Introduction
Cisplatin is a cytotoxic agent, which is used to
treat a variety of neoplasms. While the toxicities of cisplatin
include emesis, loss of appetite, ototoxicity and peripheral
neuropathy, the main dose-limiting side effect of cisplatin is
nephrotoxicity. Several studies have reported risk factors for
predicting cisplatin-induced acute kidney injury (AKI), such as old
age, female sex, smoking, hypoalbuminemia, hypokalemia,
hypomagnesemia, a high body surface area, the frequency of
cisplatin treatment, the combined use of cisplatin and paclitaxel,
advanced cancer, the total dose of cisplatin administered,
cardiovascular disease and diabetes mellitus (1-6).
Recently, some studies have focused on the
associations between genetic alterations in the genes coding for
renal drug transporters, such as organic cation transporter 2
(OCT2), and the nephrotoxicity of cisplatin (7-10).
One of the problems with these studies on genetic variants is that
their analyses are based on several different definitions of renal
injury. In clinical practice, the measurement of serum creatinine
concentration is widely used for the detection of AKI. Therefore,
the usefulness of genetic information must also be verified using a
widely accepted definition of renal damage, such as that included
in the National Cancer Institute-Common Terminology Criteria for
Adverse Events (NCI-CTCAE).
The aim of the present pilot study was to elucidate
the usefulness of genetic variants in predicting AKI using serum
creatinine measurements.
Materials and methods
Study design and participants
This was a single-center pilot study. Patients were
selected for this study according to the following criteria: i)
Administration of cisplatin (≥50 mg/m2)-containing
chemotherapy as first-line treatment; ii) creatinine clearance rate
(CCR) of ≥50 ml/min and a normal serum creatinine level; iii)
Eastern Cooperative Oncology Group (ECOG) performance status score
of 0-2; iv) adequate organ function, and v) age ≥20 years. The
exclusion criteria were as follows: Uncontrolled hypertension,
diabetes mellitus, hyperuricemia, pleural effusion and/or ascites
that required drainage to reduce the associated symptoms, and a
history of previous treatment with cisplatin-containing
chemotherapy.
The present study was conducted in accordance with
the principles outlined in the Declaration of Helsinki and the
International Conference on Harmonization and Good Clinical
Practice guidelines. The study protocol was approved by the
Institutional Ethics Committee of Mie University Hospital (approval
no. 2721). All the patients provided written informed consent.
Procedures
All the cisplatin-containing regimens were
administered in the inpatient setting. Cisplatin was administered
in 500 ml of 0.9% normal saline over 2 h. All patients were pre-
and posthydrated with infusions of ≥1,000 ml of saline. For
antiemetic prophylaxis, 5-HT3 serotonin receptor
antagonists and dexamethasone were administered 15-30 min before
the start of the cisplatin treatment, and a neurokinin 1 receptor
antagonist was administered 60 min before the start of cisplatin
treatment in all cases. Laboratory testing (hematological,
serological and urological tests) were conducted at baseline (days
-5-0) and on days 2-4, 7-9, 12-18 and 19-25 after chemotherapy.
For the genetic analysis, genomic DNA samples were
isolated from blood samples using the Qiagen DNA isolation kit
(Qiagen GmbH) and quantified using a NanoDrop spectrophotometer at
260 nm (Thermo Fisher Scientific, Inc.). The samples were genotyped
using the DMET platform (DMET™ Plus, Affymetrix; Thermo Fisher
Scientific, Inc.) according to the standard protocol described by
the manufacturer. The DMET Plus GeneChip enables the genotyping of
1,936 functionally significant genetic variants [1,931
single-nucleotide variants (SNV) and 5 copy number variations] in
231 genes, including phase I and II drug-metabolizing enzyme-coding
genes and drug transporter-coding genes. The DMET Plus platform
examines various types of genetic variations, including biallelic
and triallelic SNV, copy number variations and
insertions/deletions, and includes efficient and comprehensive
molecular inversion probe technology. Then, a genotype profile for
the 1,931 SNV was generated using the DMET™ console, v1.3
(Affymetrix; Thermo Fisher Scientific, Inc.).
Outcomes
In accordance with previous studies (1-14),
cisplatin-induced nephrotoxicity was evaluated based on two
definitions.
AKI as defined by CTCAE v4.0
In terms of the clinical-practical aspects, AKI is
defined as an increase in the serum creatinine level of >0.3
mg/dl or to 1.5-2 times the baseline level with reference to the
NCI-CTCAE v4.0.
Changes in estimated glomerular
filtration rate (eGFR)
Several previous studies on genetic factors have
adopted changes in the eGFR as outcomes (7,11,12).
Therefore, regarding genetic variants that are considered to be
associated with renal impairment, we evaluated not only their
associations with AKI (CTCAE v4.0), but also their effect on the
mean changes from baseline eGFR after the first cycle of
chemotherapy.
Statistical analysis
The patients' baseline characteristics, including
information on age, sex, ECOG performance status, height, weight,
body mass index, body surface area, complications, smoking history,
site of the primary tumor, tumor histology, clinical stage,
combination chemotherapy, concurrent radiotherapy, laboratory data
and SNV, were recorded. Two-sided Student's t-test, Mann-Whitney U
test, or Fisher's exact test were used to compare the baseline
characteristics of the groups with and without cisplatin-induced
AKI. All statistical analyses were performed with IBM SPSS
statistics v23.0 (IBM Corp.). All P-values were two-sided, and
P<0.05 was considered to indicate statistically significant
differences.
To identify genetic variants that were associated
with the changes in eGFR, each genotype of every genetic variant
was first converted to numerical values as follows: A/A to 1, A/C
to 2, A/G to 3, A/T to 4, C/C to 5, C/G to 6, C/T to 7, G/G to 8,
G/T to 9 and T/T to 10. We then converted the quantitative values
to Z scores, based on the mean and standard deviation values for
patients with each SNV. The changes in eGFR (difference between the
minimum value and the baseline value) were also converted to Z
scores based on the mean and standard deviation values for each
endpoint. The SNVs associated with the changes in eGFR were
identified using Pavlidis Template Matching in the TM4 MeV package
(15,16) using P<0.05 as a threshold.
Results
Baseline characteristics
A total of 28 patients (22 men and 6 women) were
enrolled in this trial between April 2014 and June 2016. The
baseline characteristics of the patients are listed in Table I. Their median age was 65 years
(range, 55-77 years). All the patients had a good performance
status (0 or 1) according to the ECOG scale. A total of 13 patients
(46.4%) had hypertension and 8 (28.6%) patients had diabetes
mellitus. A total of 23 (82.1%) patients had a history of smoking.
The predominant primary tumor site was the esophagus (75%). The
other primary tumor sites included the stomach in 4 cases (14.3%),
the lungs in 2 cases (7.1%) and the pancreas in 1 case (3.6%). The
predominant histological type was squamous cell carcinoma (60.7%).
There were 7 cases (25%) of adenocarcinoma, and 4 cases (14.3%) of
neuroendocrine carcinoma. A total of 11 patients (39.3%) received
cisplatin as neoadjuvant or adjuvant chemotherapy, 15 (53.6%)
received cisplatin as palliative chemotherapy and 2 (7.1%) received
cisplatin as concurrent chemoradiotherapy. The median serum
creatinine level was 0.73 mg/dl, the median eGFR was 82.3
ml/min/1.73 m2, and the median CCR was 88.1 ml/min.
 | Table IBaseline characteristics of the
patients. |
Table I
Baseline characteristics of the
patients.
| Characteristics | No. (%) |
|---|
| Age (years), median
(range) | 65 (55-77) |
| Sex | |
|
Male | 22 (78.6) |
|
Female | 6 (21.4) |
| ECOG performance
status score | |
|
0 | 9 (32.1) |
|
1 | 19 (67.9) |
| Predominant primary
tumor site | |
|
Esophagus | 21 (75.0) |
|
Stomach | 4 (14.3) |
|
Pancreas | 1 (3.6) |
|
Lung | 2 (7.1) |
| Predominant
histological type | |
|
Squamous
cell carcinoma | 17 (60.7) |
|
Adenocarcinoma | 7 (39.3) |
|
Neuroendocrine
carcinoma | 4 (14.3) |
| Setting | |
|
Neoadjuvant/adjuvant | 11 (39.3) |
|
Metastatic | 15 (53.6) |
|
Concurrent
chemoradiotherapy | 2 (2.7) |
| Co-administered
drugs | |
|
Fluorouracil | 20 (71.4) |
|
Etoposide | 4 (14.3) |
|
S-1 | 3 (10.7) |
|
Pemetrexed | 1 (3.6) |
| Cisplatin dose
(mg/m2), median (range) | 70 (60-80) |
| Creatinine (mg/dl),
median (range) | 0.73 (0.40-0.99) |
| eGFR (ml/min/1.73
m2), median (range) | 82.3
(61.2-142.5) |
| Creatinine clearance
(ml/min), | 88.1 (50.0-206) |
| median (range) |
| Comorbidities | |
|
Hypertension | 13 (46.4) |
|
Diabetes | 8 (28.6) |
| History of
smoking | |
|
Yes | 23 (82.1) |
|
No | 5 (17.9) |
Incidence of AKI
AKI, as defined by CTCAE v4.0, occurred in 10/28
patients (35.7%). Of these 10 patients with AKI, 2 suffered ileus
or vomiting during chemotherapy. As such adverse events can cause
pre-renal injuries, these 2 patients were excluded from our
analysis. In total, 8 patients (28.6%) developed cisplatin-induced
AKI, whereas 18 patients (64.2%) had no indications of
cisplatin-induced AKI. A comparison of various parameters in terms
of the presence or absence of AKI is presented in Table II. The patients' median age and the
median dosage of cisplatin were similar between the two groups. The
pretreatment CCR of the patients with AKI was similar to that of
patients without AKI. In the present study, the majority of the
patients developed AKI on days 7-9. Among the 8 patients with AKI,
the serum creatinine levels of 6 patients subsequently returned to
normal, whereas 2 patients developed irreversible renal
failure.
 | Table IIComparison of the baseline
characteristics of the patients with and without AKI. |
Table II
Comparison of the baseline
characteristics of the patients with and without AKI.
| Characteristics | AKI+
(n=8) | AKI-
(n=18) |
|---|
| Age (years), median
(range) | 66 (57-76) | 63 (55-77) |
| Sex, n (%) | | |
|
Male | 7 (87.5) | 13 (72.2) |
|
Female | 1 (12.5) | 5 (27.8) |
| ECOG performance
status score, n (%) | | |
|
0 | 4 (50.0) | 4 (22.2) |
|
1 | 4 (50.0) | 14 (77.8) |
| Tumor location, n
(%) | | |
|
Esophagus | 5 (62.5) | 15 (83.3) |
|
Stomach | 2 (25.0) | 1 (5.6) |
|
Pancreas | 1 (12.5) | 0 (0) |
|
Lung | 0 (0.0) | 2 (11.1) |
| Co-administered
drugs, n (%) | | |
|
Fluorouracil | 4 (50.0) | 15 (83.3) |
|
Etoposide | 2 (25.0) | 2 (11.1) |
|
S-1 | 2 (25.0) | 0 (0.0) |
|
Pemetrexed | 0 (0.0) | 1 (5.6) |
| Cisplatin dose
(mg/m2), median (range) | 80 (60-80) | 80 (75-80) |
| Creatinine (mg/dl),
median (range) | 0.79
(0.40-0.93) | 0.68
(0.40-0.99) |
| CCR (ml/min),
median (range) | 81.7
(61.2-116.9) | 89.2
(50-206.2) |
Risk factors Clinical factors
Univariate analyses were used to identify clinical
risk factors for cisplatin-induced AKI. The results of the
univariate analyses demonstrated that the baseline urinary
β2-microglobulin level and body surface area were significantly
higher in the AKI group (P<0.05; Table III). In addition, the baseline
serum cystatin C level and the baseline urinary levels of
N-acetyl-β-D-glucosaminidase and chloride were slightly higher in
patients with AKI compared with those in patients without AKI.
Other clinical factors, such as the serum levels of albumin,
potassium and magnesium, as well as age, which have previously been
reported as risk factors for cisplatin-induced AKI, were not found
to be significantly associated with cisplatin-induced AKI in the
present study.
 | Table IIIClinical risk factors for AKI. |
Table III
Clinical risk factors for AKI.
| Factors | AKI+
(mean ± SD) | AKI-
(mean ± SD) | P-value |
|---|
| Urinary
β2-microglobulin, µg/l | 730.0±888.0 | 150.7±189.5 | 0.016 |
| Body surface area,
m2 | 1.6±0.11 | 1.5±0.12 | 0.022 |
| Cystatin C,
mg/l | 1.1±0.26 | 0.93±0.12 | 0.075 |
| Urinary chloride,
mEq/l | 134.5±54.0 | 101.0±41.6 | 0.097 |
| Urinary NAG,
U/gCrea | 15.2±12.2 | 12.5±15.1 | 0.107 |
| Uric acid,
mg/dl | 5.5±1.3 | 4.5±1.3 | 0.114 |
| Urinary sodium,
mEq/l | 132.6±34.5 | 105.5±38.8 | 0.134 |
| Creatinine,
mg/dl | 0.76±0.16 | 0.67±0.16 | 0.203 |
| Sodium, mEq/l | 141.1±1.1 | 139.2±3.8 | 0.208 |
| eGFR, ml/min | 78.6±16.04 | 88.5±19.7 | 0.226 |
| Urinary
α1-microglobulin, mg/l | 7.8±6.7 | 5.2±5.0 | 0.289 |
| Chloride,
mEq/l | 104.5±2.5 | 102.7±3.6 | 0.300 |
| Age, years | 66.2±5.4 | 64.0±5.5 | 0.347 |
| Urinary albumin,
mg/gCrea | 175.2±469.5 | 17.1±39.6 | 0.373 |
| Creatinine
clearance, ml/min | 84.8±22.3 | 95.2±33.8 | 0.487 |
| Phosphorus,
mg/dl | 3.3±0.47 | 3.1±0.49 | 0.546 |
| Urinary potassium,
mEq/l | 38.8±29.0 | 33.7±19.8 | 0.602 |
| Potassium,
mEq/l | 4.0±0.34 | 4.1±0.42 | 0.628 |
| Magnesium,
mg/dl | 2.0±0.21 | 2.0±0.17 | 0.64 |
| Urinary creatinine,
mg/dl | 106.1±54.8 | 122.8±121.6 | 0.717 |
| Blood urea
nitrogen, mg/dl | 12.1±4.3 | 12.6±5.2 | 0.804 |
| Calcium, mg/dl | 9.4±0.85 | 9.3±0.98 | 0.865 |
| Albumin, g/dl | 3.6±0.50 | 3.5±0.35 | 0.975 |
| Urinary calcium,
mg/dl | 13.6±6.7 | 15.4±13.3 | 1.000 |
Genetic factors
In genetic analysis of the associations between AKI
and genetic variants, none of the examined genetic variants were
found to be associated with cisplatin-induced AK. OCT2 rs316019,
which was considered to be representative of renal injury-related
genetic factors, was not associated with cisplatin-induced AKI
(P=0.667, Fisher's exact test). Similarly, none of the other
examined OCT2 gene variants were found to be associated with
AKI.
By contrast, as regards the associations between the
mean changes in eGFR and SNV, 5 SNVs (ABCC4 rs3742106, CYP7A1
rs13251066, CYP39A1 rs7761731, MAT1A rs17102596 and
UGT1A3*4 rs45625338) were found to be associated with
the mean changes in eGFR (Fig.
1).
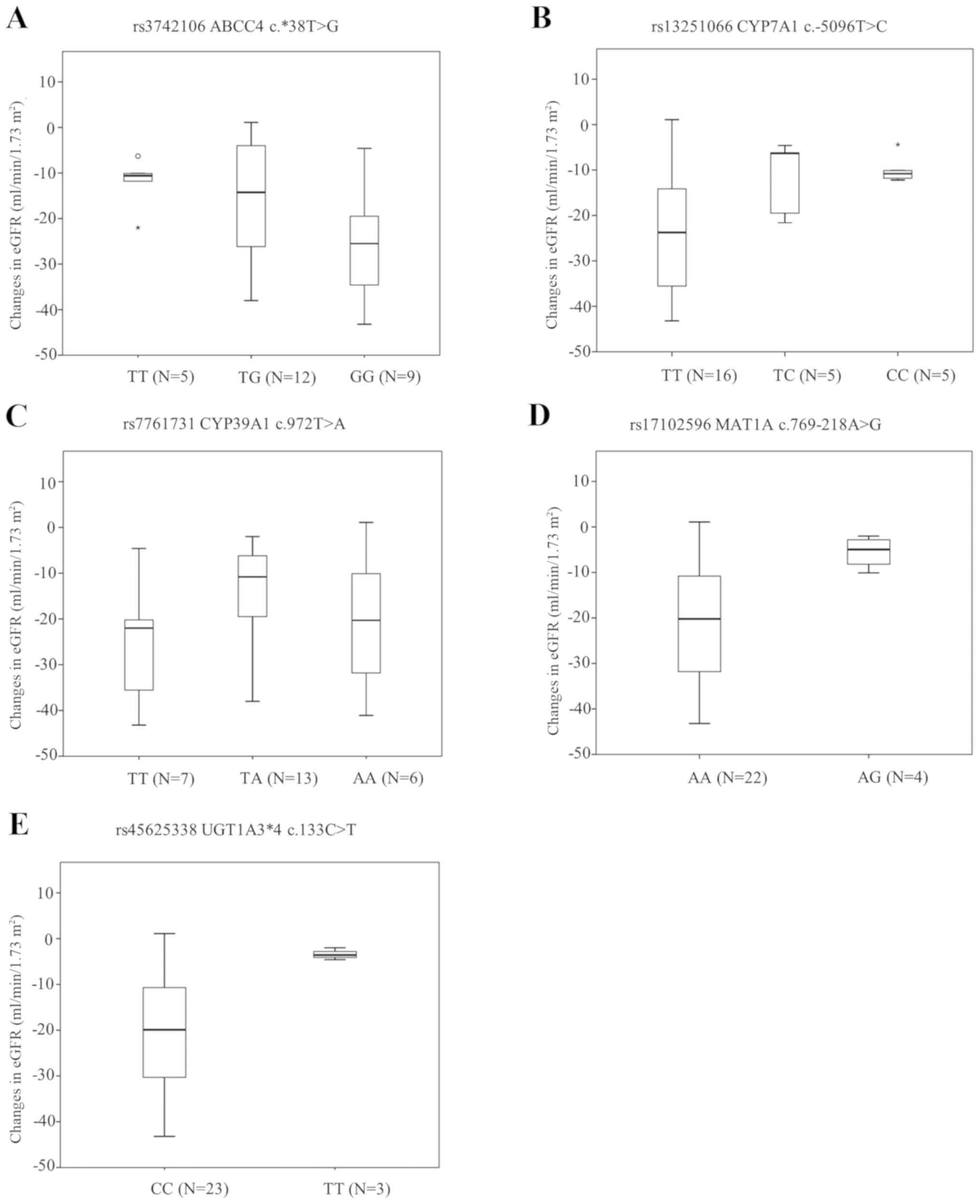 | Figure 1Associations between SNV and mean
changes in the eGFR. (A) The mean change in the eGFR was
significantly associated with rs3742106; ABCC4
c.*38T>G (TT vs. GG, P=0.048), (B) rs13251066; CYP7A1
c.-5096T>C (TT vs. CC, P=0.002), (C) rs7761731; CYP39A1
c.972T>A (TT vs. TA, P=0.034), (D) rs17102596; MAT1A
c.769-218A>G (AA vs. AG, P<0.001), and (E) rs45625338;
UGT1A3*4 c.133C>T (CC vs. TT, P<0.001). Asterisks
indicate extreme outliers (greater than three times the height of
the boxes). SNV, single-nucleotide variant; eGFR, estimated
glomerular filtration rate. |
Discussion
Cisplatin has been widely used as a cytotoxic agent
to treat several solid tumors, such as esophageal, gastric, ovarian
and lung cancer. However, other platinum-based drugs, such as
carboplatin and oxaliplatin, have recently become standard
treatments for these diseases; therefore, cisplatin is used less
frequently than before. The main reason for avoiding to use
cisplatin is its adverse effects, particularly because it is highly
emetic and nephrotoxic. Nausea and vomiting are serious adverse
events that affect the quality of life of patients during treatment
with cisplatin-containing regimens (17). However, recent advances in
antiemetic therapies, such as 5-HT3 antagonists,
aprepitant and olanzapine, appear to have resolved these problems
(17). With regards to carboplatin
and oxaliplatin, there appear to be three main limitations to their
use: Hypersensitivity reactions, thrombocytopenia and peripheral
neuropathy (18). Thus, determining
the risk factors for cisplatin nephrotoxicity would assist
clinicians in selecting patients for cisplatin-based chemotherapy
among those who are not medically fit to receive carboplatin or
oxaliplatin, which would be clinically significant.
In the present study, univariate analyses revealed
significant differences in body surface area and pretreatment
urinary β2-microglobulin levels between the patients who did and
did not develop AKI. By contrast, none of the SNVs that were
previously described as risk factors for renal injuries, including
OCT2, were found to be associated with AKI (CTCAE 4.0). Although
this is a small pilot study, the results indicate that genetic
factors may not be useful for predicting AKI as defined by CTCAE in
clinical practice.
Previous studies investigating the associations
between genetic variants and renal injuries, defined changes in
eGFR (7,11,12),
cystatin C (8) and KIM-1(13) as kidney injury. In the present
study, we found that some gene variants, including ABCC4, which is
involved in drug transport in kidney tubular cells, were
significantly associated with mean changes in eGFR. Cystatin C and
KIM-1 may be useful for early and accurate detection of kidney
injury; however, these novel biomarkers require further
investigation.
To apply nephrotoxicity-related genetic information
to clinical decision-making, a study with an appropriate definition
of nephrotoxicity, such as that defined by the CTCAE, is required.
Another approach is to construct a risk prediction model that
combines genetic variants with clinical factors. Several studies on
genetic factors associated with adverse drug reactions to various
antitumor drugs have been reported (19-21).
Unfortunately, the prediction of adverse events based on genetic
variants has not been sufficiently incorporated into daily clinical
practice; however, there are some exceptions, such as the
association between variants in the UGT1A1 gene and the effects of
irinotecan. Considering the current situation, a risk prediction
model that integrates clinical and genetic factors may be a
practical approach to using genetic information in daily clinical
practice.
In summary, we herein performed a pilot study to
elucidate the clinical usefulness of genetic factors for predicting
renal damage, with the intend of examining the associations between
cisplatin-induced AKI and genetic factors in a large-scale study
with an appropriate definition of nephrotoxicity.
Acknowledgements
The authors would like to thank Professor Masahiro
Okuda (Department of Pharmacy, Mie University Hospital), Mr. Kenji
Ikemura (Department of Pharmacy, Mie University Hospital) and Dr
Eiji Ishikawa (Department of Nephrology, Mie University Hospital),
whose comments/suggestions helped improve this study.
Funding
Funding was received from Mie University Hospital,
Tsu, Japan (The Director Research Grant in 2017).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HO designed the study and wrote the initial draft of
the manuscript. TM contributed to interpretation of data, and
assisted in the preparation of the manuscript. MaI, MN, YN and KN
contributed to analysis and interpretation of data. HO, AT, MiI,
KS, ST and YY contributed to clinical treatment. NK supervised the
patient treatment. All authors critically reviewed the manuscript
and approved the final version of the manuscript.
Ethic approval and consent to
participate
This article does not contain any studies with
animals performed by any of the authors. The study was conducted in
accordance with the principles of the Declaration of Helsinki and
the International Conference on Harmonization and Good Clinical
Practice guidelines. The study protocol was approved by the
Institutional Review Board of Mie University Hospital. Informed
consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Perazella MA and Moeckel GW:
Nephrotoxicity from chemotherapeutic agents: Clinical
manifestations, pathobiology and prevention/therapy. Semin Nephrol.
30:570–581. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Didier Portilla, A Mazin Safar and Melissa
L Shannon: Cisplatin nephrotoxicity. Post TW, ed. UpToDate.
Waltham, MA: UptoDate Inc. https://www.uptodate.com/contents/cisplatin-nephrotoxicity.
Accessed February 26, 2019.
|
|
4
|
Dobyan DC, Levi J, Jacobs C, Kosek J and
Weiner MW: Mechanism of cis-platinum nephrotoxicity: II.
Morphologic observations. J Pharmacol Exp Ther. 213:551–556.
1980.PubMed/NCBI
|
|
5
|
Sobrero A, Guglielmi A, Aschele C and
Rosso R: Current strategies to reduce cisplatin toxicity. J
Chemother. 2:3–7. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Jongh FE, van Veen RN, Veltman SJ, de
Wit R, van der Burg ME, van den Bent MJ, Planting AS, Graveland WJ,
Stoter G and Verweij J: Weekly high-dose cisplatin is a feasible
treatment option: Analysis on prognostic factors for toxicity in
400 patients. Br J Cancer. 88:1199–1206. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hinai Y, Motoyama S, Niioka T and Miura M:
Absence of effect of SLC22A2 genotype on cisplatin-induced
nephrotoxicity in oesophageal cancer patients receiving cisplatin
and 5-fluorouracil: Report of results discordant with those of
earlier studies. J Clin Pharm Ther. 38:498–503. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang J and Zhou W: Ameliorative effects
of SLC22A2 gene polymorphism 808 G/T and cimetidine on
cisplatin-induced nephrotoxicity in Chinese cancer patients. Food
Chem Toxicol. 50:2289–2293. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iwata K, Aizawa K, Kamitsu S, Jingami S,
Fukunaga E, Yoshida M, Yoshimura M, Hamada A and Saito H: Effects
of genetic variants in SLC22A2 organic cation transporter 2 and
SLC47A1 multidrug and toxin extrusion 1 transporter on
cisplatin-induced adverse events. Clin Exp Nephrol. 16:843–851.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Filipski KK, Mathijssen RH, Mikkelsen TS,
Schinkel AH and Sparreboom A: Contribution of organic cation
transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin
Pharmacol Ther. 86:396–402. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tzvetkov MV, Behrens G, O'Brien VP,
Hohloch K, Brockmöller J and Benöhr P: Pharmacogenetic analyses of
cisplatin-induced nephrotoxicity indicate a renoprotective effect
of ERCC1 polymorphisms. Pharmacogenomics. 12:1417–1427.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Windsor RE, Strauss SJ, Kallis C, Wood NE
and Whelan JS: Germline genetic polymorphisms may influence
chemotherapy response and disease outcome in osteosarcoma: A pilot
study. Cancer. 118:1856–1867. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chang C, Hu Y, Hogan SL, Mercke N, Gomez
M, O'Bryant C, Bowles DW, George B, Wen X, Aleksunes LM and Joy MS:
Pharmacogenomic variants may influence the urinary excretion of
novel kidney injury biomarkers in patients receiving cisplatin. Int
J Mol Sci. 18(1333)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zazuli Z, Vijverberg S, Slob E, Liu G,
Carleton B, Veltman J, Baas P, Masereeuw R and Maitland-van der Zee
AH: Genetic variations and cisplatin nephrotoxicity: A systematic
review. Front Pharmacol. 9(1111)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pavlidis P and Nobel WS: Analysis of
strain and regional variation in gene expression in mouse brain.
Genome Biol. 2(Research0042)2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Howe EA, Sinha R, Schlauch D and
Quackenbush J: RNA-Seq analysis in MeV. Bioinformatics.
27:3209–3210. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hesketh PJ, Kris MG, Basch E, Bohlke K,
Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina
SB, et al: Antiemetics: American society of clinical oncology
clinical practice guideline update. J Clin Oncol. 35:3240–3261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dilruba S and Kalayda GV: Platinum-based
drugs: Past, present and future. Cancer Chemother Pharmacol.
77:1103–1124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Innocenti F, Schilsky RL, Ramírez J,
Janisch L, Undevia S, House LK, Das S, Wu K, Turcich M, Marsh R, et
al: Dose-finding and pharmacokinetic study to optimize the dosing
of irinotecan according to the UGT1A1 genotype of patients with
cancer. J Clin Oncol. 32:2328–2334. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Henricks LM, Opdam FL, Beijnen JH, Cats A
and Schellens JHM: DPYD genotype-guided dose individualization to
improve patient safety of fluoropyrimidine therapy: Call for a drug
label update. Ann Oncol. 28:2915–2922. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Relling MV, Gardner EE, Sandborn WJ,
Schmiegelow K, Pui CH, Yee SW, Stein CM, Carrillo M, Evans WE and
Klein TE: Clinical Pharmacogenetics Implementation Consortium.
Clinical pharmacogenetics implementation consortium guidelines for
thiopurine methyltransferase genotype and thiopurine dosing. Clin
Pharmacol Ther. 89:387–391. 2011.PubMed/NCBI View Article : Google Scholar
|















