Introduction
Esophageal cancer is one of the highly malignant
tumors worldwide. The incidence rate and the mortality rate rank
6th and 4th among all cancers in China, respectively (1), 95% of all esophageal cancer cases are
cases of squamous cell carcinoma (2). There are regional and ethnic
variations in the incidence rate and etiological factors behind
esophageal cancer. The variation between different nationalities
and geographical regions are likely to be related to genetic
susceptibility (3). Xinjiang is a
multi-ethnic residential area and is an area with high incidence
for esophageal cancer, and Kazakh populations present the highest
risk of esophageal cancer in the area. The prognosis of esophageal
cancer types has been improved by surgery, chemotherapy,
radiotherapy, targeted therapy and immunotherapy; however, the
prognosis is still unsatisfactory. The overall 5-year survival rate
is less than 20% (4). Therefore,
early diagnosis, early treatment and new treatment methods are
needed to improve the recovery rate and the 5-year survival rate.
Targeted therapy is an important step in the development of
individualized treatment in for patients with esophageal
cancer.
Human epidermal growth factor receptor-2 (HER-2) is
a member of the epidermal growth factor receptor (EGFR) family, and
it is a transmembrane tyrosine kinase receptor with a molecular
weight of 185 kDa. Overexpression of the HER-2 protein in malignant
tumor cells accelerates cell division and proliferation, and
ultimately promotes tumor growth and metastasis (5). In recent years, HER-2 receptors and
the whole HER-2-mediated signaling pathway have been identified as
targets in the treatment of various malignant tumors (6).
Fluorescence in situ hybridization (FISH) is
a molecular pathological technique used to detect chromosome
aberration, gene deletion and amplification by using special
fluorescent labeled DNA single-stranded-nucleates as probes. It is
widely used in the detection of various cancers such as leukemia,
lung cancer, breast cancer and renal cancer. Due to its high
sensitivity and specificity, it can qualitatively detect malignant
cells (7,8). FISH is recognized as the gold standard
for detecting the HER-2 gene (9).
There are significant regional and ethnic variations in esophageal
cancer rates worldwide; however, there is currently no reports on
the HER-2 gene of Kazakh patients with esophageal cancer. Thus, the
current study detected HER-2 gene amplification in esophageal
squamous cell carcinoma (ESCC) tissues, and analyzed the
association between HER-2 gene amplification and the
clinicopathological characteristics of ESCC patients. This is to
provide a theoretical basis for the research, diagnosis and
treatment of ESCC patients in Kazakh populations, to explore the
association between HER-2 gene amplification and the survival
period of patients with esophageal cancer after radical operation,
and to evaluate the association between HER-2 gene expression
levels and the prognosis of patients with ESCC.
Patients and methods
Patients
A total of 70 specimens were obtained from Kazakh
patients who had not received chemotherapy or radiotherapy prior to
surgery from the Department of Thoracic Surgery of the First
Affiliated Hospital of Xinjiang Medical University, China, from
January 2014 to January 2016. There were 54 males and 16 females,
with a median age of 55.5 years (range of 32-79 years).
Postoperative pathological diagnosis was confirmed as ESCC, and
according to the 7th edition of AJCC Cancer Staging Manual
(10), 17 cases were poorly
differentiated, 39 cases were moderately differentiated and 14
cases were highly differentiated. There were 41 cases of positive
lymph node metastasis vs. 29 cases of negative lymph node
metastasis; 7 cases had IA-IB stage, 39 had IIA-IIB stage and 24
had IIIA-IIIB stage disease. All patients undergoing surgery had
written consent to use their tissues for this study. The collection
of samples conformed to the ethical requirements and this study was
approved by the Ethics Committee of the First Affiliated Hospital
of Xinjiang Medical University.
FISH and score for HER-2/neu
FISH analysis was performed in the Department of
Pathology of the First Affiliated Hospital of Xinjiang Medical
University, using the PathVysion HER-2/neu (Vysis) kit composed of
2 hybridization probes: A DNA probe CEP17 of 5.4 kb for the
centromeric region of chromosome 17, marked with Spectrum Green and
a DNA locus-specific probe LSI HER2/neu of 190 Kb, marked with
Spectrum Orange for HER2/neu.
Fresh cancer tissue was applied to the slide and was
dried at room temperature (25˚C) for 24 h, then was preserved at
-80˚C. After decomposition and denaturation with 70% formamide
solution, specimens were dehydrated in 70, 85 and 100% ethanol
series. The ThermoBrite automatic in situ hybridization
system was used, the hybridization conditions were as follows:
Denaturation at 75˚C for 5 min, hybridization at 37˚C for 16 h. The
next day, slides were washed with 2xSSC solution for 2 min at 72˚C
and then at room temperature for 1 min. Slides were allowed to dry
in the dark. After the slides were dried, 10 µl DAPI II was added
and the glass was covered. Ultimately this was observed using
fluorescence microscopy.
A special image acquisition and analysis system
(Leica Microsystems, Ltd.) was used to count the number of signals
under a fluorescence microscope. The counting results were
independently completed by two participants, and the results were
confirmed only when the counts were consistent.
Results criteria: At least 100 nuclei were counted.
When the ratio of red signal (HER2-neu) to green signal (17
chromosome centromere) was ≥2.0, or the number of nuclei of cells
with >15 red signals for >10% of the total number of cells,
HER2-neu was considered to be amplified. When the ratio of red
signal to green signal was less than 1.8, HER2-neu was considered
to not be amplified; if the ratio of red signal to green signal was
between 1.8 and 2.0, a total of 100 extra cells were counted
(11).
Statistical analysis
For statistical analysis a χ2 test was
used to evaluate the differences between the two groups, the
survival analysis was performed by the Kaplan-Meier with log-rank
test, and in all tests P<0.05 was considered to indicate a
statistically significant difference. Statistical calculations were
performed using SPSS 20.0 (IBM Corp.).
Results
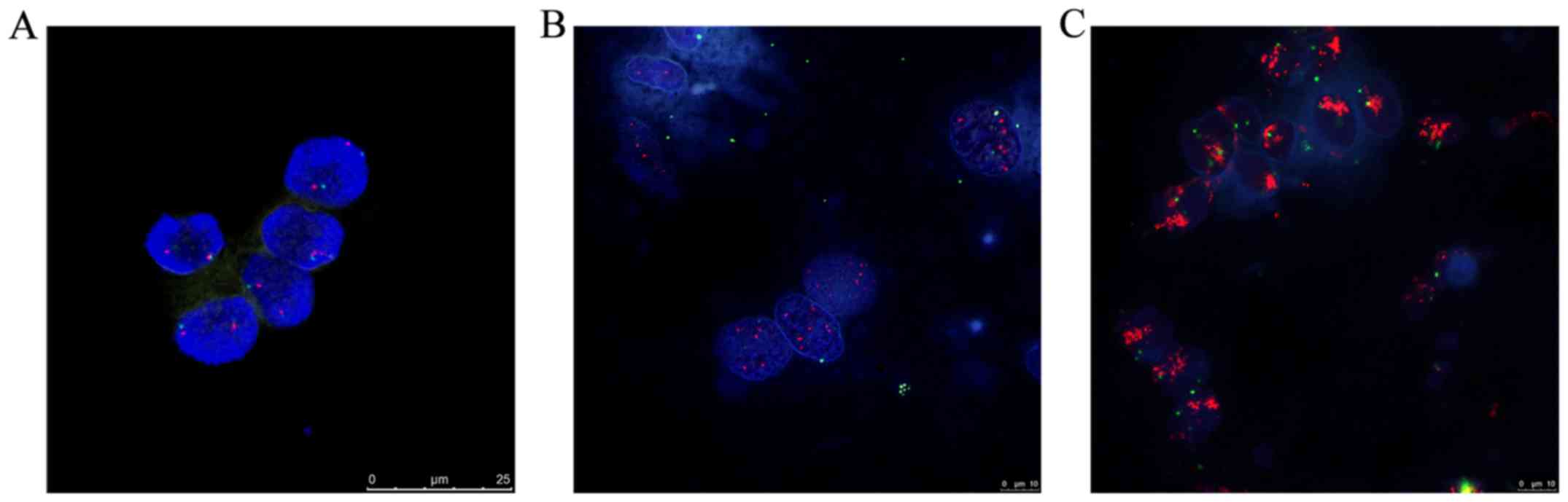
HER2 amplification pattern
The FISH technique was used to detect HER-2 gene
expression levels in 87 fresh specimens of ESCC. Among them,
results from 70 cases were recorded, with the remaining 17 being
excluded due to failure of the experiment, nuclear rupture and
unclear nuclear staining. In the current study, the positive
amplification rate of HER-2 was 54.2% (38/70). Normal epithelial
cells and non-neoplastic stroma or inflammatory cells generally
presented two HER-2 signals. There were two patterns observed in
the HER-2 gene amplification in esophageal cancer samples of Kazakh
populations: >15 red signals (HER-2) and multiple green signals
(CEP17) in >10% of tumor cell nucleus were amplified, and ≥4 red
signals (HER-2) and green signals (CEP17) in >40% of the tumor
cells that showed as polysomy, were amplified (HER2/CEP17 ratio
≥2.0, Table I and Fig. 1).
 | Table IResults of HER-2 and CEP17 by
FISH. |
Table I
Results of HER-2 and CEP17 by
FISH.
| | Ploidy No. | |
|---|
| Case | Age | Sex | 2 HER-2/CEP17 | 3 HER-2/CEP17 | 4 HER-2/CEP17 | >15
HER-2/CEP17 | FISH |
|---|
| 1 | 51 | M | 62/54 | 38/46 | 0/0 | 0/0 | N |
| 2 | 65 | M | 48/42 | 32/39 | 20/19 | 0/0 | N |
| 3 | 45 | M | 44/44 | 46/34 | 10/22 | 0/0 | N |
| 4 | 54 | F | 54/38 | 30/32 | 16/30 | 0/0 | N |
| 5 | 50 | M | 38/22 | 58/38 | 4/40 | 0/0 | N |
| 6 | 51 | F | 10/48 | 14/28 | 66/24 | 10/0 | P |
| 7 | 41 | M | 62/48 | 38/28 | 0/24 | 0/0 | N |
| 8 | 63 | M | 4/34 | 36/15 | 60/25 | 0/26 | P |
| 9 | 57 | M | 8/60 | 36/14 | 56/20 | 0/0 | P |
| 10 | 46 | M | 34/22 | 12/60 | 54/18 | 0/0 | P |
| 11 | 56 | M | 24/46 | 56/20 | 8/32 | 12/2 | P |
| 12 | 68 | M | 28/38 | 40/26 | 42/36 | 0/0 | N |
| 13 | 63 | M | 12/44 | 18/28 | 70/28 | 0/0 | P |
| 14 | 66 | M | 14/42 | 24/38 | 52/20 | 10/0 | P |
| 15 | 71 | M | 54/40 | 24/12 | 22/48 | 0/0 | N |
| 16 | 44 | M | 28/46 | 34/30 | 38/24 | 0/0 | N |
| 17 | 69 | M | 56/22 | 22/18 | 8/56 | 14/4 | P |
| 18 | 63 | M | 48/34 | 14/46 | 38/20 | 0/0 | N |
| 19 | 68 | M | 36/23 | 53/46 | 2/31 | 0/0 | N |
| 20 | 54 | M | 5/34 | 35/40 | 60/26 | 0/0 | P |
| 21 | 59 | M | 54/62 | 23/0 | 23/38 | 0/0 | N |
| 22 | 66 | M | 7/55 | 50/35 | 33/10 | 10/0 | P |
| 23 | 64 | F | 20/10 | 57/50 | 20/40 | 0/0 | N |
| 24 | 50 | M | 64/60 | 21/14 | 15/26 | 0/0 | N |
| 25 | 38 | F | 6/36 | 40/46 | 54/18 | 0/0 | P |
| 26 | 62 | M | 70/70 | 21/0 | 9/22 | 0/8 | N |
| 27 | 57 | M | 5/56 | 55/22 | 40/12 | 0/10 | P |
| 28 | 67 | F | 12/41 | 40/45 | 34/14 | 14/0 | P |
| 29 | 52 | M | 64/49 | 24/19 | 12/32 | 0/0 | N |
| 30 | 47 | M | 10/23 | 49/67 | 38/10 | 0/0 | P |
| 31 | 50 | M | 70/60 | 24/16 | 6/24 | 0/0 | N |
| 32 | 52 | M | 15/34 | 31/40 | 44/10 | 0/0 | P |
| 33 | 49 | M | 10/30 | 43/56 | 35/8 | 12/2 | P |
| 34 | 63 | M | 63/49 | 24/21 | 13/30 | 0/0 | N |
| 35 | 57 | M | 5/30 | 39/61 | 45/9 | 11/0 | P |
| 36 | 62 | M | 58/66 | 19/3 | 25/31 | 0/0 | N |
| 37 | 57 | M | 41/50 | 36/16 | 23/34 | 0/0 | N |
| 38 | 57 | F | 45/59 | 25/13 | 20/28 | 0/0 | N |
| 39 | 62 | F | 58/55 | 14/21 | 28/24 | 0/0 | N |
| 40 | 69 | F | 4/19 | 56/71 | 40/10 | 10/0 | P |
| 41 | 45 | M | 6/47 | 36/28 | 58/20 | 0/5 | P |
| 42 | 43 | M | 67/48 | 10/0 | 16/21 | 7/8 | N |
| 43 | 58 | F | 4/20 | 30/54 | 54/14 | 12/0 | P |
| 44 | 66 | M | 69/50 | 24/10 | 6/24 | 1/16 | N |
| 45 | 57 | M | 64/49 | 20/19 | 16/32 | 0/0 | N |
| 46 | 79 | M | 12/54 | 30/18 | 48/18 | 10/0 | P |
| 47 | 57 | M | 12/40 | 22/38 | 54/20 | 12/2 | P |
| 48 | 54 | F | 44/38 | 34/16 | 22/46 | 0/0 | N |
| 49 | 61 | F | 58/44 | 18/32 | 24/24 | 0/0 | N |
| 50 | 51 | M | 12/28 | 16/44 | 68/28 | 6/0 | P |
| 51 | 63 | M | 55/48 | 22/38 | 23/14 | 0/0 | N |
| 52 | 69 | M | 18/40 | 10/32 | 72/28 | 0/0 | P |
| 53 | 73 | M | 5/14 | 8/24 | 76/22 | 11/1 | P |
| 54 | 51 | M | 12/60 | 34/20 | 54/20 | 0/0 | P |
| 55 | 68 | M | 16/21 | 24/58 | 48/18 | 12/3 | P |
| 56 | 66 | M | 16/50 | 18/26 | 66/24 | 0/0 | P |
| 57 | 63 | M | 21/30 | 22/58 | 46/12 | 11/0 | P |
| 58 | 57 | F | 8/36 | 20/41 | 58/20 | 14/3 | P |
| 59 | 32 | M | 18/28 | 12/48 | 60/24 | 10/0 | P |
| 60 | 56 | M | 22/44 | 24/40 | 54/16 | 0/0 | P |
| 61 | 66 | F | 16/30 | 22/46 | 62/24 | 0/0 | P |
| 62 | 61 | F | 16/34 | 20/44 | 52/22 | 12/0 | P |
| 63 | 64 | M | 40/32 | 36/48 | 24/20 | 0/0 | N |
| 64 | 77 | F | 56/60 | 24/30 | 20/10 | 0/0 | N |
| 65 | 53 | M | 62/58 | 38/42 | 0/0 | 0/0 | N |
| 66 | 49 | F | 21/32 | 16/48 | 52/20 | 10/0 | P |
| 67 | 63 | M | 33/40 | 6/38 | 61/22 | 0 | P |
| 68 | 52 | M | 56/48 | 34/44 | 10/8 | 0/0 | N |
| 69 | 75 | M | 12/26 | 16/29 | 72/30 | 0/5 | P |
| 70 | 70 | M | 58/42 | 36/40 | 6/18 | 0/0 | N |
Association between HER2 gene
amplification and clinicopathological characteristics
The amplification rates of the HER-2 gene were 14%
(2/14), 54% (21/39) and 88% (15/17) among the highly
differentiated, moderately differentiated and in poorly
differentiated groups, respectively. Statistical differences were
observed in different degrees of differentiations (P<0.001). The
amplification rate of the HER-2 gene in the lymph node metastasis
group was higher than that of the non-lymph node metastasis group
(P<0.010); However, there were no significant differences in the
HER-2 gene positive amplification among age, sex, depth of
invasion, clinical stage and vascular infiltration status
(P>0.05; Table II).
 | Table IIRelationship between HER-2 gene
amplification and clinicopathological characteristics of patients
with esophageal squamous cell carcinoma. |
Table II
Relationship between HER-2 gene
amplification and clinicopathological characteristics of patients
with esophageal squamous cell carcinoma.
| | HER-2
amplification, n (%) | |
|---|
| Variable | Case | Positive | Negative | χ2 | P-value |
|---|
| Sex | | | | 0.032 | 0.544 |
|
Male | 54 | 29(54) | 25(46) | | |
|
Female | 16 | 9(56) | 7(44) | | |
| Age (years) | | | | 0.048 | 0.508 |
|
<60 | 36 | 20(56) | 16(44) | | |
|
≥60 | 34 | 18(53) | 16(47) | | |
|
Differentiation | | | | 16.925 | <0.001 |
|
Well | 14 | 2(14) | 12(86) | | |
|
Moderate | 39 | 21(54) | 18(46) | | |
|
Poor | 17 | 15(88) | 2(12) | | |
| Depth of
invasion | | | | 1.228 | 0.193 |
|
T1-2 | 30 | 14(47) | 16(53) | | |
|
T3-4 | 40 | 24(60) | 16(40) | | |
| Lymph node
metastasis | | | | 6.557 | 0.010 |
|
Yes | 41 | 17(41) | 24(59) | | |
|
No | 29 | 21(72) | 8(28) | | |
| pTNM | | | | 2.256 | 0.105 |
|
I, II | 46 | 22(48) | 24(52) | | |
|
III, IV | 24 | 16(67) | 8(33) | | |
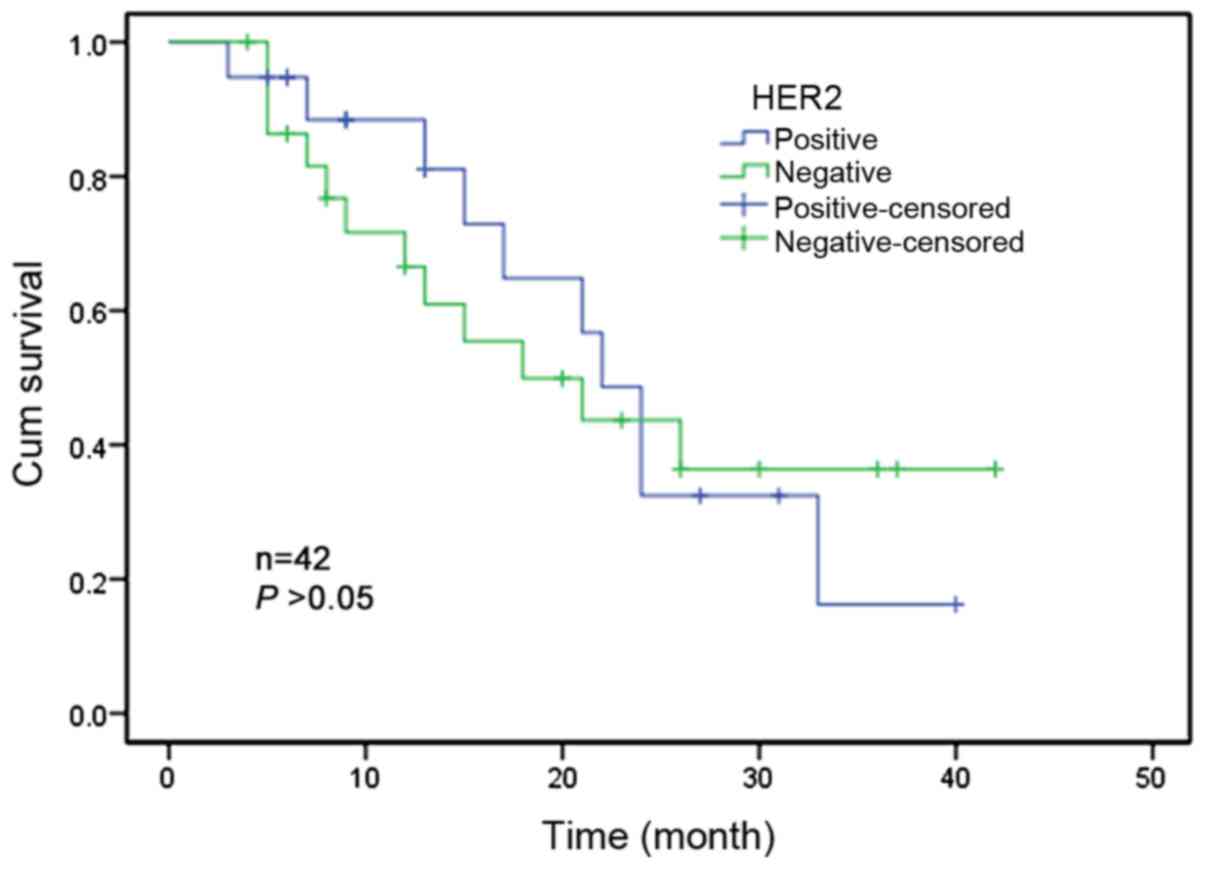
Association between HER2 gene
amplification and survival
Only 42 patients had complete follow-up records out
of the total of 70 patients. The follow-up period was 36 months. At
the time of the statistics, 22 patients had succumbed to the
disease and 20 patients remained alive. Kaplan-Meier univariate
survival analysis demonstrated that there was no significant
association between HER-2 gene amplification and the survival of
ESCC patients (P>0.05; Fig.
2).
Discussion
With the development of cancer molecular biology,
targeted therapy has entered the clinical treatment stage for
various cancer types, to provide an effective treatment for
patients with malignant tumors, including esophageal cancer
(12-14).
A large number of clinical studies have demonstrated that HER-2 is
a therapeutic target for breast cancer and the efficacy of
herceptin for treating patients with HER-2-positive breast cancer
is significantly better than conventional chemotherapy, and this
consensus has been reached in the treatment of breast cancer
(15).
Studies have shown that the HER-2 gene is associated
with the histological types, lymph node metastasis and prognosis of
various malignant tumors (16). In
the past few years, a large number of studies on HER-2 gene
amplification or protein overexpression in epithelial malignant
tumors such as gastric cancer, lung cancer and breast cancer have
been published (17-20).
However, there are few reported studies examining HER-2 gene
amplification in esophageal cancer. In the present study, HER-2
amplification was examined in ESCC specimens of Kazakh populations,
then the association between HER-2 amplification and
clinicopathological characteristics of ESCC was analyzed. The aim
of the study was to provide theoretical evidence for the targeted
therapy of esophageal cancer.
Among the studies of esophageal cancer in different
regions and ethnicities, the HER2 gene amplification rates have
been recorded as 6.5-30% (21-22). In the present
study, the HER-2 gene amplification rate was 54.2% in Kazakh
patients, which was significantly higher than that reported in
other nationalities. Whether this is related to the life habits of
Kazakhs, for example heavy drinking, smoking, eating large
quantities of smoked horse meat, high-salt diet, low vitamin
consumption or other esophageal cancer-inducing factors or
ethnicity is unclear, and this needs further study for
verification.
There are also different conclusions about the
association between HER-2 gene amplification and
clinicopathological characteristics of ESCC. By using FISH,
Reichelt et al (23)
detected that the HER-2 gene amplification levels in ESCC had no
association with clinicopathological features. Zhan et al
(24) reported that the HER-2
amplification was related to the differentiation and staging of
cancer tissues. The results of the present study showed that there
were significant differences in the degree of differentiations and
lymph node metastasis in ESCC. Niemiec et al found that
HER-2 promotes the infiltration and/or metastasis of tumor cells by
increasing the secretion of matrix metalloproteinase and changing
the tissue structure (25). The
current study suggests that the HER-2 gene was closely associated
with the occurrence and development of esophageal cancer and that
HER-2 gene expression indicated poor prognosis of esophageal cancer
to some extent. However, the Kazakh people are a nomadic people,
most of them living in mountain areas and without a stable phone
number, so the proportion of lost follow-up is a little bit high,
even though the follow-up rate was 60%, so these conclusions need
to be further confirmed by larger samples study. Besides, there was
no significant association between HER-2 gene amplification and
clinicopathological parameters such as sex, age, depth of invasion
and clinical stage. In addition, the amplification of the HER-2
gene was not related to the survival period of patients, and this
result may be due to such factors including the limited numbers of
samples, different geographical regions and targeted population.
The amplification of the HER-2 gene may be associated with the
following factors: i) The number of follow-up patients is small;
ii) The target population of the living area and the ethnicity
varied; iii) The regional economic development level, medical
conditions, diagnosis, treatment, patients' education level and
positive treatment views.
In conclusion, the amplification rate of HER-2 in
patients of Kazakh nationality with ESCC is significantly higher
than that of other nationalities, and it is related to the degree
of cancer differentiation and lymph node metastasis. The HER-2
amplification rate may have potential clinical significance in the
prognostic evaluation of esophageal cancer, and it can also be used
as a potential target therapy. But there are still some limitations
in our study, because the manuscript was focus at the association
between HER2 amplification and clinicopathological parameters and
prognosis of esophageal cancer, and the association between HER2
expression and esophageal cancer pathological parameters has been
reported in many literatures, so we only analyze the amplification
level under FISH detection. However, the evidence behind HER-2
amplification as an independent prognostic factor for patients with
esophageal cancer is still insufficient, this needs to be further
validated by large-sample, multi-center and long-term follow-up
studies.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of Xinjiang Uygur Autonomous Region (grant no.
2016D01C264).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AM and EA was designed the current study, performed
the experiments, drafted the manuscript and collected various
clinical data, including clinicopathological and patient follow-up
data. JA was analyzed and interpreted the data, performed
statistical analysis and assisted with the experiments. MN and ZL
provided study materials, revised the manuscript critically and
designed the experiments of the current study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by The Ethics
Committee of The First Affiliated Hospital of Xinjiang Medical
University, and written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng R, Zeng H, Zhang S and Chen W:
Estimates of cancer incidence and mortality in China, 2013. Chin J
Cancer. 36(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gupta B and Kumar N: Worldwide incidence,
mortality and time trends for cancer of the oesophagus. EurJ Cancer
Prev. 26:107–118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou YX, Zhou KM, Liu Q, Wang H, Wang W,
Shi Y and Ma YQ: The effect of glut1 and c-myc on prognosis in
esophageal squamous cell carcinoma of Kazakh and Han patients.
Future Oncol. 14:1801–1815. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim T, Grobmyer SR, Smith R, Ben-David K,
Ang D, Vogel SB and Hochwald SN: Esophageal cancer: The five year
survivors. J Surg Oncol. 103:179–183. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sagara Y, Kamada Y, Yamamoto Y, Tanaka M,
Kubo M, Yamaguchi R, Nishimura R and Mitsuyama S: Study on the
state of implementation of HER2 testing and positive ratios in
patients with breast cancer in the Kyushu-Okinawa region of Japan.
Breast Cancer. 19:315–320. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rosińska A, Rosiński G and Hołubowicz R:
Advdnces on the HER-2/neu and oncotherapy. Acta Academiae Medicinae
Cpaf 62, 2008.
|
|
7
|
Halling KC and Kipp BR: Fluorescence in
situ hybridization indiagnostic cytology. Surg Oncol Clin N Am.
18:411–422. 2009.
|
|
8
|
Fritcher EG, Brankley SM, Kipp BR, Voss
JS, Campion MB, Morrison LE, Legator MS, Lutzke LS, Wang KK, Sebo
TJ and Halling KC: A comparison of conventional cytology,DNA ploidy
analysis,and fluorescence in situ hybridization for the detection
of dysplasia and adenocarcinoma in patients with barrett's
esophagus. Hum Pathol. 39:1128–1135. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dietel M, Ellis IO, Höfler H, Kreipe H,
Moch H, Dankof A, Kölble K and Kristiansen G: Comparison of
automated silver enhanced in situ hybridisation (SISH) and
fluorescence ISH (FISH) for the validation ofHER2gene status in
breast carcinoma according to the guidelines of the American
society of clinical oncology and the college of American
pathologists. Virchows Arch. 451:19–25. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Amin MB, Edge S, Greene F, (eds), et al:
AJCC Esophageal and esophagogastric junction. AJCC Cancer Staging
Manual. 8th edition. NewYork, Springer; pp185-202: 2017.
|
|
11
|
Huang JX, Zhao K, Lin M, Wang Q, Xiao W,
Lin MS, Yu H, Chen P and Qian RY: HER2 gene amplification in
esophageal squamous cell carcinoma is less than in gastroesophageal
junction and gastric adenocarcinoma. Oncol Lett. 6:13–18.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shroff GS, De Groot PM,
Papadimitrakopoulou VA, Truong MT and Carter BW: Targeted therapy
and immunotherapy in the treatment of non-small cell lung cancer.
Radiol Clin North Am. 56:485–495. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Alvarez RH, Valero V and Hortobagyi GN:
Emerging targeted therapies for breast cancer. J Clin Oncol.
28:3366–3379. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin CY, Yang SJ, Peng CL and Shieh MJ:
Panitumumab-Conjugated and platinum-cored pH-sensitive apoferritin
nanocages for colorectal cancer-targeted therapy. ACS Appl Mater
Interfaces. 21:6096–6106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vu T, Sliwkowski MX and Claret FX:
Personalized drug combinations to overcome trastuzumab resistance
in HER2-positive breast cancer. Biochim Biophys Acta. 1846:353–365.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gutierrez CC and Schiff RR: HER2: Biology,
detection, and clinical implications. Arch Pathol Lab Med.
135:55–62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yan SY, Hu Y, Fan JG, Tao GQ, Lu YM, Cai
X, Yu BH and Du YQ: Clinicopathologic significance of HER-2/neu
protein expression and gene amplification in gastric carcinoma.
World J Gastroenterol. 17:1501–1506. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li HH, Ma F, Zeng X, Wang JY, Yuan P, Fan
Y and Xu BH: Comparison of fluorescence in situ hybridization and
immunohistochemistry assessment for Her-2 status in breast cancer
and its relationship to clinicopathological characteristics.
Zhonghua Yi Xue Za Zhi. 91:76–80. 2013.(In Chinese).
|
|
19
|
Koo JS and Kim SH: EGFR and HER-2 status
of non-small cell lung cancer brain metastasis and corresponding
primary tumor. Neoplasma. 58:27–34. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bouché O and Penaultllorca F: HER-2 and
gastric cancer: A novel therapeutic target for trastuzumab. Bull
Cancer. 97:1429–1440. 2010.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
21
|
Sato-Kuwabara Y, Neves JI, Fregnani JH,
Sallum RA and Soares FA: Evaluation of gene amplification and
protein expression of HER-2/neu in esophageal squamous cell
carcinoma using fluorescence in situ hybridization (FISH) and
immunohistochemistr. BMC Cancer. 7(9)2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wei Q, Chen L, Sheng L, Nordgren H, Wester
K and Carlsson J: EGFR, HER-2 and HER3 expression in esophageal
primary tumours and corresponding metastases. Int J Oncol.
31:493–499. 2007.PubMed/NCBI
|
|
23
|
Reichelt U, Duesedau P, Tsourlakis MC,
Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A,
et al: Frequent homogeneous HER-2 amplification in primary and
metastatic adenocarcinoma of the esophagus. Mod Pathol. 20:120–129.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhan N, Dong WG, Tang YF, Wang ZS and
Xiong CL: Analysis of HER-2 gene amplification and protein
expression in esophageal squamous cell carcinoma. Med Oncol.
29:933–940. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Niemiec JA, Adamczyk A, Małecki K,
Majchrzyk K and Ryś J: Relationships between immunophenotype, Ki-67
index, microvascular density, Ep-CAM/P-cadherin, and MMP-2
expression in early-stage invasive ductal breast cancer. Appl
Immunohistochem Mol Morphol. 20:550–560. 2012.PubMed/NCBI View Article : Google Scholar
|