Introduction
Intimal sarcoma of pulmonary artery (PAIS) is a rare
disease which has a poor prognosis (1). PAIS is often diagnosed as advanced
disease and has a high rate of postoperative recurrence. Although
therapeutic options for PAIS are limited, several recent studies
have reported that pazopanib, a multi-kinase inhibitor that targets
vascular endothelial growth factor receptor (VEGFR)-1, -2, -3,
platelet-derived growth factor (PDGF) receptor (PDGFR)-α, -β and
stem cell factor receptor (c-Kit), shows activity in treatment for
advanced vascular sarcomas, including PAIS (2-5).
However, these previous reports did not investigate the
relationship between the levels of PDGFR and VEGFR in tumor tissues
and the response to pazopanib. Our present report provides new
information in this regard, as we studied this relationship in two
cases of PAIS.
Case report
Case 1
A 33-year-old man visited a local hospital with a
chronic cough. A CT scan showed an intravascular lesion of the left
pulmonary artery and multiple masses in the left lung. He underwent
pneumonectomy of the left lung for tumor embolism and angioplasty
of the left pulmonary artery. In addition to the clinical course,
immunohistochemical analysis showed spinal tumor cells were
positive for murine double minute 2 (MDM2) and CD34, which led to
the final diagnosis of PAIS. Because the margin of the left
pulmonary artery base was positive for tumor cells, adjuvant
irradiation (60 Gy/30 fr) and adjuvant chemotherapy with
doxorubicin and ifosfamide (60/7,500 mg/m2/every 3
weeks, 1 cycle, AI therapy) were performed. However, recurrence was
observed 2 months later. Pazopanib treatment was initiated and this
stabilized the disease for 5.8 months, which is superior to the 2
months achieved with AI therapy. Subsequently, eribulin was
administered, but a CT scan on day 14 of the 1st cycle showed rapid
tumor growth, which was considered as flare-up upon the
discontinuation of pazopanib. The patient died 2 months after the
discontinuation of pazopanib, a total of 18 months after the
diagnosis (Table I).
 | Table IMain characteristics and patient
outcomes. |
Table I
Main characteristics and patient
outcomes.
| | Sequential
therapy | Immunohistochemical
staining | |
|---|
| Case | Age | Sex | Metastatic
lesion | Resection | Adjuvant therapy | RFS (months) | Regimen | Best response | PFS (months) | OS (months) | PDGFR-α | PDGFR-β | VEGFR-2 | PFS (months) |
|---|
| 1 | 33 | Male | Lung | R1 | RT + AI | 7.5 | 1st: Pazopanib | SD | 5.8 | 18.0 | Moderate | Strong | Negative | 5.8 |
| | | | | | | | 2nd: Eribulin | PD | 0.4 | | Positive | Positive | | |
| 2 | 44 | Female | Lung | R2 | - | 3.1 | 1st: Doxorubicin | SD | 4.7 | 14.3 | Weak | Weak | Negative | 1.1 |
| | | | | | | | 2nd: Eribulin | SD | 1.0 | | Positive | Positive | | |
| | | | | | | | 3rd: Pazopanib | SD | 1.1 | | | | | |
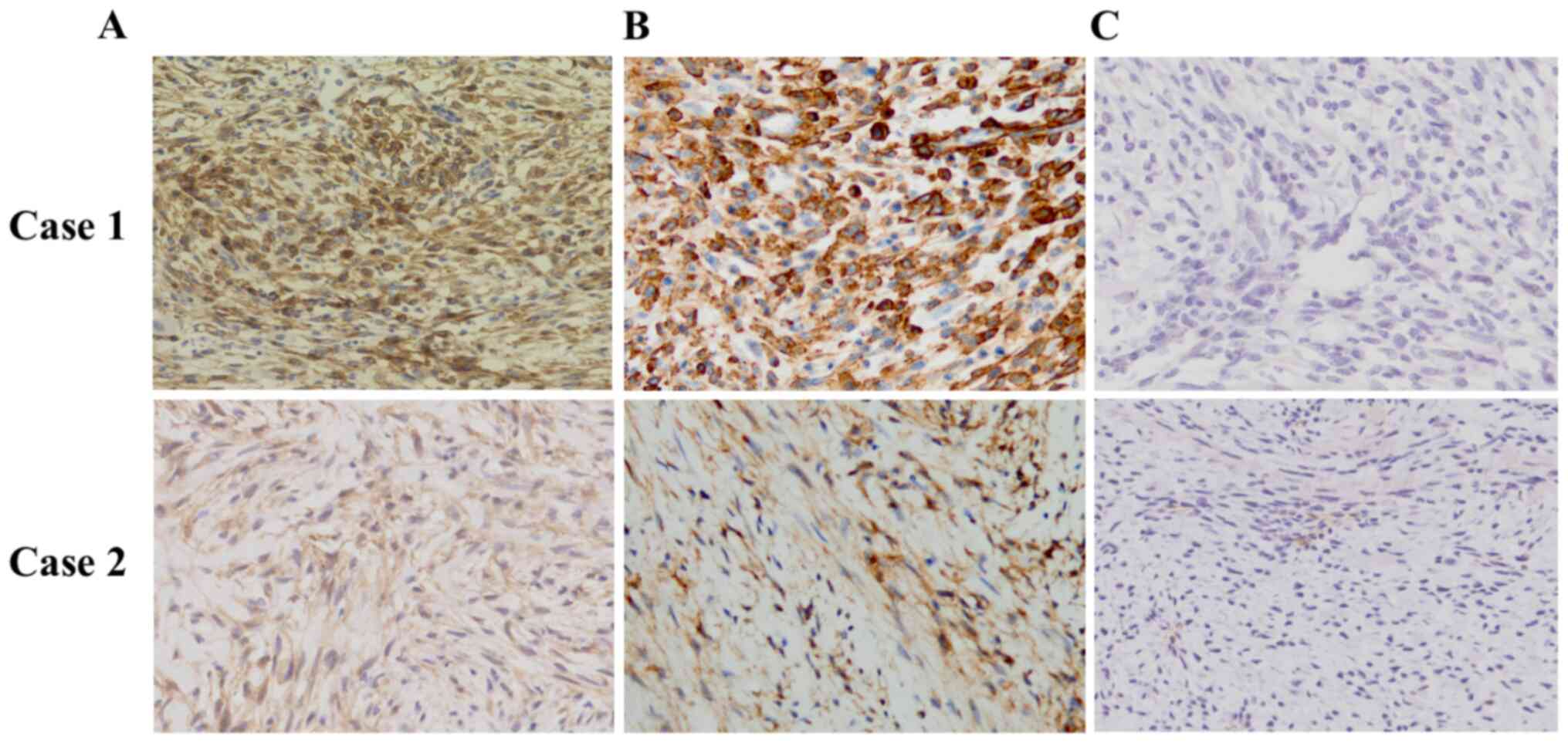
Immunohistochemistry revealed that tumor cells were
moderately and strongly positive for PDGFR-α (Cell Signaling
Technology, Inc.; cat. no. 3174; Fig.
1A) and PFGFR-β (Abcam; cat. no. ab32570; Fig. 1B), respectively, but negative for
VEGFR-2 (Cell Signaling Technology, Inc.; cat. no. 2479; Fig. 1C).
Case 2
A 44-year-old woman visited a local hospital with
dyspnea on exertion, and a CT scan revealed an intravascular lesion
of the left pulmonary artery and a mass in the right lung. She
underwent right upper lobectomy and angioplasty of the left
pulmonary artery. During postoperative recovery and preparation of
therapy for the positive tumor cells at the resection margins, lung
metastases developed 3 months after the surgery. Subsequently,
palliative chemotherapy with doxorubicin (60 mg/m2/every
3 weeks, 6 cycles) followed by eribulin (1.4 mg/m2/every
3 weeks, 2 cycles) were administered sequentially, but neither
treatments induced a response. Nine months after the diagnosis,
pazopanib was initiated as a 3rd-line palliative pharmacotherapy.
However, CT scans one month after the start of pazopanib revealed
an increased tumor size. The patient died 14.3 months after the
original diagnosis (Table I).
Immunohistochemistry revealed that the tumor cells
were weakly positive for PDGFR-α (Fig.
1A) and PDGFR-β (Fig. 1B), and
negative for VEGFR-2 (Fig. 1C).
Immunostaining was performed according to the following protocol.
Paraffin sections of tumor tissues of pulmonary artery were
subjected to immunohistochemical staining for the PDGFR-α,-β,
VEGFR-2. At first, blocked specimen in blocking buffer for 1 h at
room temperature. Blocking solution was removed and diluted primary
antibodies were added (PDGFR-α: Cell Signaling Technology cat. no.
3174; PDGFR-β: Abcam cat. no. ab32570; VEGFR-2: Cell Signaling
Technology cat. no. 3174) and incubated overnight at 4˚C. Antibody
solution was removed and the incubated specimen was mixed with
fluorochrome-conjugated secondary antibody for 1-10 min at room
temperature.
Discussion
Through two cases of PAIS, the relationship between
the expression of PDGFR or VEGFR and the response to pazopanib was
investigated (Table I). In case 1,
the tumor cells were strongly positive for PDGFR-β (Fig. 1B) and moderately positive for
PDGFR-α (Fig. 1A). Time to
progression before pazopanib was two months, but pazopanib
treatment stabilized the tumor for 5.8 months. In the second case,
the tumor cells were weakly positive for PDGFR-α (Fig. 1A) and PDGFR-β (Fig. 1B), which may explain the reduced
response to treatment. Time to progression on pazopanib treatment
was 1.1 months, while it took 3 months to progress after the
surgery. Both cases were negative for expression of VEGFR-2
(Fig. 1C). Focusing on these two
cases, high levels of PDGFR in tumor tissues might have contributed
to an improved response to pazopanib treatment.
Compared to placebo, pazopanib significantly
improved PFS (median PFS, 4.6 months vs. 1.6 months) in patients
with metastatic non-adipocytic soft-tissue sarcoma (6). According to a European Organization
for Research and Treatment of Cancer (EORTC) retrospective analysis
of pazopanib in patients with advanced vascular sarcoma, the median
PFS and OS were 3.0 and 9.9 months, respectively. In this analysis,
among the 40 patients with vascular sarcoma, 2 (3.8%) had intimal
sarcomas and both of these patients demonstrated a partial response
(2). Unfortunately, the number of
PAIS cases in this previous report was too low to investigate the
relationship between PDGFR and VEGFR expression in tumor tissue and
the response to pazopanib. To the best of our knowledge, this is
the first study to demonstrate the relationships between PDGFR and
VEGFR expression and the response to pazopanib.
Although pazopanib is a multi-kinase inhibitor
(1), our results suggest that the
activity against PDGF may account for its activity in PAIS. This is
consistent with the role of PDGFs in other malignancies. PDGFs bind
to PDGFR-α and -β, and trigger cell proliferation, migration and
differentiation. In humans, abnormal PDGF/PDGFR signaling has been
associated with numerous malignant tumors, including activating
mutations of PDGFR-α in gastrointestinal stromal tumors and the
overexpression of PDGFR-β in dermatofibrosarcoma protuberans
(7,8). In addition to hematological tumors,
PDGFR-β alterations are also involved in myofibromas, suggesting an
oncogenic role in mesenchymal tumors (9). Amplifications of PDGFR-α, PDGFR-β,
c-Kit, MDM2 and epidermal growth factor receptor (EGFR) have been
seen in intimal sarcoma (7,9-12).
Alterations in the PDGF/PDGFR signaling pathway, particularly
PDGFR-α and PDGFR-β, may play an important role in the pathogenesis
of intimal sarcoma (9,10), with amplification and co-activation
of both PDGFR-α and PDGFR-β being observed frequently in intimal
sarcoma, whereas KIT levels were similar to those in normal tissue.
Consistent with the above, PDGFR, EGFR and MDM2 have all been
proposed as therapeutic targets in intimal sarcoma (9,10).
Although early clinical trials have shown that the anti-PDGFR
antibody olaratumab has antitumor activity in advanced soft-tissue
sarcoma (13,14), a recent phase III study failed to
demonstrate an additional effect of olaratumab when combined with
doxorubicin in soft tissue sarcoma (15). However, PAIS was not included in the
phase III study, and the role of PDGFR inhibitors in PAIS warrants
further investigation. We did not observe expression of VEGFR-2 in
our patients, and no previous reports have found raised levels of
VEGFR in intimal sarcoma. We thus infer that VEGFR does not play an
important role in PAIS pathogenesis.
Interpretation of our present study is limited, as
the small case number precludes statistical analysis. Furthermore,
in case 1, pazopanib was used as a first-line treatment, whereas in
case 2, it was used as a third-line treatment; this does not allow
a simple comparison of PFS in order to evaluate pazopanib efficacy.
In addition, factors other than PDGFR and VEGFR expression are
likely to determine the response to pazopanib. Allowing for these
limitations, however, the strength of this study is that we
evaluate the relationship between PDGFR expression and the
therapeutic effect of pazopanib, in contrast to previous studies.
In future studies, the relationship between the expression levels
of PDGFR expression and the response to pazopanib needs to be
verified with a larger sample population.
In conclusion, the present data suggest that PDGFR
expression might be a potential predictor for the prediction of
pazopanib efficacy.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YI and SS made substantial contributions to the
conception and design of the study. YH, KT and HS made substantial
contributions to the acquisition of the data. SS drafted the
manuscript. YI, NK, MT, YF, NC, YH, KT, HS, NJ and HM made
substantial contributions to the analysis and interpretation of the
data and were involved in revising the manuscript critically for
important intellectual content. NJ performed the
immunohistochemistry staining of intimal sarcoma of the pulmonary
artery. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed consent for publication of this case report
was obtained from patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krüger I, Borowski A, Horst M, de Vivie
ER, Theissen P and Gross-Fengels W: Symptoms, diagnosis, and
therapy of primary sarcomas of the pulmonary artery. Thorac
Cardiovasc Surg. 38:91–95. 1990.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kollár A, Jones RL, Stacchiotti S,
Gelderblom H, Guida M, Grignani G, Steeghs N, Safwat A, Katz D,
Duffaud F, et al: Pazopanib in advanced vascular sarcomas: An EORTC
soft tissue and bone sarcoma group (STBSG) retrospective analysis.
Acta Oncol. 56:88–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schur S, Hamacher R and Brodowicz T:
Pazopanib in primary cardiac angiosarcoma of the right atrium: A
case report. Case Rep Oncol. 9:363–367. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoo KH, Kim HS, Lee SJ, Park SH, Kim SJ,
Kim SH, La Choi Y, Shin KH, Cho YJ, Lee J and Rha SY: Efficacy of
pazopanib monotherapy in patients who had been heavily pretreated
for metastatic soft tissue sarcoma: A retrospective case series.
BMC Cancer. 15(154)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nakamura Y, Toda K, Miyagawa S, Yoshikawa
Y, Hata H, Domae K, Matsuura R and Sawa Y: Surgical resection and
pazopanib treatment for recurrent cardiac angiosarcoma. Clin
Pathol. 12(2632010X19831261)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dewaele B, Floris G, Finalet-Ferreiro J,
Fletcher CD, Coindre JM, Guillou L, Hogendoorn PC, Wozniak A,
Vanspauwen V, Schöffski P, et al: Coactivated platelet-derived
growth factor receptor {alpha} and epidermal growth factor receptor
are potential therapeutic targets in intimal sarcoma. Cancer Res.
70:7304–7314. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sirvent N, Maire G and Pedeutour F:
Genetics of dermatofibrosarcoma protuberans family of tumors: From
ring chromosomes to tyrosine kinase inhibitor treatment. Genes
Chromosomes Cancer. 37:1–19. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ito Y, Maeda D, Yoshida M, Yoshida A,
Kudo-Asabe Y, Nanjyo H, Izumi C, Yamamoto F, Inoue M, Shibata H, et
al: Cardiac intimal sarcoma with PDGFRβ mutation and
co-amplification of PDGFRα and MDM2: An autopsy case analyzed by
whole-exome sequencing. Virchows Arch. 471:423–428. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tamborini E, Casieri P, Miselli F,
Orsenigo M, Negri T, Piacenza C, Stacchiotti S, Gronchi A,
Pastorino U, Pierotti MA and Pilotti S: Analysis of potential
receptor tyrosine kinase targets in intimal and mural sarcomas. J
Pathol. 212:227–235. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bode-Lesniewska B, Zhao J, Speel EJ,
Biraima AM, Turina M, Komminoth P and Heitz PU: Gains of 12q13-14
and overexpression of mdm2 are frequent findings in intimal
sarcomas of the pulmonary artery. Virchows Arch. 438:57–65.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tajima S, Takanashi Y, Takahashi T and
Neyatani H: Intimal sarcoma of the abdominal aorta with
platelet-derived growth factor receptor α overexpression and
amplification in mural invasive cells and pulmonary metastatic
cells but not in intimal spreading cells. Pathol Int. 65:426–431.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tap WD, Jones RL, Van Tine BA, Chmielowski
B, Elias AD, Adkins D, Agulnik M, Cooney MM, Livingston MB, Pennock
G, et al: Olaratumab and doxorubicin versus doxorubicin alone for
treatment of soft-tissue sarcoma: An open-label phase 1b and
randomised phase 2 trial. Lancet. 388:488–497. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yonemori K, Kodaira M, Satoh T, Kudo T,
Takahashi S, Nakano K, Ando Y, Shimokata T, Mori J, Inoue K, et al:
Phase 1 study of olaratumab plus doxorubicin in Japanese patients
with advanced soft-tissue sarcoma. Cancer Sci. 109:3962–3970.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tap WD, Wagner AJ, Schöffski P,
Martin-Broto J, Krarup-Hansen A, Ganjoo KN, Yen CC, Abdul Razak AR,
Spira A, Kawai A, et al: Effect of doxorubicin plus olaratumab vs.
doxorubicin plus placebo on survival in patients with advanced soft
tissue sarcomas: The ANNOUNCE randomized clinical trial. JAMA.
323:1266–1276. 2020.PubMed/NCBI View Article : Google Scholar
|