Introduction
Occult lymph node (LN) metastases, which are
clinically negative nodes, are estimated to exist in 20-30% of oral
cavity cancers. For T1 and T2 oral cancers, there are two surgical
treatment strategies (1,2). In some patients in which the ‘wait and
see’ strategy is employed, unnecessary surgery can be avoided.
However, delayed neck metastases can occur and occasionally
develops into advanced stages. To avoid recurrence, selected neck
dissection (ND) is often performed for high risk cases.
A comparative examination of these two surgical
treatment strategies using a randomized trial (3) and meta-analysis (4) revealed a prognostic advantage of
selective ND.
Selective neck dissection at the time of primary
surgery revealed no metastases to the neck in approximately 70% of
patients and consequently, ND appears to be an unnecessary surgical
procedure for most patients. This possibility of patients
undergoing unnecessary surgery reaffirms the importance of
performing cervical LN staging before treatment. Therefore, the
development of a new staging system with high reliability that
extends beyond conventional diagnostic imaging is necessary.
The effectiveness of SNB as an accurate staging tool
has been demonstrated (2,5). To date, sentinel node (SN) biopsies
have required radioactive isotopes (RIs). If an SN in the head and
neck region could be identified using the fluorescence properties
of indocyanine green (ICG), it would be possible to overcome the
issues of radiation exposure for medical personnel and patients,
institutional limitations, and the complex procedures that arise
from the conventional use of RIs as tracers. This new method could
be performed at any institution without radiation exposure, and we
expect that this method would benefit patients.
We designed the present study to examine the
usefulness of the transcutaneous neck compression technique and the
diagnostic accuracy of ICG fluorescence navigated SNBs in
comparison to the RI method.
The ICG method as a non-RI method is convenient and
widely applicable and may be useful for the diagnosis and treatment
of the early stages not only in oral cancer, but also head and neck
cancer.
Materials and methods
Patients
This study was a prospective, multicentre, phase II
clinical trial (UMIN00006509) conducted in Japan. Between November
2011 and November 2012, 20 patients from 3 medical facilities were
enrolled. Patients were included if they had been diagnosed with
cN0 stage disease, had a pathologically confirmed squamous cell
carcinoma, and had a tumour in the clinical ‘late-T2’ stage (i.e.,
a T2 tumour with a diameter ≥3 cm or any T2 tumour with a tumour
depth ≥5 mm) or T3 stage. The clinical stage was determined by
physical examination and radiological computed tomography (CT). The
7th UICC classification was adopted in this study. Tumour depth was
defined as the distance from the surrounding normal tissue to the
bottom of the tumour and was estimated using CT or magnetic
resonance imaging (MRI) in addition to palpation. The procedures
used in this study were approved by the institutional review boards
of each institution, and written consent was obtained from all the
patients.
Study procedures
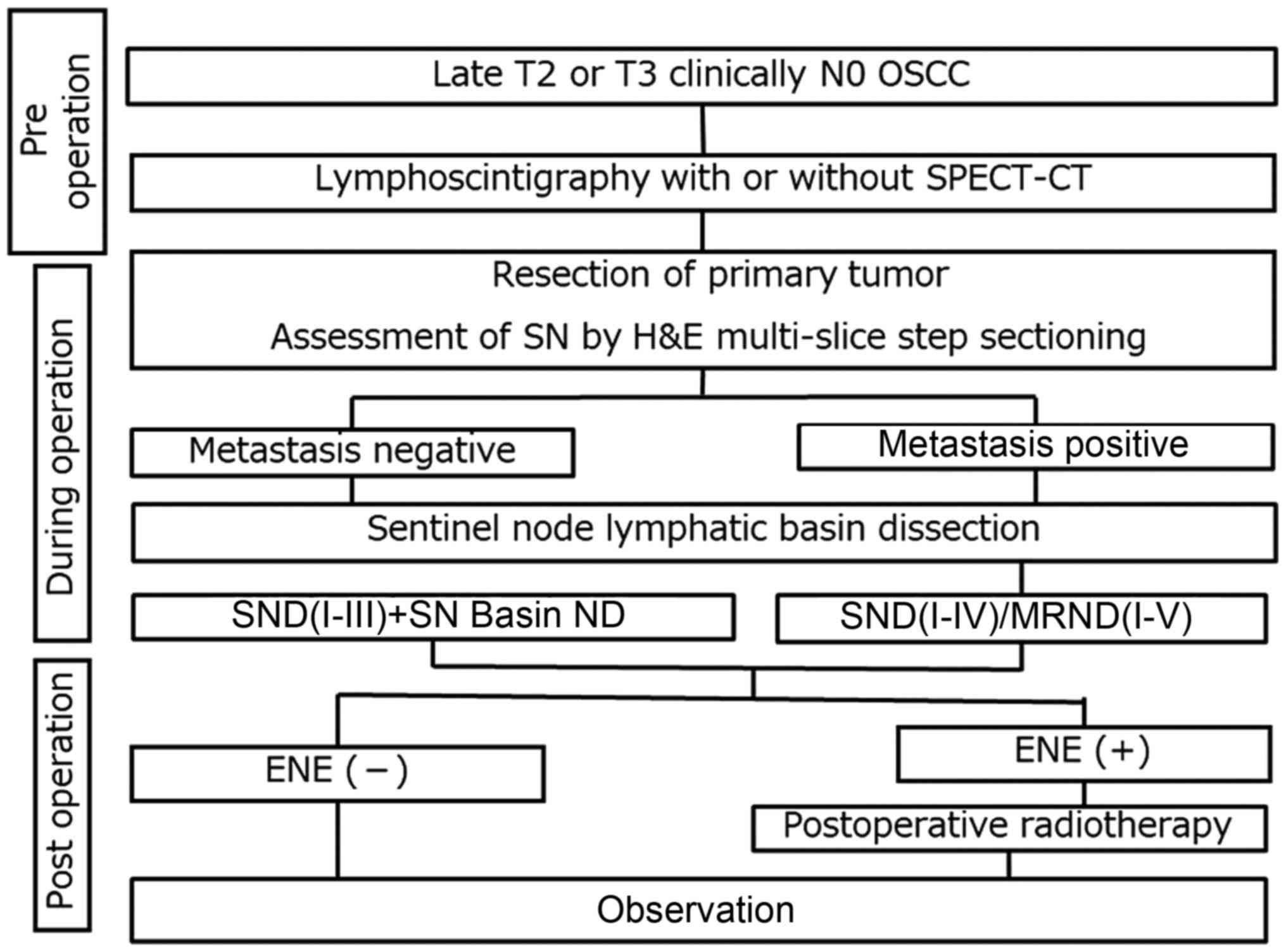
A flow diagram of the procedures used in this study
is presented in Fig. 1. Before
surgery, the enrolled patients underwent lymphoscintigraphy to map
the SNs. During surgery, the SNs were initially detected via RI
mapping, and ICG was subsequently injected. Next, ICG mapping of
the SNs was performed through the skin. Skin marking was done by
ICG mapping method.
The primary tumour was resected, a neck flap was
elevated for neck dissection, and SN biopsy was performed using RI
and ICG mapping. Selective ND with SN basin dissection was
performed according to the SN status (6). In cases of pull-through resection, the
SN biopsy was performed before the resection. The patients with
positive LNs but no extranodal extension (ENE) were carefully
followed up after surgery. However, if ENE was observed,
radiotherapy with/without chemotherapy was administered.
Chemoradiotherapy was applied to patients with multiple neck
metastasis (N2b) and ENE.
RI SN mapping
Preoperatively, the SNs were localized using
conventional lymphoscintigraphy. In some cases, single-photon
emission CT with CT (SPECT/CT) was performed to obtain additional
information. Technetium 99 m (99mTc) phytate (FUJIFILM
RI Pharma Co., Ltd. and Nihon Medi-Physics Co., Ltd.), which was
used as the radiotracer, was injected submucosally (74 MBq in 1 ml)
24 h before surgery at 4 points (one point in each quadrant) around
the primary tumour.
During surgery, radioactive SNs were detected with
handheld gamma probes, a neo2000 probe (Neoprobe) or a
NavigatorTM GPS probe (RMD Instruments).
ICG fluorescence SN mapping
An infrared fluorescence imaging system
(Photodynamic Eye, Hamamatsu Photonics, Japan) that consisted of
light-emitting diodes (LED) set at 760 nm was used as the light
source, and a charge-coupled device camera with a cut filter set
below 820 nm was used as a detector to obtain near-infrared (NIR)
fluorescence images. ICG (5 mg/2 ml) was injected submucosally at 4
points around the primary tumour intraoperatively. Several minutes
were usually required after ICG injection until adequate amounts of
ICG accumulated in the SNs. After 10 min, transcutaneous detection
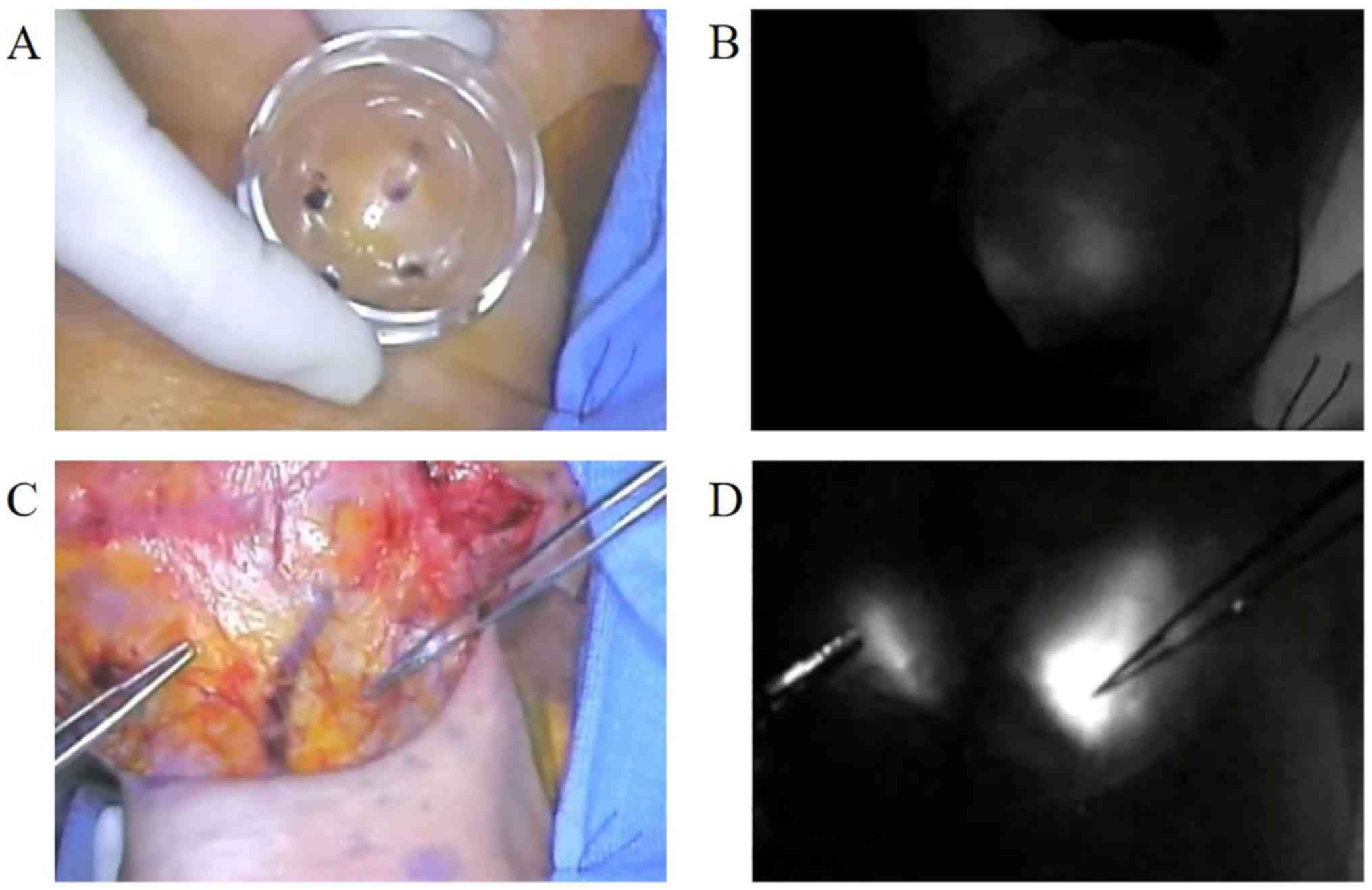
of the SNs was initiated by performing the neck compression
technique. When the neck skin was compressed against the posterior
neck or oral floor with a transparent plastic cone device
(Hamamatsu Photonics) (Fig. 2), a
fluorescence signal appeared at the bottom of the device because
the LN moved close to the surface (7). The LNs appeared as round areas of
intense signal.
The lymphatic vessels of the oral cavity and the
laryngopharynx drain directly into the deep nodes (i.e., the
submental, submandibular and jugular nodes). When the ICG was
injected submucosally, the subcutaneous lymphatic vessels did not
exhibit a fluorescent signal.
After neck flap elevation for neck dissection, most
of the ICG-mapped SNs were detected without the neck compression
technique. RI- and/or ICG-mapped SNs were biopsied using a gamma
probe or a fluorescence imaging system.
Because ICG is not a colloid, it spreads to the
secondary LNs over time. Therefore, at biopsy, LNs other than the
SN also emitted weak fluorescence. Numbers of SNs are maximally
limited to six. Skin marking was used to ensure the exact locations
of the SNs to be extracted; these SNs exhibited strong fluorescence
intensities.
Histopathological evaluation
All the SNs were subjected to intraoperative
pathological evaluation. All the other LNs, including the
non-radioactive and non-fluorescent LNs, were considered non-SNs.
The non-SNs were divided longitudinally into 2 specimens, and a
single representative cross section was stained with
haematoxylin-eosin (HE) for a final postoperative diagnosis. The
SNs were cut into 2-mm blocks, and 4-µm sections from each block
were used for intraoperative frozen section analysis. Additional
sections were stained with HE and the cytokeratinAE1/AE3 (Agilent
Technologies) stain for a final postoperative diagnosis.
Occult metastases were classified into 3 categories
(8): Isolated tumour cells (ITCs;
<0.2 mm in diameter), micrometastases (0.2-2 mm), and
macrometastases (>2 mm). Macrometastasis and micrometastasis
were considered indicative of pathological positivity for LN
metastasis but ITCs were not.
Statistical analyses
The primary endpoint of this study was the SN
identification rate of the ICG fluorescence method (cases with the
ICG method/cases with the RI method). The SN identification rates
are evaluated by the concordance rates between the RI and ICG
methods.
The assumed SN identification rate of the ICG
mapping was 95%, the threshold identification rate was 70%, the α
error was 0.05, the detection power was 0.80, and the required
number of cases was 15.
Considering the possibility of some unevaluable
cases, 18 was the target number of cases.
Kaplan-Meier survival curves were used to analyse
the survival outcomes. We defined overall survival (OS) as the
duration between the date of registration and the date of death or
last follow-up. Disease-free survival (DFS) was defined as the
duration between the date of registration and the date of relapse,
second primary tumour, death, or last follow-up. P<0.05 was
considered to indicate a statistically significant difference. All
the statistical analyses were performed using EZR (v1.32) (9) on R commander.
Results
Subject characteristics
From July 2011 to July 2012, 20 patients were
enrolled in the study. Of these, 2 patients were excluded because
of final preoperative diagnoses of positive nodes. SNs were
identified in all 18 enrolled patients. The median follow-up period
was 62.1 months (range: 52.3-72.6 months). The patient
characteristics are summarized in Table
I.
 | Table IPatients characteristics. |
Table I
Patients characteristics.
| Characteristics | Number | % |
|---|
| Age, median (range),
years | 54.5 (26-82) | |
| Sex | | |
|
Male | 11 | 61 |
|
Female | 7 | 39 |
| Tumor location | | |
|
Tongue | 15 | 83 |
|
Gingiva | 2 | 11 |
| Clinical T
stagea | | |
|
Late T2 | 17 | 94 |
|
T3 | 1 | 6 |
| Pathologic TN
stagea (pT) | | |
|
Tis | 1 | 6 |
|
T1 | 11 | 61 |
|
T2 | 3 | 17 |
|
T3 | 1 | 6 |
|
T4a | 2 | 11 |
| Pathologic TN
stagea (pN) | | |
|
N0 | 12 | 67 |
|
N1 | 4 | 22 |
|
N2b | 2 | 11 |
| Positive sentinel
nodes (cases) | 6 | 33 |
| Positive sentinel
nodes (nodes) | 8 | |
|
ITC | 1 | 13 |
|
0.2-2
mm | 2 | 25 |
|
<2
mm | 5 | 63 |
| Tumor resection
method | | |
|
Trans-oral/cervical | 14 | 78 |
|
Pull-through | 4 | 22 |
| Reconstruction
method | | |
|
Primary
suture | 14 | 78 |
|
Flap | 4 | 22 |
| Node
dissection | | |
|
Ipsilateral | 11 | 61 |
|
Bilateral | 6 | 33 |
|
Sentinel
node biopsy | 1 | 6 |
| Adjuvant
chemoradiotherapy | 1 | 6 |
Primary endpoint
The SN identification concordance rates between the
RI and ICG methods are presented in Table II. The concordance rate of the ICG
and RI methods at the case level was 94% (17/18, 95% CI:
0.73-1.00), and the concordance rate for the overall RI and ICG
methods was 72% (13/18, 95% CI: 0.47-0.90); thus, the agreement
rates were high.
 | Table IIConcordance rate of RI and ICG
mapping SNs. |
Table II
Concordance rate of RI and ICG
mapping SNs.
| | Cases | Nodes |
|---|
| Detection
methods | No. | % | 95% CI | No. | % | 95% CI |
|---|
| ICG/RI | 17/18 | 94 | 0.73-1.00 | 61/63 | 97 | 0.89-1.00 |
| RI/ICG | 13/18 | 72 | 0.47-0.90 | 61/67 | 91 | 0.82-0.97 |
The SN identification concordance rates of the ICG
and RI methods and the RI and ICG methods at the LN level were 97%
(61/63) and 91% (61/67), respectively. (Table II); thus, the agreement rates were
high. These results indicate that the primary endpoint goal was
achieved.
Distributions and metastatic statuses
of the SNs
The detailed distributions and metastatic statuses
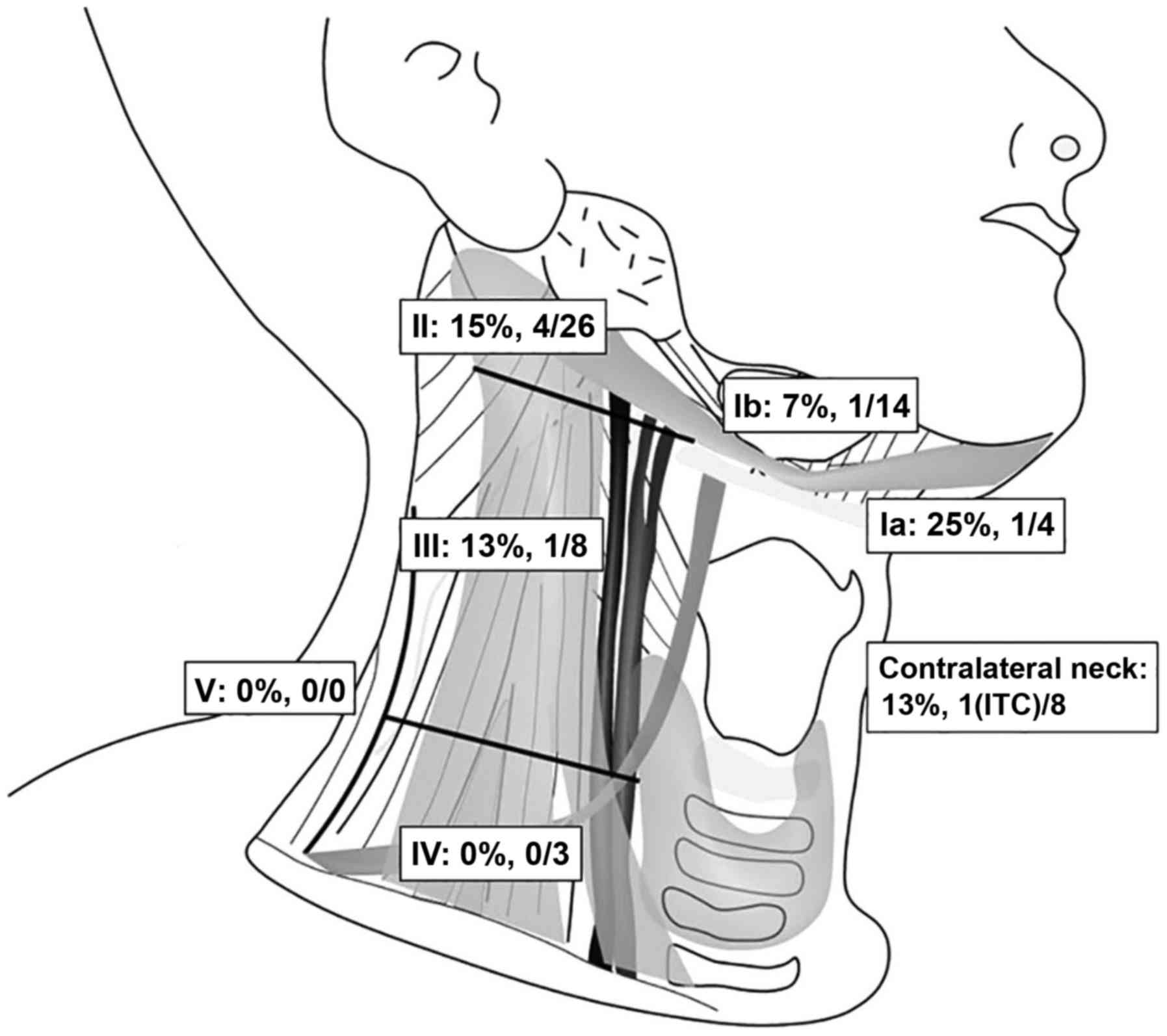
of the SNs are illustrated in Figs.
3 and 4. With the RI method, a
total of 63 SNs were detected (18 level I, 26 level II, 8 level
III, 3 level IV, and 8 SNs in the contralateral region of the
neck). Among these SNs, 8 (12.7%) were positive for metastasis,
including ITCs (2 were level I, 4 were level II, 1 was level III, 0
were level V and 1 was in the contralateral region of the neck;
Fig. 3). The median number of SNs
per patient identified by SNB was 4 (range: 2-6).
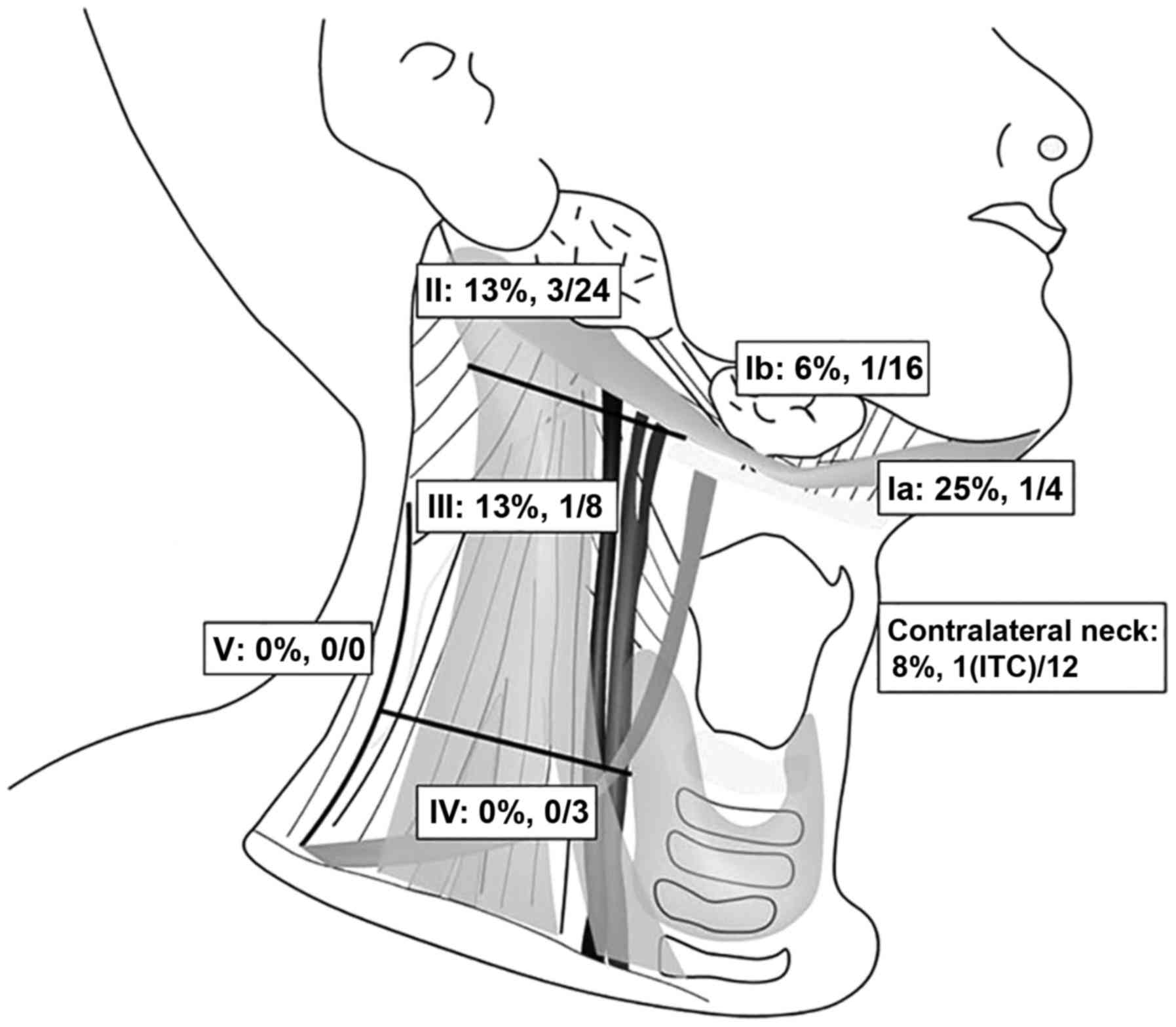
With the ICG method, a total of 67 SNs were detected
(20 were level I, 24 were level II, 8 were level III, 3 were level
IV, and 12 were in the contralateral region of the neck). Among
these SNs, 7 (10.4%) were positive for metastasis, including ITCs
(2 were level I, 3 were level II, 1 was level III, 0 were level V
and 1 was in the contralateral region of the neck; Fig. 4). The median number of SNs per
patient identified by SNB was 4 (range: 1-6).
Outcomes
There was 1 case of ENE, which was treated with
postoperative chemoradiotherapy. Five patients experienced
recurrences or second primary tumours during the follow-up period,
3 of which occurred at the primary site, 1 of which occurred in the
neck, and 1 of which occurred at a second primary site.
The cervical recurrence was a case of pN2b, which
was from the dissected area of the affected neck.
Of the 3 patients who experienced recurrence at the
primary site, 2 had suspicious multicentric cancer.
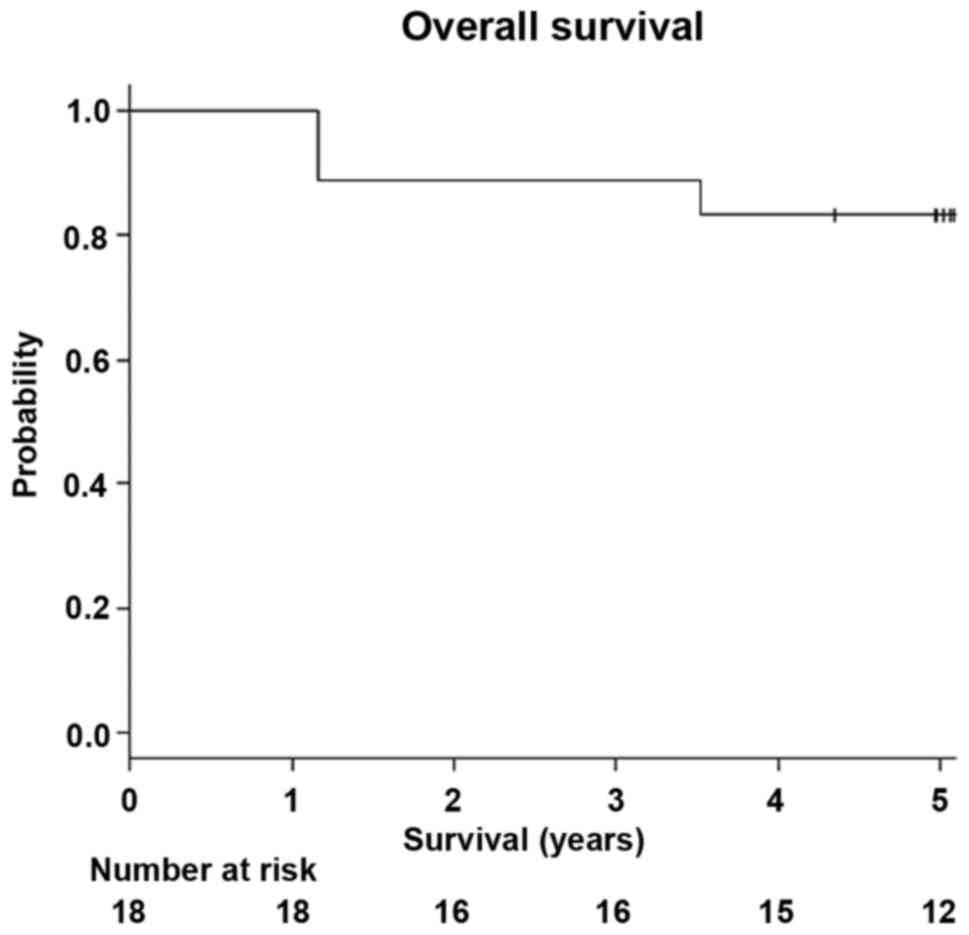
The 5-year OS of all the patients was 83.3% (95% CI:
0.57-0.88; Fig. 5), and the 5-year
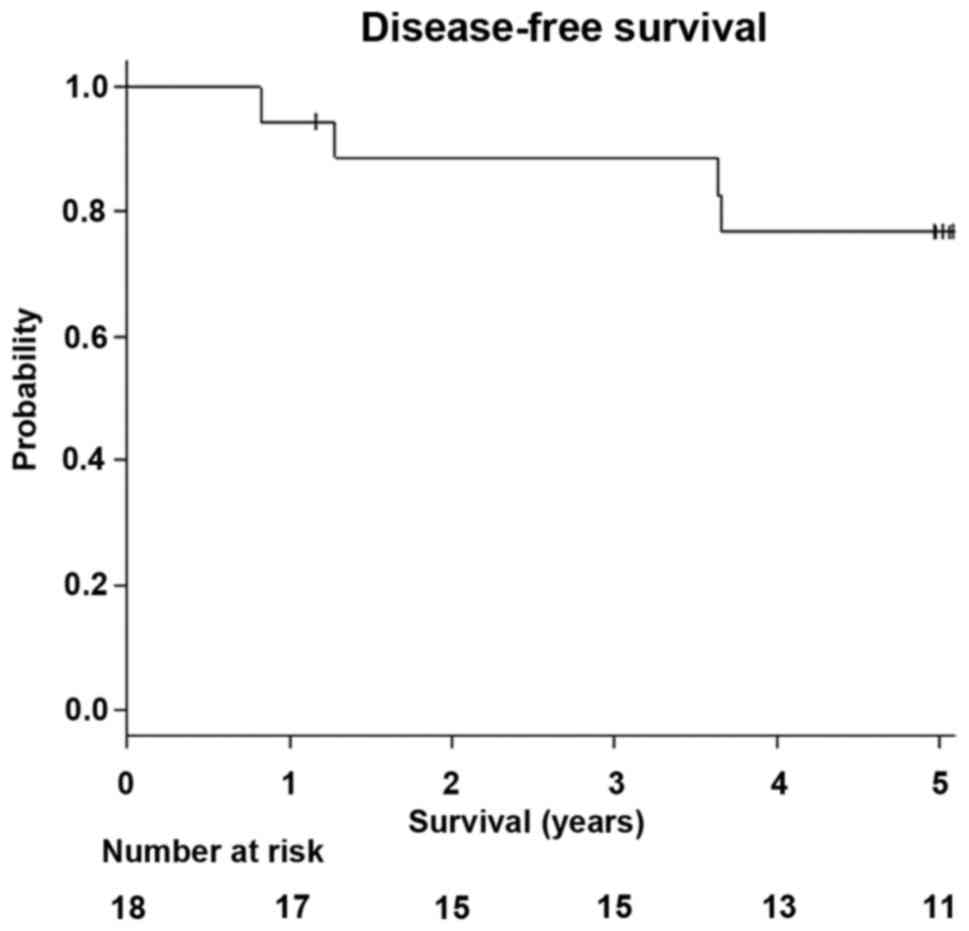
DFS of all the patients was 76.7% (95% CI: 0.49-0.91; Fig. 6).
Discussion
The results of a phase III study (3) in India demonstrated a prognostic
advantage of selective neck dissection (SND) for early oral cancer
in 2015. However, the surgical indication for SND could be limited
if the diagnosis of N0 cases or the regular follow-up of patients
could be strictly performed.
If SND is performed for all N0 cases of early
cancer, unnecessary surgery will be performed for more than 70% of
patients, and surgery occasionally causes postoperative dysfunction
or complications. In this respect, SN biopsy has been demonstrated
to be less invasive than SND (10-12).
Moreover, avoiding unnecessary procedures is expected to reduce
medical expenses (13-15).
Furthermore, potential metastasis to level IV, which occurs outside
the range of supraomohyoid neck dissection (SOND), has been
reported to occur in 9-10% of cases (16-18).
This missed metastasis is also a problem for improving survival. SN
biopsy is considered a useful solution to these problems.
SND and SN biopsy have been studied in early-stage
oral cancer and also in early pharyngeal and laryngeal cancers with
the transoral approach.
In an investigation of 40 N0 laryngopharyngeal
carcinoma patients who underwent transoral resection, the author
indicated that the indications for SND include a tumour depth
exceeding 1 mm and microvascular invasion. Because patients with
tumour depths of 0.5-1.0 mm require sufficient observation, the
author also indicated that these cases should be good candidates
for SN biopsy (19).
Need for an SN biopsy method and a
non-RI method in throat cancer
In recent years, the transoral approach for
pharyngo-laryngeal cancers has been reported to enable minimally
invasive surgery for primary tumours. Additionally, this approach
has the advantage of performing SN navigation neck dissection for
occult LN metastasis while being even less invasive to the neck
(20,21). Future investigations are needed to
verify whether the combination of primary tumour resection with a
transoral approach and sentinel LN navigation neck dissection is
possible.
When an RI method is applied for laryngeal or
hypopharyngeal cancer, an endoscopic injection is needed, whereas a
direct injection can be used for oropharyngeal cancer. This
requirement limits the equality of medical care due to technical
and equipment issues at institutions. Therefore, there is a need to
develop non-RI methods for laryngeal cancers.
ICG fluorescence method
When SN biopsies are performed, RIs are
conventionally used as tracers to identify the SNs. However, this
method has the disadvantage of radiation exposure and thus requires
improvement. ICG can be administered to the human body and is used
as a reagent for examinations of the liver, ocular fundus, and
other structures. ICG is excited by infrared light (760-780 nm) and
emits near-infrared fluorescence at different wavelengths (800-850
nm) that are easily transmitted in the human body. The dynamic
state can be observed non-invasively below the tissue surface using
an infrared light detection camera following the injection of ICG
into the body.
Because ICG accumulates in LNs, SNs can be
visualized intraoperatively. Therefore, this method could be the
best alternative to RI methods.
The ICG method has great advantages as a non-RI
method, but two problems must be overcome. First, the depth of
penetration of near-infrared light is limited, and second, the
near-infrared signal spreads rapidly and diffusely because ICG is
not a colloid. The longer the time between injection and detection,
the more lymph nodes that are detected. It requires more skillful
techniques because it requires detection in a short time. As more
lymph nodes are detected in ICG, the number of SNs selected is
approximately 4. In our study, out of several ICG infrared lymph
nodes in level II, 2 bigger ICG infrared lymph nodes were
incorrectly selected as SNs and a smaller metastatic node was not
selected (Fig. 4).
ICG method also detected more SNs in the
contralateral neck than RI method. It was estimated that this is
why it depends on the duration from injection to biopsy.
The application of SN biopsy methods using ICG have
been reported in breast cancer (22) and gastric cancer (23). However, Al-Dam et al
(24) reported that the sensitivity
and specificity of ICG in oral cavity cancer were 50% and 100%,
respectively. As ICG spreads quickly, the number of SLNs were
usually more than RI colloid methods. However, the mean number of
SLNs was 1.95 (1-3).
The number of SLNs in 4 false negative cases were 1,1,2 and 3,
respectively while in 4 positive cases they were 2, 3, 2 and 3,
respectively. The mean number of SLNs of the two groups were 1.75
and 2.5, respectively. The number of SLNs were fewer than the
average number 3.4(25) and our
cases (mean number 4). As it was difficult to select one or two
metastatic SLNs from many fluorescence lymph nodes, the metastatic
SLNs were probably not selected accurately as SLNs. van der Vorst
et al (26), also reported
that after injection of ICG with human serum albumin (complex:
ICG:HSA), SLNs were observed to increase significantly over time.
As SLNs increase significantly over time, it is necessary to set a
limit on the number of SLNs needed. We recommend 3 or 4 SLNs in ICG
method.
According to the eighth international symposium for
SNB in head and neck cancer (8th SNB) in 2018, ICG fluorescence
method has shown promise in identifying lymph nodes located close
to the primary tumor (ie, FOM) that may otherwise be missed due to
high gamma signal at the injection site (shine-through effect)
(27). However, Schilling et
al (28) recommend that ICG
fluorescence method alone is not applicable for SNB, yet support
its use in combination with a radionuclide tracer. Murase et
al (29), also reported that
since using ICG fluorescent imaging and 99 m-technetium-tin colloid
were able to identify all positive SLNs, this combined method was
useful for detecting SLNs in oral cavity cancer. However, ICG
fluorescent imaging could not detect any SLNs transcutaneously.
Compression method could facilitate the intraoperative localization
of the SN in breast cancer (7).
However, compression methods using a transparent plastic cone
device have not been reported in oral cavity cancer. This is the
first report describing the potential usefulness of compression
methods using a transparent plastic cone device in oral cavity
cancer.
In a basic experiment using the HyperEye Medical
System (HEMS), the locations of cervical LNs could be visualized
transcutaneously in rabbits. In pigs, which have thick skin and fat
layers, the locations of LNs could not be identified
transcutaneously. However, the LNs themselves emitted fluorescence,
and it was possible to visualize the LNs based on accumulated ICG
in the fat layer (30).
NIR fluorescence penetrates human tissues to a depth
of 1-2 cm and allows for the transcutaneous visualization of SLNs
and lymphatic vessels (31).
Usually, NIR fluorescence does not reach the jugular nodes.
Nakamura reported that fluorescent SNs could be visualized
transcutaneously with the HEMS camera when the skin over the SN
received finger pressure in 6 head and neck cancers (32). The jugular nodes are the frequent
targets of metastasis in head and neck cancer. As the jugular nodes
are located in the deep neck and behind the sternocleidomastoid
muscle of the neck, it is difficult to confirm the SNs through the
skin. Fortunately, there are some neck spaces without muscles
including carotid triangle, submental triangle, and lessor
supraclaviclar fossa in the neck. The neck compression technique
using a plastic cone can be applied to confirm deep SNs. As a
result, we can definitely detect lymphnodes through these spaces by
using a plastic cone.
As previously mentioned, one of the major drawbacks
of this method is the rapid migration of ICG through the lymphatic
system, which limits the diagnostic window and can lead to the
undesirable detection of downstream nodes. The exact detection of
SNs with ICG mapping requires a sufficient learning curve. Araki
et al (33), resolved this
problem by mixing ICG with phytate colloid to retard its migration
and demonstrated the feasibility of this technique in a nude mouse
study.
This prospective multicenter study has some
limitations such as the learning curve associated with operative
procedures. Before conducting this study, we held meetings in order
to establish uniform surgical techniques and to shorten the
learning curve. Inexperienced operators went to study SNB using ICG
fluorescence method with a plastic cone device at experienced
surgical centers. Furthermore, in the first trials conducted at
institutions with limited experience, skilled surgeons went to
instruct inexperienced surgeons and to promote uniform
accessibility of SNB using the ICG fluorescence method.
As previously mentioned, the ICG method has two
problems associated with the limited depth of penetration of
near-infrared light and the rapid spread of ICG. By compression
through several neck spaces with a plastic cone device, the jugular
nodes and submandibular nodes can be positively detected
transcutaneously. As SLNs increase over time due to spreading ICG,
there need to be limitations in the number of SLNs. Before
conducting this prospective, multicentre, phase II clinical trial,
we discussed and practiced detecting transcutaneously SLNs by ICG
fluorescence method with a plastic cone device. As detecting
rapidly spreading near-infrared lymph nodes, we decided to set the
number of SLNs at approximately 4.
As overcoming these two problems by using ICG
fluorescence method with a plastic cone device, we demonstrated the
high concordance between RI and ICG mapping of SNs in oral cavity
cancer. As a result, we expect ICG fluorescence method with a
plastic cone can be employed as an alternative technique to SN
mapping in oral cavity cancer in near future.
The neck compression technique demonstrated high
concordance between RI and ICG mapping of SNs in oral cavity
cancer. This simple method can facilitate the surgical procedures
of ICG fluorescence-navigated SNB transcutaneously in oral cavity
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Health and
Labour Sciences Research Grant for Clinical Cancer Research (grant
nos. H21-Gannrinshou-Ippan-016 and H24-Gannrinshou-Ippan-006) from
the Ministry of Health, Labour and Welfare in Japan. The present
study was also supported by KAKENHI (Grants-in-Aid for Scientific
Research) 16K11247 from the Ministry of Education, Culture, Sports,
Science and Technology (MEXT).
Availability of data and materials
The datasets during and/or analyzed during the
present study available from the corresponding author on reasonable
request.
Authors' contributions
YH and JY designed the study. AS, MS and SO
contribute to acquisition of data. JY and YH drafted the
manuscript. MS and NK performed the statistical analysis. YM
conducted the pathological diagnosis. JY and YH revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of each School and Hospital of Head and Neck Surgery and
registered in the prospective, multicentre, phase II clinical trial
(UMIN00006509) in Japan. All patients provided written informed
consent before the study.
Patient consent for publication
Written informed consent for the publication of any
associated data was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Den Toom IJ, Heuveling DA, Flach GB, van
Weert S, Karagozoglu KH, van Schie A, Bloemena E, Leemans CR and de
Bree R: Sentinel node biopsy for early-stage oral cavity cancer:
The VU university medical center experience. Head Neck. 37:573–578.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schilling C, Stoeckli SJ, Haerle SK,
Broglie MA, Huber GF, Sorensen JA, Bakholdt V, Krogdahl A, von
Buchwald C, Bilde A, et al: Sentinel European node trial (SENT):
3-year results of sentinel node biopsy in oral cancer. Eur J
Cancer. 51:2777–2784. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
D'Cruz AK, Vaish R, Kapre N, Dandekar M,
Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh
A, et al: Elective versus therapeutic neck dissection in
node-negative oral cancer. N Engl J Med. 373:521–529.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abu-Ghanem S, Yehuda M, Carmel NN, Leshno
M, Abergel A, Gutfeld O and Fliss DM: Elective neck dissection vs
observation in early-stage squamous cell carcinoma of the oral
tongue with no clinically apparent lymph node metastasis in the
neck: A systematic review and meta-analysis. JAMA Otolaryngol Head
Neck Surg. 142:857–865. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Civantos FJ, Zitsch RP, Schuller DE,
Agrawal A, Smith RB, Nason R, Petruzelli G, Gourin CG, Wong RJ,
Ferris RL, et al: Sentinel lymph node biopsy accurately stages the
regional lymph nodes for T1-T2 oral squamous cell carcinomas:
Results of a prospective multi-institutional trial. J Clin Oncol.
28:1395–400. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miura K, Hirakawa H, Uemura H, Yoshimoto
S, Shiotani A, Sugasawa M, Homma A, Yokoyama J, Tsukahara K,
Yoshizaki T, et al: Sentinel node biopsy for oral cancer: A
prospective multicenter phase II trial. Auris Nasus Larynx.
44:319–326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kitai T and Kawashima M: Transcutaneous
detection and direct approach to the sentinel node using axillary
compression technique in ICG fluorescence-navigated sentinel node
biopsy for breast cancer. Breast Cancer. 19:343–348.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hermanek P, -Hutter RV, Sobin LH and
Wittekind C: International union against cancer. Classification of
isolated tumor cells and micrometastasis. Cancer. 86:2668–2673.
1999.PubMed/NCBI
|
|
9
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schiefke F, Akdemir M, Weber A, Akdemir D,
Singer S and Frerich B: Function, postoperative morbidity, and
quality of life after cervical sentinel node biopsy and after
selective neck dissection. Head Neck. 31:503–512. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Murer K, Huber GF, Haile SR and Stoeckli
SJ: Comparison of morbidity between sentinel node biopsy and
elective neck dissection for treatment of the n0 neck in patients
with oral squamous cell carcinoma. Head Neck. 33:1260–1264.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hernando J, Villarreal P, Alvarez-Marcos
F, Gallego L, García-Consuegra L and Junquera L: Comparison of
related complications: Sentinel node biopsy versus elective neck
dissection. Int J Oral Maxillofac Surg. 43:1307–1312.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Govers TM, Takes RP, Baris Karakullukcu M,
Hannink G, Merkx MA, Grutters JP and Rovers MM: Management of the
N0 neck in early stage oral squamous cell cancer: A modeling study
of the cost-effectiveness. Oral Oncol. 49:771–777. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kosuda S, Kusano S, Kohno N, Ohno Y,
Tanabe T, Kitahara S and Tamai S: Feasibility and
cost-effectiveness of sentinel lymph node radiolocalization in
stage N0 head and neck cancer. Arch Otolaryngol Head Neck Surg.
129:1105–1109. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
O'Connor R, Pezier T, Schilling C and
McGurk M: The relative cost of sentinel lymph node biopsy in early
oral cancer. J Craniomaxillofac Surg. 41:721–727. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah JP: Patterns of cervical lymph node
metastasis from squamous carcinomas of the upper aerodigestive
tract. Am J Surg. 160:405–409. 1990.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Byers RM, Weber RS, Andrews T, McGill D,
Kare R and Wolf P: Frequency and therapeutic implications of ‘skip
metastases’ in the neck from squamous carcinoma of the oral tongue.
Head Neck. 19:14–19. 1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Crean SJ, Hoffman A, Potts J and Fardy MJ:
Reduction of occult metastatic disease by extension of the
supraomohyoid neck dissection to include level IV. Head Neck.
25:758–762. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tomifuji M, Imanishi Y, Araki K, Yamashita
T, Yamamoto S, Kameyama K and Shiotani A: Tumor depth as a
predictor of lymph node metastasis of supraglottic and
hypopharyngeal cancers. Ann Surg Oncol. 18:490–496. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shiotani A, Tomifuji M, Araki K, Yamashita
T and Saito K: Videolaryngoscopic transoral en bloc resection of
supraglottic and hypopharyngeal cancers using laparoscopic surgical
instruments. Ann Otol Rhinol Laryngol. 119:225–232. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamashita T, Tomifuji M, Araki K, Kurioka
T and Shiotani A: Endoscopic transoral oropharyngectomy using
laparoscopic surgical instruments. Head Neck. 33:1315–1321.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tagaya N, Aoyagi H, Nakagawa A, Abe A,
Iwasaki Y, Tachibana M and Kubota K: A novel approach for sentinel
lymph node identification using fluorescence imaging and image
overlay navigation surgery in patients with breast cancer. World J
Surg. 35:154–158. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tajima Y, Murakami M, Yamazaki K, Masuda
Y, Kato M, Sato A, Goto S, Otsuka K, Kato T and Kusano M: Sentinel
node mapping guided by indocyanine green fluorescence imaging
during laparoscopic surgery in gastric cancer. Ann Surg Oncol.
17:1787–1793. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Al-Dam A, Precht C, Barbe A, Kohlmeier C,
Hanken H, Wikner J, Schön G, Heiland M and Assaf AT: Sensitivity
and specificity of sentinel lymph node biopsy in patients with oral
squamous cell carcinomas using indocyanine green fluorescence
imaging. J Craniomaxillofac Surg. 46:1379–1384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Peng H, Wang SJ, Niu X, Yang X, Chi C and
Zhang G: Sentinel node biopsy using indocyanine green in
oral/oropharyngeal cancer. World J Surg Oncol.
17(278)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
van der Vorst JR, Schaafsma BE, Verbeek
FP, Keereweer S, Jansen JC, van der Velden LA, Langeveld AP,
Hutteman M, Löwik CW, van de Velde CJ, et al: Near-infrared
fluorescence sentinel lymph node mapping of the oral cavity in head
and neck cancer patients. Oral Oncol. 49:15–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Christensen A, Juhl K, Charabi B,
Mortensen J, Kiss K, Kjær A and von Buchwald C: Feasibility of
real-time near-infrared fluorescence tracer imaging in sentinel
node biopsy for oral cavity cancer patients. Ann Surg Oncol.
23:565–572. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schilling C, Stoeckli SJ, Vigili MG, de
Bree R, Lai SY, Alvarez J, Christensen A, Cognetti DM, D'Cruz AK,
Frerich B, et al: Surgical consensus guidelines on sentinel node
biopsy (SNB) in patients with oral cancer. Head Neck. 41:2655–2664.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Murase R, Tanaka H, Hamakawa T, Goda H,
Tano T, Ishikawa A, Hino S, Sumida T, Nakashiro K and Hamakawa H:
Double sentinel lymph node mapping with indocyanine green and 99
m-technetium-tin colloid in oral squamous cell carcinoma. Int J
Oral Maxillofac Surg. 44:1212–1217. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamauchi K, Nagafuji H, Nakamura T, Sato T
and Kohno N: Feasibility of ICG fluorescence-guided sentinel node
biopsy in animal models using the hypereye medical system. Ann Surg
Oncol. 18:2042–2047. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fujisawa Y, Nakamura Y, Kawachi Y and
Otsuka F: Indocyanine green fluorescence-navigated sentinel node
biopsy showed higher sensitivity than the radioisotope or blue dye
method, which may help to reduce false-negative cases in skin
cancer. J Surg Oncol. 106:41–45. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nakamura T, Kogashiwa Y, Nagafuji H,
Yamauchi K and Kohno N: Validity of sentinel lymph node biopsy by
ICG fluorescence for early head and neck cancer. Anticancer Res.
35:1669–1674. 2015.PubMed/NCBI
|
|
33
|
Araki K, Mizokami D, Tomifuji M, Yamashita
T, Ohnuki K, Umeda IO, Fujii H, Kosuda S and Shiotani A: Novel
indocyanine green-phytate colloid technique for sentinel node
detection in head and neck: Mouse study. Otolaryngol Head Neck
Surg. 151:279–285. 2014.PubMed/NCBI View Article : Google Scholar
|