Introduction
Myopericytoma is a rare type of benign tumor, named
by Granter in 1998(1) and
classified by the World Health Organization in 2002(2). This type of tumor is derived from
perivascular myoid cells and shares features with both smooth
muscle and glomus cells (3). The
most commonly affected sites of myopericytoma are the skin and soft
tissues of the lower extremities and upper extremities; however, it
can also occur in the intracranial space, nose, kidney, and urinary
tract (4-8).
Reports of myopericytoma occurrence in the liver are extremely
rare. Chen and Liang (9) reported a
case of myopericytoma, which occurred in the area between the liver
and stomach in 2017, while Mannan et al (10) reported a case of myopericytoma,
which was identified during surgery on segment IV of the liver and
was 1.0 cm in diameter without imaging data in 2016. Due to the
limited number of cases reported, the preoperative diagnosis of
liver myopericytoma is difficult, and could be mistaken for other
types of neoplasms, which occur in the liver, such as intrahepatic
cholangiocarcinoma or liver metastasis. In the present report, a
case of multiple myopericytoma, which occurred in the liver with a
maximum diameter of 4.5 cm has been described, and the accompanying
contrast-enhanced computed tomography (CT) scan and positron
emission tomography/CT (PET/CT) scan images are also included to
further discuss the imaging features and preoperative differential
diagnosis of myopericytoma in the liver.
Case report
The patient was a 55-year-old female who presented
with right upper quadrant tenderness for 2 weeks and was
hospitalized at the Hebei Medical University 4th Hospital in
December 2018. A non-enhanced CT scan acquired at another local
hospital prior to hospitalization showed two low-density lesions
adjacent to each other on segments IV and VIII of the liver,
suggesting a malignant tumor. Physical examination revealed no
palpable abdominal mass and positive right upper quadrant
tenderness without rebound tenderness. After being hospitalized,
the patient's laboratory blood tests showed no abnormal blood
routine results, coagulation function or liver function. Blood
tumor markers, including α-fetoprotein (AFP), carcinoembryonic
antigen (CEA), cancer antigens (CA)-19-9, -72-4 and -125, were all
within normal ranges (<10, <3 ng/ml, 37, <7 and <46
U/ml, respectively) and antibodies (Ab) against hepatitis A virus
(HAV) and hepatitis C virus (HCV) were negative. The blood tests
for hepatitis B virus (HBV) showed positive results for HBeAb and
HBcAb and negative results for HBsAg, HBsAb and HBeAg.
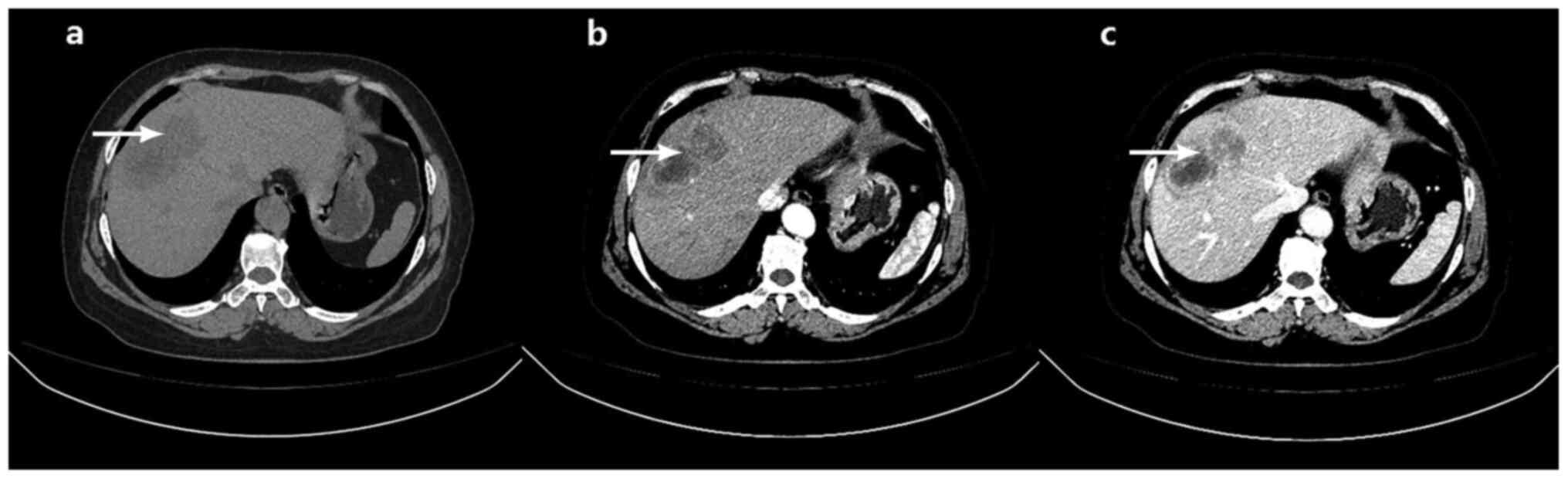
A contrast-enhanced CT scan was performed (Fig. 1), which was identical to the
previous CT scan, in which the non-enhanced phase revealed two
adjacent low-density lesions, with diameters of 3.5 and 4.5 cm on
segments IV and VIII of the liver, respectively. In the arterial
phase, the edges of the two lesions were enhanced, but the centers
were not. In the portal venous phase, the enhancement at the edge
was markedly stronger, but the center remained unenhanced.
Furthermore, a narrow low-density area could be observed between
the lesions and normal liver tissue in the portal venous phase,
showing a clear tumor boundary (Fig.
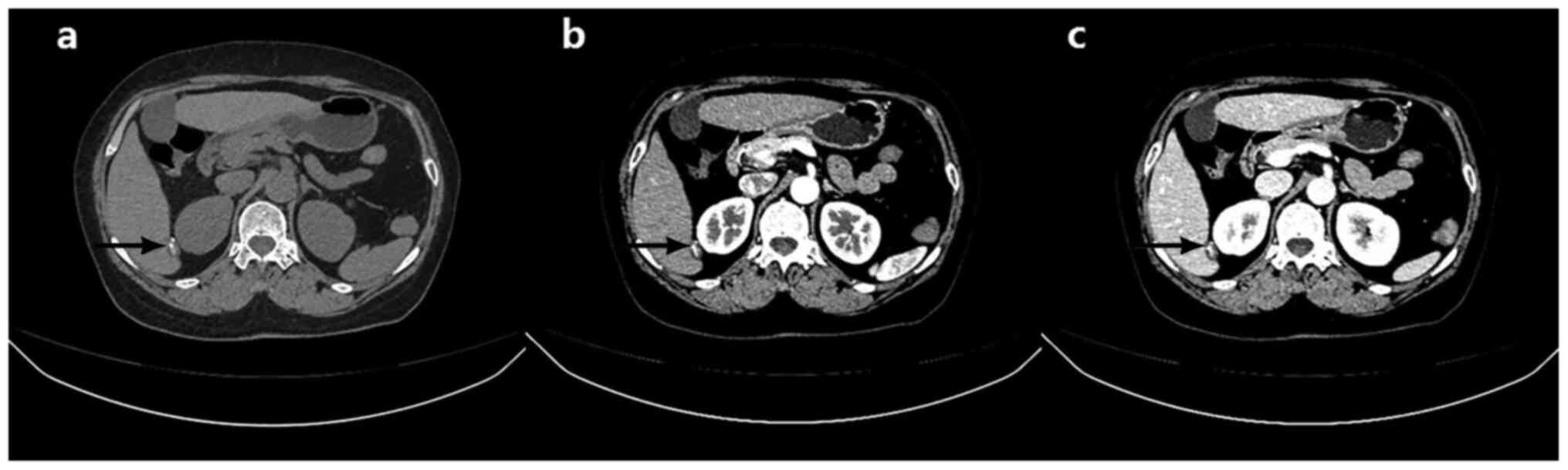
1). In addition, a high-density nodule was found on the edge of
segment VI and was considered to be a calcification (Fig. 2).
As the diagnosis was not definitive based on the CT
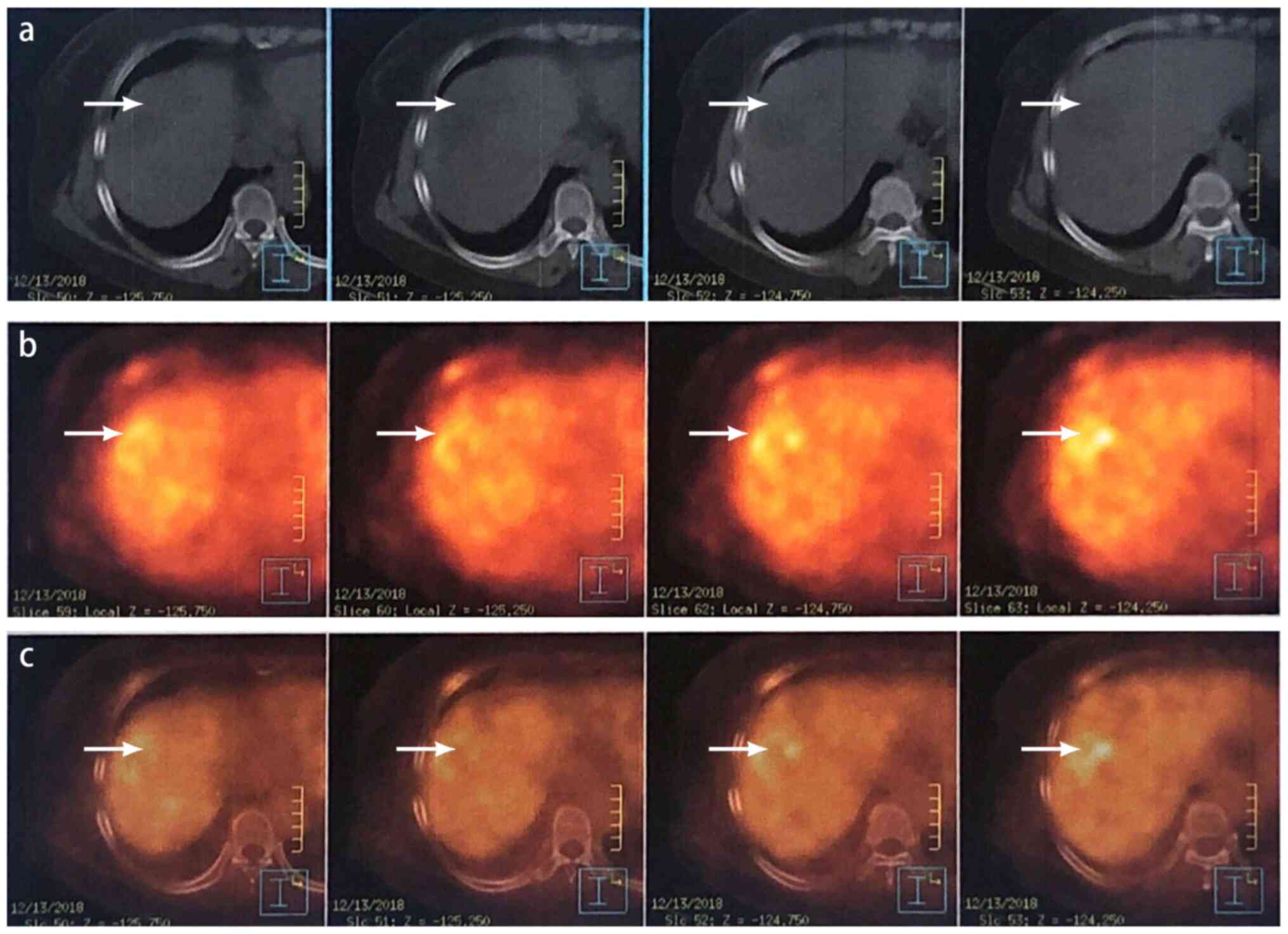
images alone, a PET/CT was subsequently performed, which revealed
consistent results with the CT findings. The PET/CT scan revealed
low-density lesions on segments IV and VII of the liver, with an
increased glucose metabolism rate [early standard uptake value
(SUV)max 4.4, delay SUVmax 5.8] (Fig. 3). The nodule on segment VI revealed
similar characteristics, and all lesions were considered to be
malignant.
To rule out the possibility of metastatic liver
tumors, gastroscopy and colonoscopy were also performed. No lesions
were found during gastroscopy, although a polyp was found in the
sigmoid colon using colonoscopy and was confirmed to be chronic
mucosal inflammation using pathological examination by a
pathologist from Department of Pathology at Hebei Medical
University 4th Hospital. The following method was used for
immunohistochemistry staining: Samples were fixed in 10% neutral
buffered formalin at room temperature for 24 h and cut into 1x1 cm
sections. Slides were incubated with the primary antibodies (SMA,
cat. no. kit-0006; 1:500; vimentin, cat. no. mab-0139; 1:500; CD34,
cat. no. kit-0004; 1:100; CD31, cat. no. mab-0031; 1:500; desmin,
cat. no. kit-0023; 1:2,000; HMB45, cat. no. mab-0098; 1:1,000;
AE1/AE3, cat. no. kit-0009; 1:100; CK-7, cat. no. kit-0021;
1:1,000; Ki67, cat. no. kit-0005; 1:500; MART-1, cat. no. mab-0275;
1:1,000; glypican-3, cat. no. kit-0036; 1:100 and Hep-1, cat. no.
mab-0249; 1:200) overnight at 4˚C, washed using 0.05% TBS-Tween-20
and then incubated with secondary antibodies and DAB using a kit
(cat. no. TT-0801; prediluted) for 45 min (all Fuzhou Maixin
Biotech Co., Ltd.). A light microscope was used for observation at
x200 magnification. Positive staining was determined by the
pathologist, as there are no guidelines for IHC staining of
myopericytoma.
Following the aforementioned examinations, the
diagnosis indicated a primary malignant tumor of the liver and a
partial hepatectomy was performed. The tumor on segments IV and
VIII and the nodule on segment VI were completely excised. The
tumor on segments IV and VIII had no clear capsule, and the section
was tough and gray. The nodule on segment VI was hard, and
calcifications were found on the tumor section. Pathological
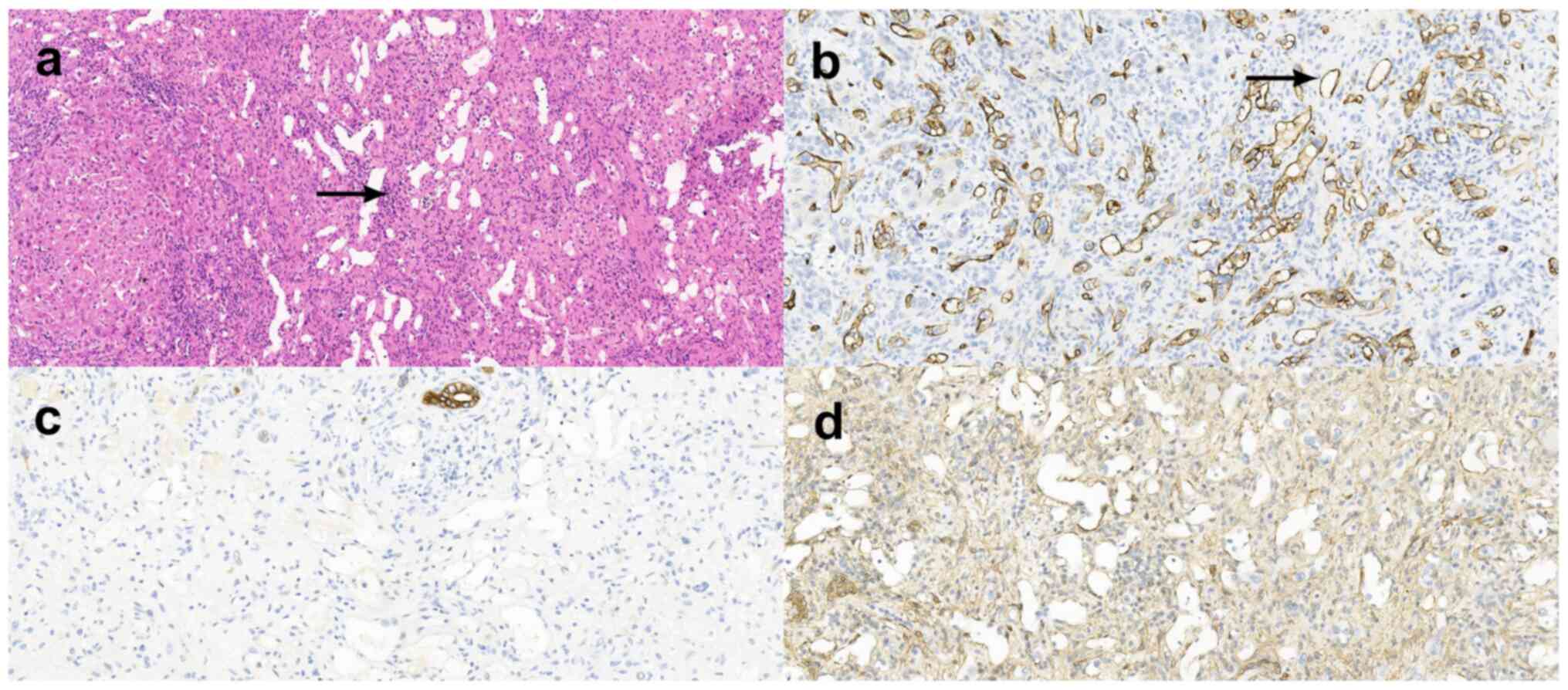
examination revealed blood vessels scattered throughout the tumor.
Spindle myoid tumor cells with eosinophilic cytoplasm were
concentrated around the blood vessels or were found to be arranged
in spirals or bundles in some areas, as indicated by the arrow
(Fig. 4A). Immunohistochemical
results showed that the tumor was positive for smooth muscle actin
(Fig. 4D), vimentin, CD-34
(Fig. 4B) and -31 (within the blood
vessel) and negative for desmin, human melanoma black 45,
cytokeratin (CK) AE1/AE3, CK7 (Fig.
4C), Ki67 (positive rate, 1%), melanoma-associated antigen
recognized by T cells 1, glypican-3, and Hep-1. All the lesions on
segments IV, VI and VIII were confirmed to be myopericytomas from
the aforementioned pathological examination. The patient was
discharged 9 days following surgery, with no complications.
Discussion
Myopericytoma is a rare type of tumor that primarily
occurs on the four limbs (6), and
can occur in individuals of all ages but it is more commonly found
in middle-aged men (>50 years of age), with no symptoms
(3,6,9).
Reports of myopericytoma occurring in the abdominal organs,
particularly the liver, are extremely rare. Moreover, no imaging
profiles of liver myopericytoma have been reported thus far.
Therefore, the differential diagnosis of liver myopericytoma could
be difficult prior to surgery, even with contrast-enhanced CT and
PET/CT images. The imaging results from the present study can
appear similar to those of several types of liver neoplasms,
including hepatocellular carcinoma (HCC), hepatic hemangioma,
intrahepatic cholangiocarcinoma and liver metastatic tumor. Thus,
in the present report, the preoperative differential diagnosis of
liver myopericytoma based on the imaging profile and laboratory
examinations was difficult.
According to previous reports, the contrast-enhanced
CT images of myopericytoma generally have a similar pattern. The
tumors appear to be low-density lesions in the non-enhanced phase;
however, the peripheral area of the tumor could be enhanced,
showing rim-like enhancement in the arterial phase (11-16).
Small tumors are displayed as full-tumor enhancement in the
arterial phase (13). Enhancement,
in which the contrast agent fills in centripetally, can be
identified in some of the tumors over time, especially the large
ones, leading to a heterogeneous enhancement in the center in the
portal venous phase (40-50 sec) and delayed phase (100 sec to 10
min) (11,15,16).
Calcifications were also observed in some tumors (12,15,16).
The contrast-enhanced CT image in the present case report was
consistent with previous studies; however, the enhancement fill-in
was not obvious in the portal venous phase. In this instance, the
contrast-enhanced CT of the patient only included the portal venous
phase but not the delayed phase, since the typical hallmark of
liver neoplasms is similar between these two phases, therefore only
the portal venous phase is shown in Fig. 2.
Both HCC and hepatic hemangioma have unique imaging
and clinical features. Patients with HCC are known to have a
background of liver cirrhosis caused by HBV/HCV or alcohol intake
(17). Increased AFP levels
(>400 ng/ml) and the fast washout of contrast enhancement on CT
are methods to diagnose HCC (17).
For hepatic hemangioma, peripheral globular enhancement and a
centripetal fill-in pattern on contrast-enhanced CT are unique
diagnostic features (18). Liver
myopericytoma lacks all of the aforementioned imaging and clinical
characteristics, therefore the differential diagnosis is
definitive.
In addition, the differential diagnosis of
myopericytoma from intrahepatic cholangiocarcinoma and metastatic
liver tumors, identified in the present study, can be confusing as
these lesions all have peripheral rim-like enhancement on
contrast-enhanced CT. However, unique imaging features still exist
on each type of tumor.
For intrahepatic cholangiocarcinoma, peripheral
rim-like enhancement can be observed in the arterial phase in the
majority of cases, and a centripetal fill-in enhancement pattern
can be observed in the portal phase and delayed phase in some cases
(19,20). In addition, intrahepatic bile duct
dilatation proximal to the tumor and regional lymph node
enlargement can occur in some cases (19,20)
and could be the primary difference between intrahepatic
cholangiocarcinoma and liver myopericytoma. With respect to PET/CT,
previous studies have found that intrahepatic cholangiocarcinoma
had a 18F-fluorodeoxyglucose median SUVmax
uptake ranging from 8.2 to 14.4 (21-23),
which is higher compared with the SUVmax of liver
myopericytoma in the present case (SUVmax, 5.8).
Therefore, PET/CT has the potential to assist with the differential
diagnosis; however, due to the lack of reports of myopericytoma
PET/CT image profiles, the sensitivity and specificity requires
further validation.
A complete ring of enhancement in the arterial phase
of contrast-enhanced CT is the primary imaging feature of liver
metastases, and centripetal fill-in can be observed in some cases
during the portal venous phase or the delayed phase (24). As the imaging characteristics of
liver metastases are very similar to those of myopericytoma, the
differential diagnosis can be difficult if this is only based on
the image of the tumor in the liver. Therefore, identifying the
primary tumor is important for diagnosis and PET/CT could play an
important role as the primary tumor can be observed at the same
time. Gastroscopy and colonoscopy are also recommended to rule out
metastases from gastric cancer and colorectal cancer.
In the present case report, other imaging
techniques, such as MRI or contrast enhanced ultrasound examination
in addition to contrast-enhanced CT and PET/CT were not performed.
According to previously published clinical guidelines from the
European Association for the Study of the Liver and the European
Organization for Research and treatment of Cancer (17), only contrast-enhanced CT and
magnetic resonance imaging (MRI) are recommended as non-invasive
diagnostic methods for liver tumors (25). Due to the uncertainty of diagnosis
following contrast-enhanced CT, PET/CT was used to identify the
malignant nature of the tumor. However, additional imaging
examinations, such as MRI or contrast-enhanced ultrasound, might
provide beneficial imaging information for the differential
diagnosis of this rare case.
Beyond imaging techniques, biomarkers are also
important for the differential diagnosis of liver myopericytoma. At
present, there are no peripheral blood biomarkers proven to be
specific to myopericytoma. However, for intrahepatic
cholangiocarcinoma, CEA and CA19-9 could significantly increase
above the normal levels in the peripheral blood of the patients
(20), and certain biomarker
increases in the primary tumor could also be observed in patients
with metastatic liver tumors, such as CA-19-9 and CEA (26).
In conclusion, to the best of our knowledge, this is
the first case report of liver myopericytoma, with preoperative
contrast-enhanced CT and PET/CT image profiles. The characteristic
imaging results of liver myopericytoma includes peripheral rim-like
enhancement in the arterial phase and a centripetal fill-in pattern
in the portal venous phase and delayed phase, which is similar to
intrahepatic cholangiocarcinoma and liver metastases. Intrahepatic
bile duct dilatation, regional lymph node enlargement and
extrahepatic primary tumor found by imaging techniques can be
beneficial to exclude the diagnosis of liver myopericytoma prior to
surgery. Blood biomarkers, including AFP, CEA, CA19-9, could also
assist with the differential diagnosis between these tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XK and SW performed the literature research and
wrote the study. HG collected the data and image information. FL
performed pathological diagnosis and described the images. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication of this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Granter SR, Badizadegan K and Fletcher CD:
Myofibromatosis in adults, glomangiopericytoma, and myopericytoma:
A spectrum of tumors showing perivascular myoid differentiation. Am
J Surg Pathol. 22:513–525. 1998.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fletcher CDM, Unni KK and Mertens F (eds):
World Health Organization Classification of Tumours Pathology and
Genetics of Tumours of Soft Tissue and Bone. International Agency
for Research on Cancer (IARC) Press, Lyon, 2002.
|
|
3
|
Dray MS, McCarthy SW, Palmer AA, Bonar SF,
Stalley PD, Marjoniemi V, Millar E and Scolyer RA: Myopericytoma: A
unifying term for a spectrum of tumours that show overlapping
features with myofibroma. A review of 14 cases. J Clin Pathol.
59:67–73. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Numata I, Nakagawa S, Hasegawa S and Aiba
S: A myopericytoma of the nose. Acta Derm Venereol. 90:192–193.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao M, Williamson SR, Sun K, Zhu Y, Li C,
Xia W, Qi H, Wang L, Linos K and Cheng L: Benign perivascular myoid
cell tumor (myopericytoma) of the urinary tract: A report of 2
cases with an emphasis on differential diagnosis. Hum Pathol.
45:1115–1121. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mentzel T, Dei Tos AP, Sapi Z and Kutzner
H: Myopericytoma of skin and soft tissues: Clinicopathologic and
immunohistochemical study of 54 cases. Am J Surg Pathol.
30:104–113. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rousseau A, Kujas M, Van Effenterre R,
Bocht AL, Carpentier A, Leroy JP and Poirier J: Primary
intracranial myopericytoma: Report of three cases and review of the
literature. Neuropathol Appl Neurobiol. 31:641–648. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lau SK, Klein R, Jiang Z, Weiss LM and Chu
PG: Myopericytoma of the kidney. Hum Pathol. 41:1500–1504.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen Z and Liang W: Myopericytoma
occurrence in the liver and stomach space: Imaging performance. BMC
Cancer. 17(143)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mannan AASR, McGinty J and Theise N:
Myopericytoma of the Liver. Am J Clin Pathol. 146 (Suppl
1)(S246)2016.
|
|
11
|
Wu F, Sun J, Dong J, Wang X and Gao Q:
Management of multicentric myopericytoma in the maxillofacial
region: A case report. Case Rep Oncol. 6:350–355. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Prado-Calleros HM, Galarza-Lozano D,
Arrieta-Gómez JR, Pombo-Nava A, Parraguirre-Martínez S and
Gutiérrez CJ: Myopericytoma arising adjacent to the common carotid
artery: Case report and systematic review of deep located neck
myopericytomas. Head Neck. 38:E2479–E2482. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nagai T, Kamimura T, Itou K, Fujii M,
Tsukino H, Mukai S, Akiyama Y, Kataoka H and Kamoto T:
Myopericytoma in urinary bladder: A case report. J Med Case Rep.
11(46)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chu ZG, Yu JQ, Yang ZG, Zhu ZY and Yuan
HM: Myopericytoma involving the parotid gland as depicted on
multidetector CT. Korean J Radiol. 10:398–401. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cao JH, Xu JP, Li YC, Lai J and Li Q:
Pulmonary myopericytoma: A case report and review of the
literatures. Chin Med J (Engl). 122:755–757. 2009.PubMed/NCBI
|
|
16
|
Zhang Z, Yu D, Shi H and Xie D: Renal
myopericytoma: A case report with a literature review. Oncol Lett.
7:285–287. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
European Association for Study of Liver
and European Organisation for Research and Treatment of Cancer.
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. Eur J Cancer. 48:599–641. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Quinn SF and Benjamin GG: Hepatic
cavernous hemangiomas: Simple diagnostic sign with dynamic bolus
CT. Radiology. 182:545–548. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Valls C, Gumà A, Puig I, Sanchez A, Andía
E, Serrano T and Figueras J: Intrahepatic peripheral
cholangiocarcinoma: CT evaluation. Abdom Imaging. 25:490–496.
2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bridgewater J, Galle PR, Khan SA, Llovet
JM, Park JW, Patel T, Pawlik TM and Gores GJ: Guidelines for the
diagnosis and management of intrahepatic cholangiocarcinoma. J
Hepatol. 60:1268–1289. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Albazaz R, Patel CN, Chowdhury FU and
Scarsbrook AF: Clinical impact of FDG PET-CT on management
decisions for patients with primary biliary tumours. Insights
Imaging. 4:691–700. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee SW, Kim HJ, Park JH, Park D I, Cho YK,
Sohn CI, Jeon WK and Kim BI: Clinical usefulness of 18F-FDG PET-CT
for patients with gallbladder cancer and cholangiocarcinoma. J
Gastroenterol. 45:560–566. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Petrowsky H, Wildbrett P, Husarik DB, Hany
TF, Tam S, Jochum W and Clavien PA: Impact of integrated positron
emission tomography and computed tomography on staging and
management of gallbladder cancer and cholangiocarcinoma. J Hepatol.
45:43–50. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sica GT, Ji H and Ros PR: CT and MR
imaging of hepatic metastases. Am J Roentgenol. 174:691–698.
2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Galle PR, Forner A, Llovet JM, Mazzaferro
V, Piscaglia F, Raoul JL, Schirmacher P and Vilgrain V: EASL
Clinical Practice Guidelines: Management of hepatocellular
carcinoma. J Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF and Bast RC Jr:
ASCO. ASCO 2006 update of recommendations for the use of tumor
markers in gastrointestinal cancer. J Clin Oncol. 24:5313–5327.
2006.PubMed/NCBI View Article : Google Scholar
|