Introduction
Angiosarcoma (AS), a highly malignant and rare tumor
of vascular or lymphatic endothelial cell origin with a poor
prognosis (1), arises in the scalp
and face in the elderly, in association with chronic lymphedema,
and in irradiated areas (1,2). AS associated with chronic lymphedema
is known as Stewart-Treves syndrome (STS) (2). STS is primarily described in patients
with lymphedema of an upper extremity occurring after breast cancer
surgery including radical axially lymph node dissection and
subsequent radiation therapy (RT). STS accounts for approximately
5% of AS (3), and is rarely
described in the presence of chronic lymphedema of the lower
abdominal wall.
Eribulin mesylate (eribulin) is a structurally
modified analog of halichondrin B, originally isolated from the
marine sponge Halichondria okadai. Its mode of action is
distinct from other tubulin inhibitors and involves binding to
specific sites on the growing positive ends of microtubules to
inhibit their growth (4-6).
Eribulin was approved in Japan for the treatment of patients with
soft tissue sarcoma in 2016. The efficacy of eribulin for AS has
not been evaluated sufficiently till date.
Herein, we report a rare case of STS in the lower
abdominal wall successfully treated with eribulin.
Case report
The patient, a 76-year-old woman, underwent a
radical hysterectomy with bilateral salpingo-ophorectomy and pelvic
lymphadenectomy for cervical cancer 12 years earlier. Postoperative
RT of 50.4 Gy in 28 fractions was administered. After treatment,
lymphedema progressed in her lower abdominal wall and bilateral
legs and she was treated with antibiotics for cellulitis
repeatedly. Then, she noticed a mass on her left lower abdominal
wall, where chronic lymphedema had developed. The mass grew rapidly
for 3 months and she was referred to our outpatient clinic.
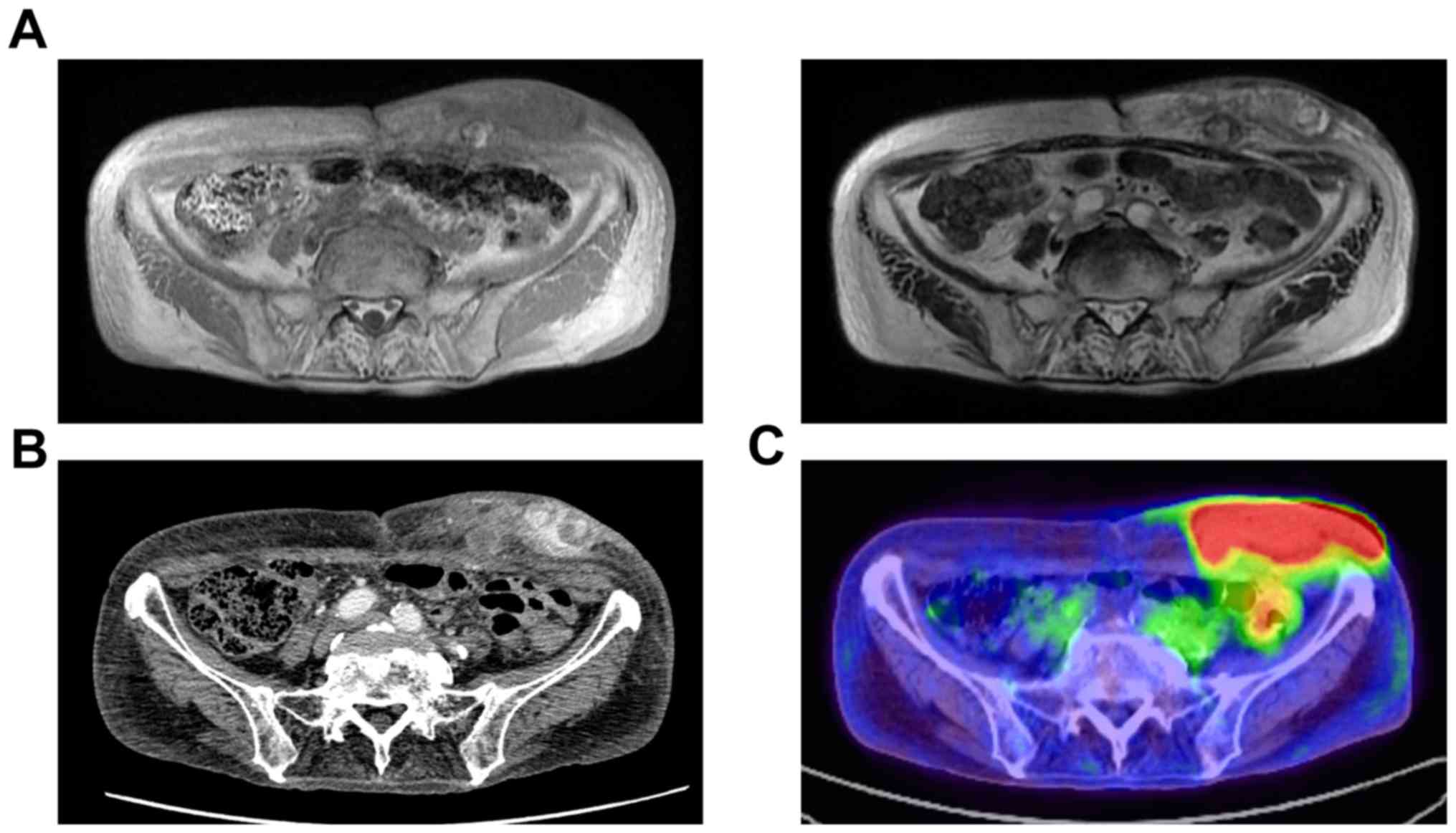
Magnetic resonance imaging (MRI) demonstrated that
the mass was heterogenous isointense on T1-weighted image and
heterogenous hyperintense on T2 weighted image (Fig. 1A). Diffuse thickening of cutis and
subcutis as well as edema of subcutaneous tissues could be seen in
the abdomen, groin, buttocks, and legs. Multiple enhancing nodules
in the left lower abdominal wall and edema of subcutaneous tissues
were revealed by contrast-enhanced computed tomography (CT) scan of
the abdomen (Fig. 1B). On
fluorodeoxyglucose positron emission tomography (FDG-PET)/CT scan,
hypermetabolism (maximum standardized uptake values=22.0) was
observed in the mass of the left lower abdominal wall (Fig. 1C), but distant metastasis was not
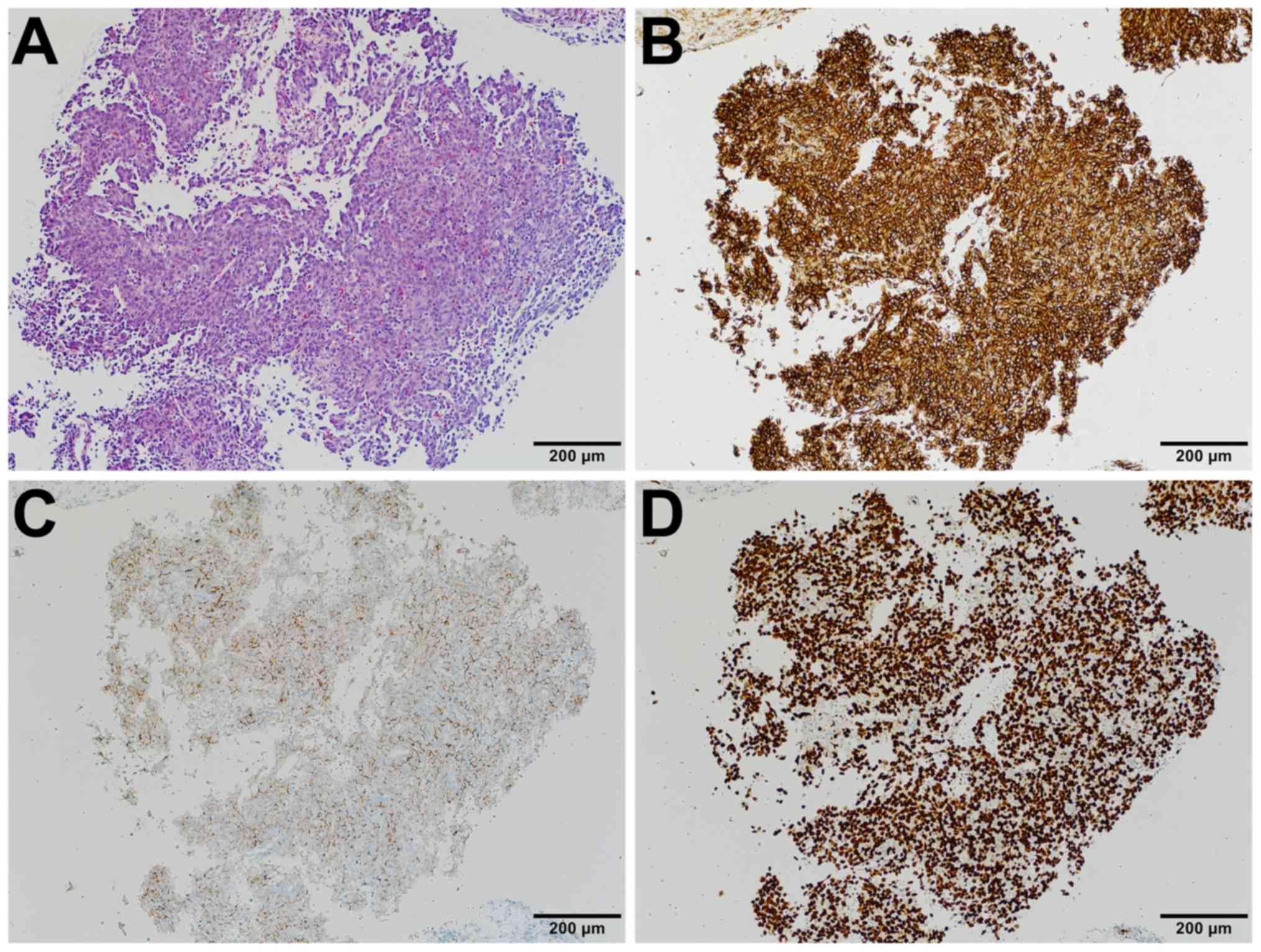
detected. Immunohistologic examination of needle biopsy specimens
of AS tested positive for CD31, D2-40, and ERG (Fig. 2A-D). Based on the morphology,
immunoprofile, and clinical presentation, the patient was diagnosed
with STS.
We judged the tumor unresectable and the combination
chemotherapy with gemcitabine (720 mg/m2) and docetaxel
(80 mg/m2) was started. She demonstrated stable disease
(SD) for 3 months. However, grade 4 neutropenia and anemia were
observed. In addition, severe side effects such as fatigue,
diarrhea, anorexia, vomiting, and weight loss occurred, and thus,
the patient discontinued treatment after 3 cycles. Then, RT of 70
Gy in 35 fractions was carried out for the left lower abdominal
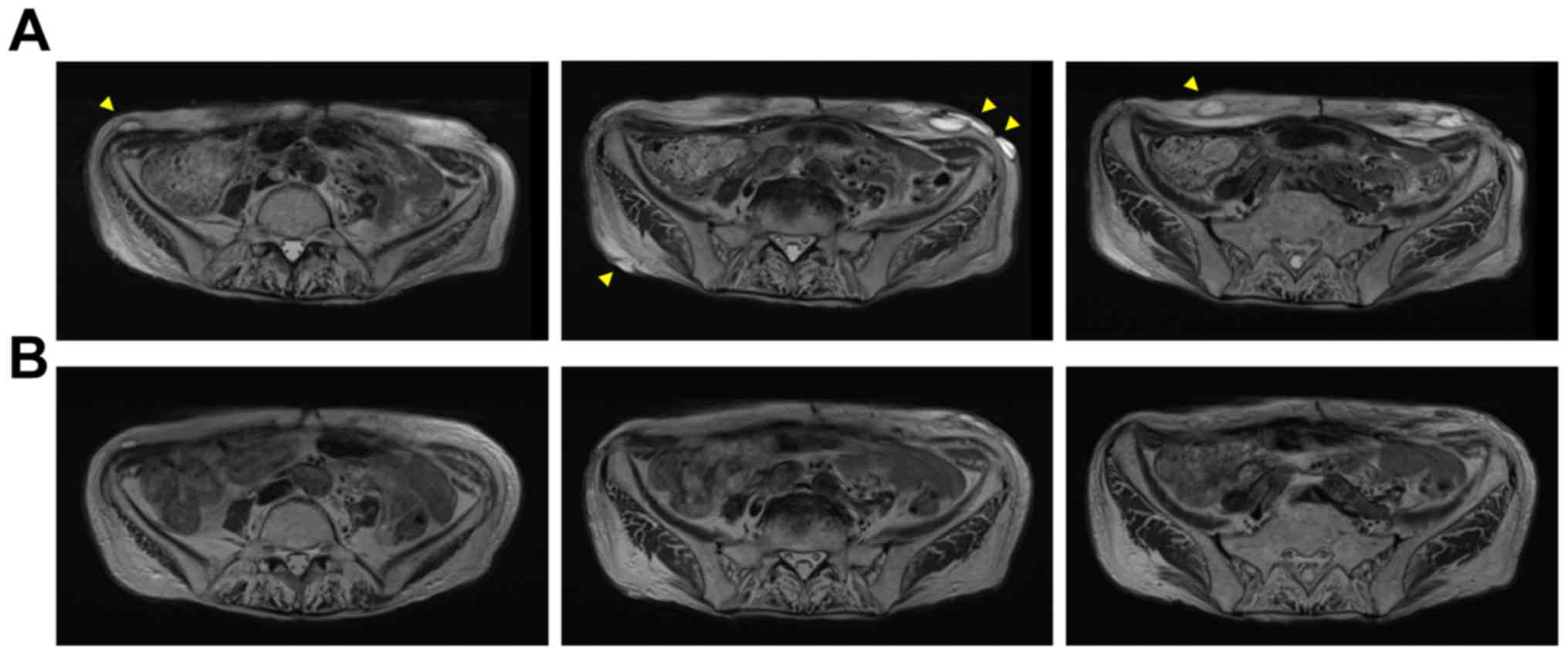
wall tumor. The size of the mass decreased once but multiple
reddish nodules, some of which were diagnosed as AS by excisional
biopsies, developed in the whole abdominal wall 3 months after RT
(Fig. 3A). At this point, treatment
with eribulin was initiated at a dose of 1.4 mg/m2.
However, grade 3 and 4 neutropenia occurred on day 8 and 15,
respectively. In addition, anorexia and diarrhea continued for 1
week after eribulin administration. Then, the dose was gradually
reduced. Eribulin was dosed at 0.8 mg/m2 and was
administered on day 1 of a 21-day cycle for 10 months. There was
shrinkage of nodules in the lower abdominal wall after 4 courses of
eribulin treatment, and no new lesions have developed. Treatment
with eribulin is effective with tumor partial response (PR). MRI
demonstrated that these effects lasted for 1 year without severe
side effects (Fig. 3B), and she
still continues to receive eribulin treatment.
Discussion
AS is a rare malignant tumor with a predilection for
skin in the head and neck region, though it can appear anywhere on
the body and has often been described as looking like a skin
infection, a bruise, or a lesion (1). Its association with chronic lymphedema
is well known (1,2). STS was first described when Fred
Stewart and Norman Treves reported a case series describing 6
patients with AS in the setting of chronic lymphedema that
developed post radical mastectomy (2). Although it is widely accepted that
lymphedema may induce AS, the mechanism remains unknown. Local
immunodeficiency resulted from long-standing chronic lymphedema may
be involved in oncogenesis of STS, and the development of AS has
been associated with chronic lymphedema of any origin. STS
typically occurs as a complication of long-lasting lymphedema of
the arm after axillary lymph node dissection following a radical
mastectomy and/or RT for breast cancer. STS can also appear in
lower limbs with chronic lymphedema. However, STS has rarely been
described in the presence of chronic lymphedema of the lower
abdominal wall and postoperative cervical cancer. For women with
cervical cancers, surgery that removes lymph nodes and/or RT to the
pelvis may damage lymph nodes in that area and lymphedema often
develops in the legs, abdomen, or groin area. Severe cellulitis
infection may damage tissue around the lymph nodes or vessels and
increase the risk of lymphedema. In this case, cellulitis occurred
repeatedly in the lower abdomen, probably leading to
lymphedema.
To date, more than 400 cases of STS have been
reported in the English literature. Collectively, STS has very poor
prognosis and is prone to local recurrence and distant metastasis
(1,7,8).
Because of the occurrence of metastatic spread and poor prognosis,
proper diagnosis of AS is important. Due to the rarity of STS,
there is no standardized therapy for this disease. Radical surgery
by local resection with a negative margin is the only curable
treatment available and complete tumor resection predicts improved
prognosis (9,10). Therefore, once the biopsy specimen
has been diagnosed as STS, extensive resection, early amputation,
or dissection of the joint and diseased limb are highly
recommended, which may increase the survival rate of patients.
However, in our case, we judged the tumor unresectable with a
safety margin. The median survival is 2.5 years after diagnosis,
with most patients dying from metastatic disease within 2 years
(1,3,7,8). The
long-term survivorship is rare, and survival time of untreated
patients is only 5-8 months post diagnosis.
AS is a highly aggressive and exceedingly rare soft
tissue sarcoma. Despite a strong need for systemic therapies, the
rarity of AS represents a major limitation to randomized trials and
therefore very few prospective clinical studies focusing on the
medical treatment are available. Standard chemotherapy for soft
tissue sarcoma is based on anthracyclines as the first line
treatment (11). Beside
anthracyclines, taxanes can be active, both as single agents and in
combination with gemcitabine in AS (12-19).
Taxanes are the current first line treatment for patients with
advanced AS who are considered difficult to treat with
anthracyclines owing to advanced age or comorbidity. Anthracyclines
can be difficult to administer in this patient due to high age and
cardiac dysfunction. Tolerability of gemcitabine plus docetaxel is
fair, with less cardiac toxicity compared with anthracyclines.
Therefore, we administered combination therapy of gemcitabine and
docetaxel in this patient.
Eribulin, a nontaxane microtubule-targeting agent,
was originally approved for the treatment of breast cancer by the
United States Food and Drug Administration in 2010. A phase III
study comparing eribulin with dacarbazine in patients with advanced
liposarcoma or leiomyosarcoma showed significant improvement in
overall survival (OS) for the eribulin arm, with a manageable
toxicity profile (20,21). In the liposarcoma subgroup, OS was
significantly improved with eribulin vs dacarbazine (15.6 vs. 8.4
months) and progression free survival was also improved with
eribulin vs. dacarbazine (2.9 vs. 1.7 months) (20,21).
Based on the results of this phase III study, in 2016 eribulin was
approved for the treatment of patients with unresectable or
metastatic liposarcoma who have received a prior
anthracycline-containing regimen in both the United States and
Europe. On the other hand, exploratory analysis from a Japanese
phase II study showed some efficacy of eribulin in patients with
several subtypes of rare soft tissue sarcoma including synovial
sarcoma, endometrial stromal sarcoma, solitary fibrous tumor, and
fibrosarcoma (22). As a result,
eribulin was approved for the treatment of all soft tissue sarcomas
in Japan. However, little data exists regarding eribulin efficacy
in rare histologic subtypes of soft tissue sarcomas such as AS.
Recently, 2 case reports from Japan described
patients with AS whose disease was controlled successfully with
eribulin mesylate (23,24). Kobayashi et al demonstrated
interim results of a post-marketing surveillance study of eribulin
in soft tissue sarcomas including rare subtypes such as AS
(25). In that study, of 12 AS
patients, PR was achieved in 1 patient (8.3%) and SD was achieved
in 2 patients (16.7%). However, treatment duration of eribulin in
those 3 patients was less than 9 months. Fujisawa et al
showed the promising efficacy of eribulin for treating patients
with advanced cutaneous AS who previously received taxanes
(26). The best overall response
rate was 20%, and the median overall survival and progression-free
survival were 8.6 and 3.0 months, respectively. It has been
concluded that caution should be exercised regarding eribulin use
in elderly patients. In the present case, PR was achieved and
maintained for 1 year with eribulin. Since the dose of eribulin was
low, we could continue eribulin treatment for the long time without
severe side effects. Moreover, this is the first case in which
eribulin demonstrated an excellent antitumor effect for STS,
suggesting that eribulin should be one of the most promising
options for the treatment of STS. Further studies are needed to
confirm the benefit of eribulin for patients with AS and to
establish predictive markers for eribulin sensitivity.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
Japan Orthopaedics and Traumatology Research Foundation, Inc.
(grant no. 372) and JSPS KAKENHI (grant no. JP19K18481).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YI and SN conceived the present study and wrote the
manuscript. SN performed immunohistochemical analysis and
pathological diagnosis of the patient. YI, TW, TT, HT, NN, and ST
collected clinical data. YI and SN revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
our institution.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of patient data and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stewart FW and Treves N: Lymphangiosarcoma
in postmastectomy lymphedema; A report of six cases in
elephantiasis chirurgica. Cancer. 1:64–81. 1948.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McHaffie DR, Kozak KR, Warner TF, Cho CS,
Heiner JP and Attia S: Stewart-treves syndrome of the lower
extremity. J Clin Oncol. 28:e351–e352. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Towle MJ, Salvato KA, Budrow J, Wels BF,
Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsky BM, Palme MH, et
al: In vitro and in vivo anticancer activities of synthetic
macrocyclic ketone analogues of halichondrin B. Cancer Res.
61:1013–1021. 2001.PubMed/NCBI
|
|
5
|
Jordan MA, Kamath K, Manna T, Okouneva T,
Miller HP, Davis C, Littlefield BA and Wilson L: The primary
antimitotic mechanism of action of the synthetic halichondrin E7389
is suppression of microtubule growth. Mol Cancer Ther. 4:1086–1095.
2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Okouneva T, Azarenko O, Wilson L,
Littlefield BA and Jordan MA: Inhibition of centromere dynamics by
eribulin (E7389) during mitotic metaphase. Mol Cancer Ther.
7:2003–2011. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharma A and Schwartz RA: Stewart-treves
syndrome: Pathogenesis and management. J Am Acad Dermatol.
67:1342–1348. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Taswell HF, Soule EH and Coventry MB:
Lymphangio-sarcoma arising in chronic lymphedematous extremities.
Report of thirteen cases and review of literature. J Bone Joint
Surg Am. 44-A:277–294. 1962.PubMed/NCBI
|
|
9
|
Grobmyer SR, Daly JM, Glotzbach RE and
Grobmyer AJ III: Role of surgery in the management of
postmastectomy extremity angiosarcoma (stewart-treves syndrome). J
Surg Oncol. 73:182–188. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kerchner K, Fleischer A and Yosipovitch G:
Lower extremity lymphedema update: Pathophysiology, diagnosis, and
treatment guidelines. J Am Acad Dermatol. 59:324–331.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: Doxorubicin alone versus intensified
doxorubicin plus ifosfamide for first-line treatment of advanced or
metastatic soft-tissue sarcoma: A randomised controlled phase 3
trial. Lancet Oncol. 15:415–423. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fata F, O'Reilly E, Ilson D, Pfister D,
Leffel D, Kelsen DP, Schwartz GK and Casper ES: Paclitaxel in the
treatment of patients with angiosarcoma of the scalp or face.
Cancer. 86:2034–2037. 1999.PubMed/NCBI
|
|
13
|
Fury MG, Antonescu CR, Van Zee KJ, Brennan
MF and Maki RG: A 14-year retrospective review of angiosarcoma:
Clinical characteristics, prognostic factors, and treatment
outcomes with surgery and chemotherapy. Cancer J. 11:241–247.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Skubitz KM and Haddad PA: Paclitaxel and
pegylated-liposomal doxorubicin are both active in angiosarcoma.
Cancer. 104:361–366. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schlemmer M, Reichardt P, Verweij J,
Hartmann JT, Judson I, Thyss A, Hogendoorn PC, Marreaud S, Van
Glabbeke M and Blay JY: Paclitaxel in patients with advanced
angiosarcomas of soft tissue: A retrospective study of the EORTC
soft tissue and bone sarcoma group. Eur J Cancer. 44:2433–2436.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Penel N, Bui BN, Bay JO, Cupissol D,
Ray-Coquard I, Piperno-Neumann S, Kerbrat P, Fournier C, Taieb S,
Jimenez M, et al: Phase II trial of weekly paclitaxel for
unresectable angiosarcoma: The ANGIOTAX study. J Clin Oncol.
26:5269–5274. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nagano T, Yamada Y, Ikeda T, Kanki H, Kamo
T and Nishigori C: Docetaxel: A therapeutic option in the treatment
of cutaneous angiosarcoma: Report of 9 patients. Cancer.
110:648–651. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stacchiotti S, Palassini E, Sanfilippo R,
Vincenzi B, Arena MG, Bochicchio AM, De Rosa P, Nuzzo A, Turano S,
Morosi C, et al: Gemcitabine in advanced angiosarcoma: A
retrospective case series analysis from the Italian rare cancer
network. Ann Oncol. 23:501–508. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Leu KM, Ostruszka LJ, Shewach D, Zalupski
M, Sondak V, Biermann JS, Lee JS, Couwlier C, Palazzolo K and Baker
LH: Laboratory and clinical evidence of synergistic cytotoxicity of
sequential treatment with gemcitabine followed by docetaxel in the
treatment of sarcoma. J Clin Oncol. 22:1706–1712. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schoffski P, Chawla S, Maki RG, Italiano
A, Gelderblom H, Choy E, Grignani G, Camargo V, Bauer S, Rha SY, et
al: Eribulin versus dacarbazine in previously treated patients with
advanced liposarcoma or leiomyosarcoma: A randomised, open-label,
multicentre, phase 3 trial. Lancet. 387:1629–1637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Demetri GD, Schoffski P, Grignani G, Blay
JY, Maki RG, Van Tine BA, Alcindor T, Jones RL, D'Adamo DR, Guo M
and Chawla S: Activity of eribulin in patients with advanced
liposarcoma demonstrated in a subgroup analysis from a randomized
phase III study of eribulin versus dacarbazine. J Clin Oncol.
35:3433–3439. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kawai A, Araki N, Naito Y, Ozaki T,
Sugiura H, Yazawa Y, Morioka H, Matsumine A, Saito K, Asami S and
Isu K: Phase 2 study of eribulin in patients with previously
treated advanced or metastatic soft tissue sarcoma. Jpn J Clin
Oncol. 47:137–144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wada N, Uchi H and Furue M: Case of
angiosarcoma of the scalp successfully controled by eribulin. J
Dermatol. 45:116–117. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Inagaki C, Shimoi T, Okuma H, Kitano A,
Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu
C, et al: A case of heavily pretreated metastatic cardiac
angiosarcoma treated successfully using eribulin. Anticancer Drug.
29:97–101. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kobayashi E, Naito Y, Asano N, Maejima A,
Endo M, Takahashi S, Megumi Y and Kawai A: Interim results of a
real-world observational study of eribulin in soft tissue sarcoma
including rare subtypes. Jpn J Clin Oncol. 49:938–946.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fujisawa Y, Fujimura T, Matsushita S,
Yamamoto Y, Uchi H, Otsuka A, Funakoshi T, Miyagi T, Hata H, Gosho
M, et al: The efficacy of eribulin mesylate for patients with
cutaneous angiosarcoma previously treated with taxane: A
multicentre prospective observational study. Br J Dermatol 2020
(Epub ahead of print).
|