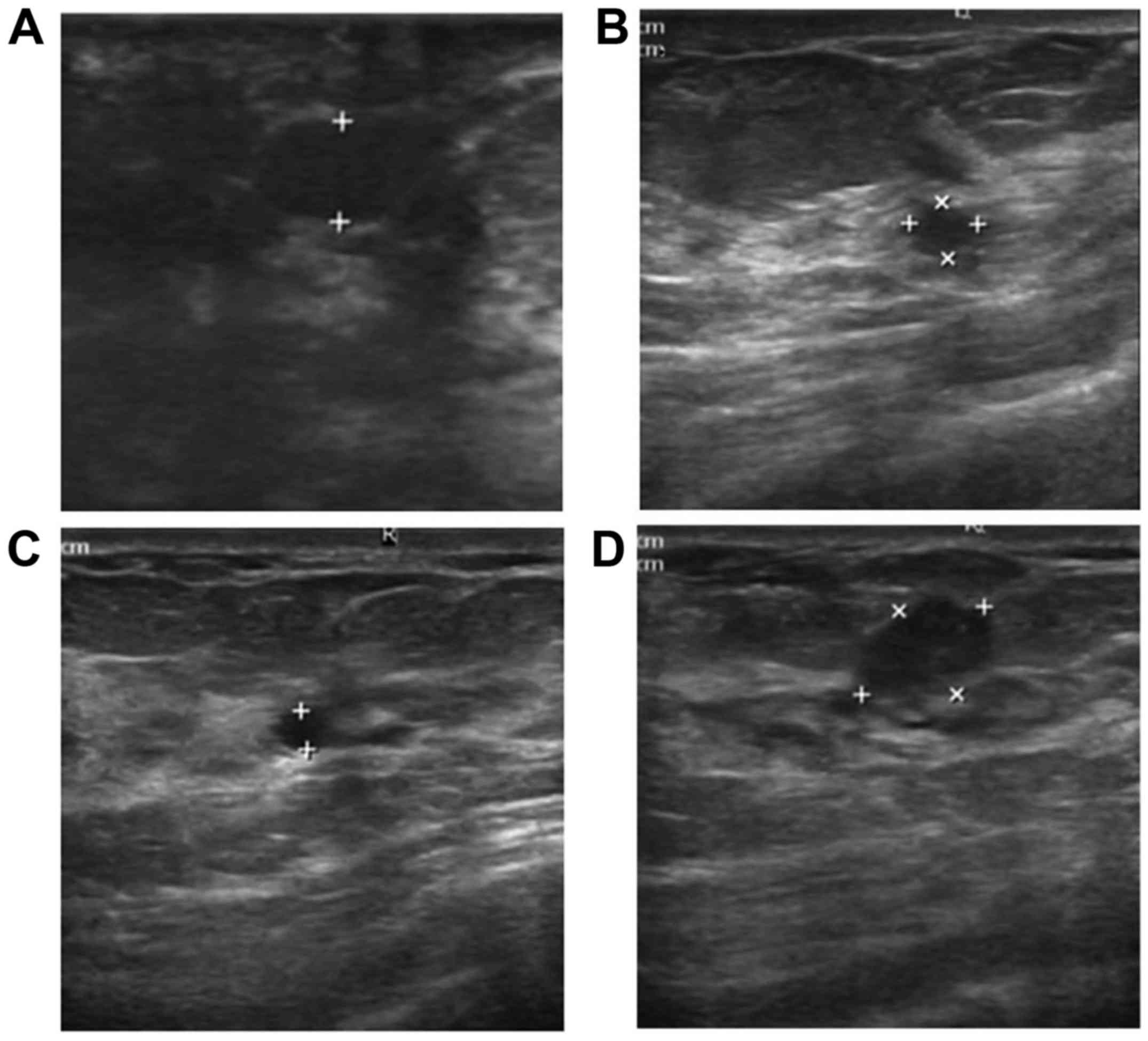
lesions, 26 of the samples were found to contain duct ectasia
(Fig. 1). However, only 6 samples
were found to have duct ectasia in the control group. The lesion
size (>1 cm) in the control group was more than that of the
study group (68.9 vs. 31.1%), while the distance from nipple (>2
cm) was the same (68.7 vs. 31.3%).
 | Table IIIUnivariate analysis of imaging
(mammography and ultrasonography) characteristics of the patients
without PND. |
Table III
Univariate analysis of imaging
(mammography and ultrasonography) characteristics of the patients
without PND.
| Imaging
characteristics | Study group no.
(%) | Control group no.
(%) | χ2 | P-value |
|---|
| Duct ectasia | | | | |
|
No | 130 (38.5) | 208 (61.5) | | |
|
Yes | 26 (81.2) | 6 (18.8) | 21.947 |
<0.001a |
| The number of
nodules | | | | |
|
Single | 58 (39.7) | 88 (60.3) | | |
|
Multiple | 98 (43.8) | 126 (56.2) | 0.587 | 0.444 |
| Distance from
nipple and areola, cm | | | | |
|
≤2 cm | 104 (51.2) | 99 (48.8) | | |
|
>2
cm | 52 (31.1) | 115 (68.9) | 15.171 |
<0.001a |
| Lesion size,
cm | | | | |
|
≤1 cm | 100 (52.4) | 91 (47.6) | | |
|
>1
cm | 56 (31.3) | 123 (68.7) | 16.824 |
<0.001a |
| Margin and
shape | | | | |
|
Indistinct/irregular | 68 (45.3) | 82 (54.7) | | |
|
Clear/regular | 88 (40.0) | 132 (60.0) | 1.040 | 0.308 |
| Blood flow | | | | |
|
No | 120 (43.2) | 158 (56.8) | | |
|
Yes | 36 (39.1) | 56 (60.9) | 0.462 | 0.497 |
| BI-RADS
category | | | | |
|
≤3 | 112 (41.3) | 159 (58.7) | | |
|
≥4 | 44 (44.4) | 55 (55.6) | 0.289 | 0.591 |
| Calcification | | | | |
|
No | 21 (31.3) | 46 (68.7) | | |
|
Yes | 59 (36.9) | 101 (63.1) | 0.633 | 0.426 |
For the other imaging characteristics, BI-RADS,
calcification, blood flow, the number of nodules, margin and shape
of masses, no significant difference was found between the study
and control groups (P>0.05) (Table
III).
Risk factors for intraductal
lesions
Nine out of 14 factors were found to be
statistically different as shown by the univariate analyses
(Tables II and III). Five factors were identified as
relative risk factors through multivariate logistic regression
analysis (Table IV). The
predominant relative risk of intraductal lesions for women without
PND but with duct ectasia of breast was found to be 9.455 times
higher than that for women without duct ectasia of the breast (95%
CI=3.194-27.987, P<0.001). The second highest relative risk for
intraductal lesions was found to be patients aged over 50 years
(OR=6.207, 95% CI=2.587-14.891, P<0.001). Age between 35 and 50
years also constituted a risk factor. The data confirm that the
relative risk for older patients was higher than that of younger
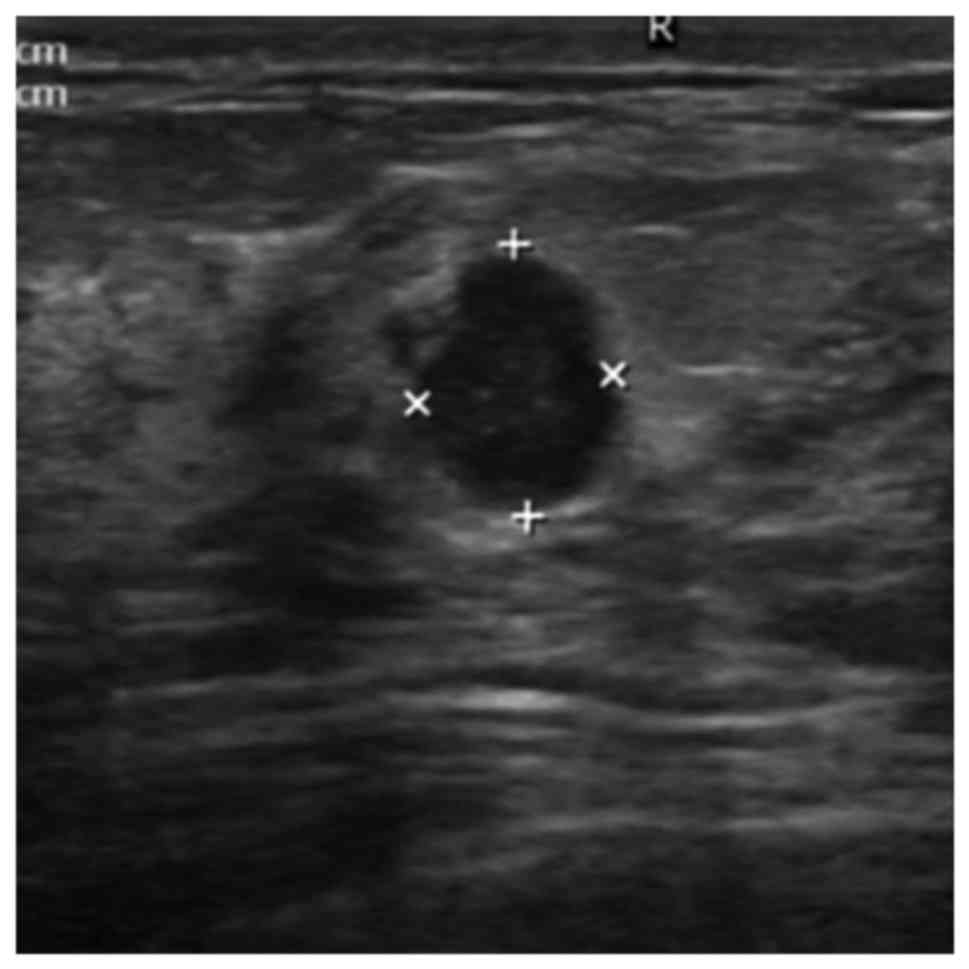
patients. The relative risk of having intraductal lesions ≤2 cm
(Fig. 2) from the nipple was
2.745-fold higher than that of patients who have intraductal
lesions at a distance >2 cm from the nipple (95% CI=1.668-4.526,
P<0.001). The multivariate analysis also shows that
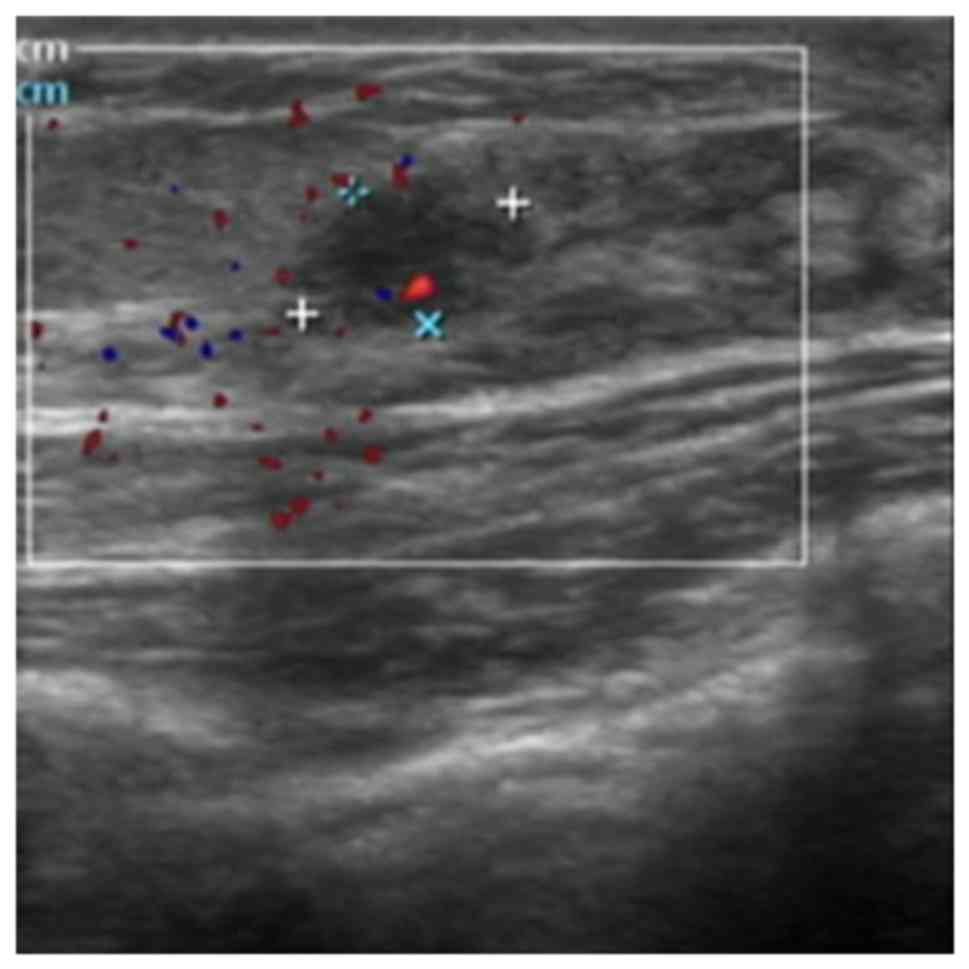
non-menstrual breast pain (OR=1.922, 95% CI=1.037-3.564, P=0.038),
and a lesion size of ≤1 cm (Fig. 3)
(OR=1.903, 95% CI=1.155-3.136, P=0.012) were more highly associated
with intraductal lesions than non-intraductal lesions (Table IV).
 | Table IVMultivariate analysis of
characteristics of intraductal lesions compared with
non-intraductal lesions. |
Table IV
Multivariate analysis of
characteristics of intraductal lesions compared with
non-intraductal lesions.
| | | | EXP (B) 95%
C.I. | EXP (B) 95%
C.I. |
|---|
| Factors | P-value | OR | Lower limit | Upper limit |
|---|
| Age, years | | | | |
|
≤34 | <0.001 | | | |
|
35-49 | <0.001 | 4.749 | 2.371 | 9.513 |
|
≥50 | <0.001 | 6.207 | 2.587 | 14.891 |
| Number of
pregnancies (≥1) | 0.724 | 0.832 | 0.299 | 2.314 |
| Number of abortions
(≥1) | 0.401 | 1.272 | 0.726 | 2.227 |
| Non-menstrual
breast pain (present) | 0.038 | 1.922 | 1.037 | 3.564 |
| Breast duct ectasia
(present) | <0.001 | 9.455 | 3.194 | 27.987 |
| Distance from
nipple, cm (≤2 cm) | <0.001 | 2.747 | 1.668 | 4.526 |
| Lesion size, cm (≤1
cm) | 0.012 | 1.903 | 1.155 | 3.136 |
Discussion
Along with the increase in the knowledge of breast
cancer, it is a generally accepted consensus that early detection
and early treatment is of great benefit to patients. To the best of
our knowledge, DCIS is considered a true precursor lesion for
invasive cancer (19), as well as
ADH. In addition, all IDPs are considered cancer precursor lesions
(10), so all precursor lesions are
intraductal lesions (10,19). However, there is no generally
accepted consensus regarding criteria used to distinguish between
intraductal and non-intraductal lesions.
It has been demonstrated that clinical parameters,
such as age, estrogen levels, a history of family genetics,
including HER-2 overexpression and BRCA1/2 mutations, increase the
risk of breast cancer. Although technology used for gene mutation
detection is highly advanced, it is very expensive. Therefore, it
is important to identify relative risk factors for precursor
lesions, which may provide guidance for clinicians. Although many
investigators have assessed risk factors for malignancies in benign
papilloma of breast in CNB, the results remain conflicting
(7,10-13,23-27).
PND is an important common symptom seen in
intraductal lesions and has been confirmed as a risk factor for
breast cancer in many studies (4-6).
However, a recent study confirmed that the frequency of intraductal
lesions without PND is much higher than that with PND (28). Pareja et al reported that
only 8 out of 166 women with nipple discharge in their study were
diagnosed with IDP (29). A number
of cases have reported similar findings (Table V). Our findings confirm that the
number of intraductal lesions is similar to that of non-intraductal
lesions (156 vs. 214), which indicates that there may be many
intraductal lesions in patients without PND. Although many studies
have investigated risk factors associated with malignant changes in
intraductal lesions, they have not specifically separated patients
with PND from those without PND. Therefore, we studied risk factors
associated with intraductal lesions in patients without PND, whose
diagnosis was confirmed through histopathological methods. In
addition, in our study, non-intraductal lesions were considered as
the control group, and their characteristics were compared with
precursor lesions.
 | Table VThe number of patients with PND and
without PND in recently published studies. |
Table V
The number of patients with PND and
without PND in recently published studies.
| Study | Total | PND | no-PND | Journal | Year | (Refs.) |
|---|
| Pareja et
al | 166 | 8 | 158 | Cancer | 2016 | (29) |
| Chang et
al | 38 | 16 | 22 | European
Radiology | 2010 | (13) |
| Glenn et
al | 179 | 14 | 165 | Annals of Surgical
Oncology | 2015 | (28) |
| Shiino et
al | 145 | 30 | 115 | Pathology
International | 2015 | (10) |
| Zhu et
al | 44 | 7 | 37 | American Journal of
Roentgenology. | 2012 | (23) |
| Swapp et
al | 224 | 61 | 163 | Annals of Surgical
Oncology | 2013 | (11) |
| Sakr et
al | 130 | 59 | 71 | European Journal of
Surgical Oncology | 2008 | (14) |
| Yi et
al | 136 | 28 | 113 | World Journal of
Surgery | 2013 | (17) |
| Rizzo et
al | 276 | 58 | 218 | American College of
Surgeons | 2012 | (31) |
We studied clinical and imaging variables to analyze
risk factors of intraductal lesions. We found clinical parameters,
including age, non-menstrual breast pain, breast duct ectasia,
distance from nipple and lesion size to be related to the risk of
intraductal lesions.
Clinically, intraductal papilloma could occur at any
age, but the majority of patients are 40-50 years of age when it
occurs (23). We reported that a
more advanced age is associated with a higher risk of intraductal
lesions. Some studies have indicated that age is correlated with
the severity of intraductal lesions (12,14,17,28,30,31).
Those studies have demonstrated that the older the patient, the
higher the degree of severity of the intraductal lesions. In
contrast to that, another study reported that age is not
significantly related to the severity of intraductal lesions, but
they found that all patients with carcinoma were aged over 50, and
34.9% of the patients in their study had prior or concurrent breast
carcinoma (29). Therefore, their
research, to a certain extent, does not provide clinical guidance
for the early detection of intraductal lesions.
We also observed that the occurrence rate of both
pregnancies and abortions, but not menopausal state was associated
with intraductal lesions in the univariate analysis. However, in
the multivariate logistic regression analysis, they were found to
be confounding factors. Unlike in this study, a previous study by
Shiino et al (10) founded
that menopausal status (menopause) is a relative risk factor for
precursors and carcinoma. One major reason for menopausal state not
reaching statistical significance (P=0.186) in our study is that
most patients were diagnosed with benign lesions at a younger age,
at which they were still menstruating. Another reason is that the
age factor may be more important when compared with menstrual and
reproductive history. Although in this study menstrual and
reproductive history was found to have no statistical significance,
they should be taken into consideration when evaluating clinical
cases, since many recent studies have shown that these factors are
associated with an increased risk of breast cancer (32-35).
Therefore, further investigations are required to explore the
mechanisms that underlie the association between menstrual and
reproductive histories and the risk of breast cancer.
Over half of the patients with intraductal lesions
in our study (57.7%), had suffered non-menstrual breast pain, a
statistically significant difference was found among clinical
characteristics between lesions with and those without intraductal
lesions. A recent study reported that breast pain may be associated
with breast cancer and it has been suggested that clinicians and
radiologists should remain attentive to female patients who
complain of breast pain (36).
Similarly, Preece et al (37) cautioned that focally isolated pain
can be a presenting symptom of cancer. When the symptom of breast
pain is present, further imaging examinations should be
suggested.
Furthermore, there are clear trends towards an
increased risk of intraductal lesions with duct ectasia, as shown
through ultrasound in the present study. In our study, duct ectasia
has been demonstrated to be a predominant risk factor for
intraductal lesions in women without PND (OR=9.455, P<0.01). Hsu
et al (38) studied 172
patients with duct ectasia through ultrasound and found that there
is a relationship between ductal ectasia and intraductal lesions,
especially non-invasive cancerous lesions. However, they did not
categorize the lesions as with or without nipple discharge.
Therefore, it is not possible to determine the relationships
between nipple discharge, duct ectasia and intraductal lesions
using this study. Since our study was a preliminary study on risk
factors for intraductal lesions in patients without PND, no
categorization of the specific types of duct ectasia was made and
further analysis of categories is required in future studies.
Intraductal lesions originate from the ductal epithelium,
therefore, regardless of the presence of PND, it is commonly
accepted that ductal ectasia indicates intraductal lesions, which
was confirmed by the results of our study, in which ductal ectasia
was found to be statistically significant. Duct ectasia with a
well-defined hypoechoic solid mass is a typical sonographic
characteristic of intraductal tumors (23,39,40).
Therefore, these findings support the results of our study.
Central intraductal lesions arise in the large
mammary duct, typically at a distance of less than 2 cm from the
nipple. By contrast, peripheral intraductal lesions arise in the
terminal duct lobular units, typically at a distance of more than 2
cm from the nipple (12). Some
authors argue that the distance from the nipple is not associated
with intraductal breast lesions (12,13,23,29,30,41).
However, the results of our study indicate that a distance of ≤2 cm
from the nipple increases the relative risk of intraductal lesions.
However, other authors have concluded that lesions 3 cm or more
away from the nipple are more likely to be atypical. The
differences between patient groups may account for these
conflicting results.
Most intraductal papilloma are small (<5 mm). Zhu
et al (23) reported that 32
of 44 intraductal papilloma analyzed were <1.0 cm in diameter.
Similarly, we found that a higher number (52.4%) of intraductal
lesion were <1.0 cm in diameter. Chang et al (13) demonstrated that a size >1.5 cm
appears to be significantly associated with malignancy, which is
consistent with our results (lesion ≤1 cm is a risk factor for
intraductal lesions). Although sizes larger than 3-4 cm have been
reported by Wang et al (24), no clinical significance has been
found based on larger diameter tumors, both benign and malignant,
which indicate that surgery may be required.
Our study found that calcification is not
significantly related to a risk of intraductal lesions because we
excluded invasive breast cancer patients and only examined
characteristics of mammography, with or without calcification.
Similarly, in the study by Pareja et al (29), the authors confirmed that there is
no statistically significant difference in radiological
characteristics of intraductal lesions. However, Li et al
(12) confirmed that
micro-calcification is a risk factor for the degree of severity of
tumors (P=0.002). Maxwell et al (7) and Sakr et al (14) have reported similar results.
Therefore, further studies are required to determine whether
micro-calcifications or calcifications can be used as an indication
of the relative risk of intraductal lesions.
To the best of our knowledge, one of the strengths
of the present study is that it is the first to study risk factors
of patients without PND but diagnosed with intraductal lesions, and
all lesions were removed through surgical excision, assuring the
accuracy of their pathological diagnosis.
However, our study also has some limitations. First,
our study is a purely retrospective study with selection bias.
Second, the number of cases included in the current study is
limited, and a majority of cases in the control contained lesions
that were not clear precursors. Furthermore, our observations of
the associated risk factors of intraductal lesions should be
regarded as preliminary, since no relevant studies exist to confirm
that these factors can help clinicians to improve the early
detection rate of breast cancer. Furthermore, large samples of a
variety of population studies are needed to confirm our results. We
are currently conducting this scale of research with intraductal
lesions that have PND, non-intraductal lesions that have PND,
intraductal lesions that do not have PND and non-intraductal
lesions that do not have PND.
Our study results demonstrate that there is a
statistically significant difference in clinical features and
imaging between intraductal and non-intraductal lesions in patients
without PND. Our data indicate that an age >35 years,
non-menstrual breast pain, breast duct ectasia, distance from
nipple of ≤2 cm and lesion size of ≤1 cm are risk factors of
intraductal lesions in patients without PND. Since the vast
majority of intraductal lesions are associated with PND, and
usually only in the presence of this symptom do clinicians and
imaging physicians attach more importance to a lesion, we suggest
that patients without PND but with the above-mentioned risk factors
require investigation, in order to prevent misdiagnosis and improve
early detection rates of breast cancer.
Acknowledgements
The authors would like to thank Dr Enqi Chen and Dr
Yuanqiang Lin (Department of Ultrasonography, China-Japan Union
Hospital of Jilin University, Changchun, China) for their
assistance of evaluating the breast ultrasound. The authors would
also like to thank the other pathologist, Dr Chengwei Jiang.
Funding
Authors are grateful to the Science and Technology
of Jilin Province Health and Family Planning Commission 2017Q035
(to ZYY).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, ZY and LS conceived and designed the study and
drafted the manuscript. LS, ZK and JW collected the data. XL and WL
performed the statistical analysis and helped to draft the
manuscript. SY contributed to obtaining the pathological materials
and pathology assessment. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
amended Declaration of Helsinki. The approval of the Ethical
Committee of China-Japan Union Hospital of Jilin University (Jilin,
China) was obtained (project approval no. 201620218). We waived the
need for ethical approval and informed consent of patients, based
on our institutional policy, strict maintenance of anonymity and
the observational nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interests.
References
|
1
|
Dundar MM, Badve S, Bilgin G, Raykar V,
Jain R, Sertel O and Gurcan MN: Computerized classification of
intraductal breast lesions using histopathological images. IEEE
Trans Biomed Eng. 58:1977–1984. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang L, Wu D and Fan ZM: Retrospective
analysis of pathologic nipple discharge. Genet Mol Res.
14:1443–1449. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Han Y, Li J, Han S, Jia S, Zhang Y and
Zhang W: Diagnostic value of endoscopic appearance during
ductoscopy in patients with pathological nipple discharge. BMC
Cancer. 17(300)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lang JE and Kuerer HM: Breast ductal
secretions: Clinical features, potential uses, and possible
applications. Cancer Control. 14:350–359. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Escobar PF, Crowe JP, Matsunaga T and
Mokbel K: The clinical applications of mammary ductoscopy. Am J
Surg. 191:211–215. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Montroni I, Santini D, Zucchini G, Fiacchi
M, Zanotti S, Ugolini G, Manaresi A and Taffurelli M: Nipple
discharge: Is its significance as a risk factor for breast cancer
fully understood? Observational study including 915 consecutive
patients who underwent selective duct excision. Breast Cancer Res
Treat. 123:895–900. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maxwell AJ, Mataka G and Pearson JM:
Benign papilloma diagnosed on image-guided 14 G core biopsy of the
breast: Effect of lesion type on likelihood of malignancy at
excision. Clin Radiol. 68:383–387. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sparks CA: Using ductoscopy to detect
breast mass at an early stage. AORN J. 76:851–854. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dubowy A, Raubach M, Topalidis T, Lange T,
Eulenstein S and Hünerbein M: Breast duct endoscopy: Ductoscopy
from a diagnostic to an interventional procedure and its future
perspective. Acta Chir Belg. 111:142–145. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shiino S, Tsuda H, Yoshida M, Jimbo K,
Asaga S, Hojo T and Kinoshita T: Intraductal papillomas on core
biopsy can be upgraded to malignancy on subsequent excisional
biopsy regardless of the presence of atypical features. Pathol Int.
65:293–300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Swapp RE, Glazebrook KN, Jones KN, Brandts
HM, Reynolds C, Visscher DW and Hieken TJ: Management of benign
intraductal solitary papilloma diagnosed on core needle biopsy. Ann
Surg Oncol. 20:1900–1905. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li X, Weaver O, Desouki MM, Dabbs D, Shyum
S, Carter G and Zhao C: Microcalcification is an important factor
in the management of breast intraductal papillomas diagnosed on
core biopsy. Am J Clin Pathol. 138:789–795. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chang JM, Moon WK, Cho N, Han W, Noh DY,
Park IA and Jung EJ: Risk of carcinoma after subsequent excision of
benign papilloma initially diagnosed with an ultrasound (US)-guided
14-gauge core needle biopsy: A prospective observational study. Eur
Radiol. 20:1093–1100. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sakr R, Rouzier R, Salem C, Antoine M,
Chopier J, Darai E and Uzan S: Risk of breast cancer associated
with papilloma. Eur J Surg Oncol. 34:1304–1308. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fatemi Y, Hurley R, Grant C, Henrichsen T,
Chen B and Ghosh K: Challenges in the management of giant
intraductal breast papilloma. Clin Case Rep. 3:7–10.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Sydnor MK, Wilson JD, Hijaz TA, Massey HD
and Shaw de Paredes ES: Underestimation of the presence of breast
carcinoma in papillary lesions initially diagnosed at core-needle
biopsy. Radiology. 242:58–62. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yi W, Xu F, Zou Q and Tang Z: Completely
removing solitary intraductal papillomas using the mammotome system
guided by ultrasonography is feasible and safe. World J Surg.
37:2613–2617. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mosier AD, Keylock J and Smith DV: Benign
papillomas diagnosed on large-gauge vacuum-assisted core needle
biopsy which span <1.5 cm do not need surgical excision. Breast
J. 19:611–617. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ward EM, DeSantis CE, Lin CC, Kramer JL,
Jemal A, Kohler B, Brawley OW and Gansler T: Cancer statistics:
Breast cancer in situ. CA Cancer J Clin. 65:481–495.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Foote FW and Stewart FW: Lobular carcinoma
in situ: A rare form of mammary cancer. Am J Pathol. 17:491–496.3.
1941.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wen HY and Brogi E: Lobular carcinoma in
situ. Surg Pathol Clin. 11:123–145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang WT and Zhu XZ: The introduction of
2012 WHO classification of tumours of the breast. Zhonghua Bing Li
Xue Za Zhi. 42:78–80. 2013.(In Chinese). PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu Y, Zhang S, Liu P, Lu H, Xu Y and Yang
WT: Solitary intraductal papillomas of the breast: MRI features and
differentiation from small invasive ductal carcinomas. AJR Am J
Roentgenol. 199:936–942. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang LC, DeMartini WB, Partridge SC,
Peacock S and Lehman CD: MRI-detected suspicious breast lesions:
Predictive values of kinetic features measured by computer-aided
evaluation. AJR Am J Roentgenol. 193:826–831. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jung SY, Kang HS, Kwon Y, Min SY, Kim EA,
Ko KL, Lee S and Kim SW: Risk factors for malignancy in benign
papillomas of the breast on core needle biopsy. World J Surg.
34:261–265. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lewis JT, Hartmann LC, Vierkant RA,
Maloney SD, Shane Pankratz V, Allers TM, Frost MH and Visscher DW:
An analysis of breast cancer risk in women with single, multiple,
and atypical papilloma. Am J Surg Pathol. 30:665–672.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Page DL, Salhany KE, Jensen RA and Dupont
WD: Subsequent breast carcinoma risk after biopsy with atypia in a
breast papilloma. Cancer. 78:258–266. 1996.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Glenn ME, Throckmorton AD, Thomison JB III
and Bienkowski RS: Papillomas of the breast 15 mm or smaller:
4-Year experience in a community-based dedicated breast imaging
clinic. Ann Surg Oncol. 22:1133–1139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pareja F, Corben AD, Brennan SB, Murray
MP, Bowser ZL, Jakate K, Sebastiano C, Morrow M, Morris EA and
Brogi E: Breast intraductal papillomas without atypia in
radiologic-pathologic concordant core-needle biopsies: Rate of
upgrade to carcinoma at excision. Cancer. 122:2819–2827.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chang JM, Moon WK, Cho N, Han W, Noh DY,
Park IA and Jung EJ: Management of ultrasonographically detected
benign papillomas of the breast at core needle biopsy. AJR Am J
Roentgenol. 196:723–729. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rizzo M, Linebarger J, Lowe MC, Pan L,
Gabram SG, Vasquez L, Cohen MA and Mosunjac M: Management of
papillary breast lesions diagnosed on core-needle biopsy: Clinical
pathologic and radiologic analysis of 276 cases with surgical
follow-up. J Am Coll Surg. 214:280–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Suh JS, Yoo KY, Kwon OJ, Yun IJ, Han SH,
Noh DY and Choe KJ: Menstrual and reproductive factors related to
the risk of breast cancer in Korea. Ovarian hormone effect on
breast cancer. J Korean Med Sci. 11:501–508. 1996.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Huang Y, Zhang X, Li W, Song F, Dai H,
Wang J, Gao Y, Liu X, Chen C, Yan Y, et al: A meta-analysis of the
association between induced abortion and breast cancer risk among
Chinese females. Cancer Causes Control. 25:227–236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Arthur R, Wang Y, Ye K, Glass AG, Ginsberg
M, Loudig O and Rohan T: Association between lifestyle,
menstrual/reproductive history, and histological factors and risk
of breast cancer in women biopsied for benign breast disease.
Breast Cancer Res Treat. 165:623–631. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jung S, Egleston BL, Chandler DW, Van Horn
L, Hylton NM, Klifa CC, Lasser NL, LeBlanc ES, Paris K, Shepherd
JA, et al: Adolescent endogenous sex hormones and breast density in
early adulthood. Breast Cancer Res. 17(77)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Noroozian M, Stein LF, Gaetke-Udager K and
Helvie MA: Long-term clinical outcomes in women with breast pain in
the absence of additional clinical findings: Mammography remains
indicated. Breast Cancer Res Treat. 149:417–424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Preece PE, Baum M, Mansel RE, Webster DJ,
Fortt RW, Gravelle IH and Hughes LE: Importance of mastalgia in
operable breast cancer. Br Med J (Clin Res Ed). 284:1299–1300.
1982.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hsu HH, Yu JC, Hsu GC, Chang WC, Yu CP,
Tung HJ, Tzao C and Huang GS: Ultrasonographic alterations
associated with the dilatation of mammary ducts: Feature analysis
and BI-RADS assessment. Eur Radiol. 20:293–302. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Francis A, England D, Rowlands D and
Bradley S: Breast papilloma: Mammogram, ultrasound and MRI
appearances. Breast. 11:394–397. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhu QL, Zhang J, Lai XJ, Wang HY, Xiao MS
and Jiang YX: Characterisation of breast papillary neoplasm on
automated breast ultrasound. Br J Radiol.
86(20130215)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chang JM, Han W, Moon WK, Cho N, Noh DY,
Park IA and Jung EJ: Papillary lesions initially diagnosed at
ultrasound-guided vacuum-assisted breast biopsy: Rate of malignancy
based on subsequent surgical excision. Ann Surg Oncol.
18:2506–2514. 2011.PubMed/NCBI View Article : Google Scholar
|