Introduction
Lung cancer is an important public health problem
and the leading cause of cancer death worldwide, with more than
1.38 million deaths worldwide (1,2). The
prognosis of lung cancer remains dismal in which 15% of patients
have a 5-year survival rate regardless of stage (3). Mutational and proteomic profiling
studies have led to the identification of molecular driver
mutations in lung cancer (4). EGFR
mutations, as the first driver mutation gene in lung cancer, has
brought about a large paradigm shift in lung cancer treatment
(5). With this as a breakthrough,
the research of driver genes for lung cancer was greatly
accelerated. Among the driver genes that are currently being
confirmed, EGFR mutations and anaplastic lymphoma kinase (ALK)
rearrangement are so far the most frequent and clinically important
carcinogenic driver mutations in non-small-cell lung cancer (NSCLC)
patients. The identification of anaplastic lymphoma kinase (ALK)
rearrangement mutations and the introduction of ALK tyrosine kinase
inhibitors (ALK-TKI) have dramatically changed the treatment of
advanced or recurrent NSCLC patients with ALK rearranged mutations
(6-8).
The value of ALK-TKIs has been demonstrated in randomized trials
(6-8),
and they are currently first-choice drugs for patients with
advanced or recurrent NSCLC harboring ALK-rearranged mutations. In
surgically-resected lung adenocarcinoma patients with ALK
rearranged mutations, however, controversy persists as to whether
the ALK-rearranged mutations is an unfavorable prognostic factor
(9-23).
Previous reports have variously indicated ALK-rearranged mutations
was a favorable prognostic factor (9,10), an
unfavorable factor (11-18),
or a factor not associated with prognosis (19-23).
Such different results might be related to the small number of
patients with ALK rearranged mutations, and the large difference in
the background between patients with and without ALK rearranged
mutations. That is, the frequency of ALK rearranged mutations was
higher among young patients, female patients and non-smokers
(6,13-15,24-26).
In addition, it is still unclear whether the presence or absence of
the ALK mutations is associated with post-recurrence survival
(PRS). Differences in patient background might also be related to
the relationship between ALK mutations and PRS. Therefore, in order
to clarify these two issues, it was necessary to match the patient
backgrounds and compare the survival of patients with and without
ALK mutations. There are several ways to match different patient
backgrounds and compare different groups, including matched-pair
analyses. In the present study, we compared disease-free survival
(DFS), overall survival (OS), and PRS in patients with surgically
resected NSCLC with ALK mutations and those without the mutations.
The primary aims of the present study were to elucidate whether
ALK-rearranged mutations in patients with surgically resected
disease was associated with OS, and to clarify whether patients
with ALK mutations had a longer PRS.
Patients and methods
Patients
This was a retrospective study in which all medical
institutions covering one prefecture in our country participated.
We included patients who were diagnosed as having ALK-rearranged
NSCLC between April 2008 and March 2019. Patients, who had
undergone surgery before the diagnostic method of ALK fusion
mutations was approved by medical insurance in our country in May
2012 and were diagnosed as having ALK-rearranged NSCLC during the
study period, were included in the study. Controls were 232
patients who were diagnosed as having neither ALK rearrangement
mutations nor epidermal growth factor (EGFR) mutations and had had
surgical resections during the study period at the Mito Medical
Center and Mito Kyodo General Hospital. The control matched-pair
patients were those among the 232 control patients whose main
clinical characteristics were consistent with ALK-rearranged
mutated patients, including age, sex, performance status,
pathological stage, and smoking habit, which are known prognostic
factors for NSCLC patients. Histopathological diagnoses were
defined according to the World Health Organization classification
system, and the patients were staged according to the Union for
International Cancer Control (UICC) tumor-node-metastasis (TNM)
staging system (8th edition). The patient characteristics,
efficacy, safety, DFS, OS were evaluated using patient data
extracted from the database of each institution. DFS was calculated
from the date of surgery to the recurrence or any cause of death
and OS was the interval from the date of surgery to the date of
death, or latest follow-up contact. PRS was defined as the interval
between the date of recurrence and to the date of death or latest
follow-up contact.
The present non-interventional and observational
retrospective study conformed to the Ethical Guidelines for
Clinical Studies issued by the Ministry of Health, Labor and
Welfare of Japan. This study was approved by the Institutional
Review Board of the Mito Kyodo General Hospital-Mito Medical
Center, University of Tsukuba (no. 16-66, 18-15) or independent
ethics committees associated with each study institute. Written
comprehensive informed consent for reporting clinical course was
obtained from each patient at the time of admission.
Measurement of ALK fusion gene
Analysis of ALK fusion mutations was performed by
the respective assay method used by each institution, such as
fluorescence in situ hybridization (FISH) and
immunohistochemistry (IHC), using biopsy specimens, cytology
specimens and plasma specimens.
Statistical analysis
All analyses were performed using SPSS version 23
(IBM Corporation). Differences in proportions between two
independent groups were compared using the χ2 test. The
Mann-Whitney U test was used to compare two groups of sample data.
The survival rate was analyzed by the Kaplan-Meier method and
comparisons performed using the log-rank test. The effects of
clinicopathological factors on survival were analyzed using the Cox
proportional hazards model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological features in
patients with ALK-rearranged mutations
During the study period, 129 patients were diagnosed
as having ALK-rearranged NSCLC. The analysis of ALK fusion mutation
was examined using FISH in 78 patients, IHC in 30 patients, and
both tests in 21 patients. Fifty-eight patients had surgical
resection. Among them, 51 patients had curative complete resection.
Seven patients were excluded from this study because they did not
have no curative resection. Table I
shows the clinicopathological features of the 51 with ALK mutations
who had curative complete resection and 232 completely resected
controls who had neither ALK-rearranged mutations nor EGFR
mutations. Proportions of all the features in these two groups of
patients except for performance status were different.
 | Table IClinicopathological characteristics of
enrolled patients: 51 surgically resected patients with ALK
mutation and 232 surgically resected controls who had neither ALK
rearranged mutation nor EGFR mutations. |
Table I
Clinicopathological characteristics of
enrolled patients: 51 surgically resected patients with ALK
mutation and 232 surgically resected controls who had neither ALK
rearranged mutation nor EGFR mutations.
| Characteristics | Resected patients
with ALK mutation | Resected controls
with neither ALK nor EGFR | P-value |
|---|
| Age (year), median,
range | 65, 32-82 | 70, 39-89 | <0.001 |
| Sex | | | <0.001 |
|
Male | 16 | 748 | |
|
Female | 35 | 84 | |
| Performance
status | | | 0.078 |
|
0 | 41 | 149 | |
|
1 | 9 | 78 | |
|
2 | 1 | 5 | |
| Smoking status | | | 0.516 |
|
Never | 36 | 151 | |
|
Past or
current | 15 | 81 | |
| P-stage | | | <0.001 |
|
IA-IB | 21 | 162 | |
|
IIA-IIB | 14 | 37 | |
|
IIIA | 16 | 33 | |
| 5-year survival
(%) | 84.0 | 69.8 | 0.059 |
In 51 patients with ALK-rearranged mutations, the
median age was 65 (range, 32-82) years. Presence of ALK fusion
mutation was confirmed in FISH, IHC, and both tests in 27, 19 and 5
patients, respectively. There were 16 (31.4%) males and 35 females.
Fifty (98.0%) patients had good performance status (Eastern
Cooperative Oncology Group 0-1). Fifteen (29.4%) of them were
non-smokers. All 51 patients had adenocarcinoma. The median maximum
diameter of the primary lesion was 20 mm (range, 12-82 mm). At the
time of initial diagnosis, 21 patients (41.2%) were diagnosed as
having pathological stage IA-B, 14 (27.5%) patients with IIA-B, and
16 (31.4%) patients with IIIA. The average 5-year survival rate of
the 51 patients who underwent complete resections was 84.0%.
Clinicopathological features and
survival in matched-pair patients
Table II shows the
clinicopathological characteristics of 38 ALK-rearranged mutated
patients and 38 matched-pair control patients whose main clinical
characteristics were matched to patients with ALK-rearrangements.
In these two matched groups of patients, 11 and 12 patients
experienced recurrence during the study period. DFS and OS from the
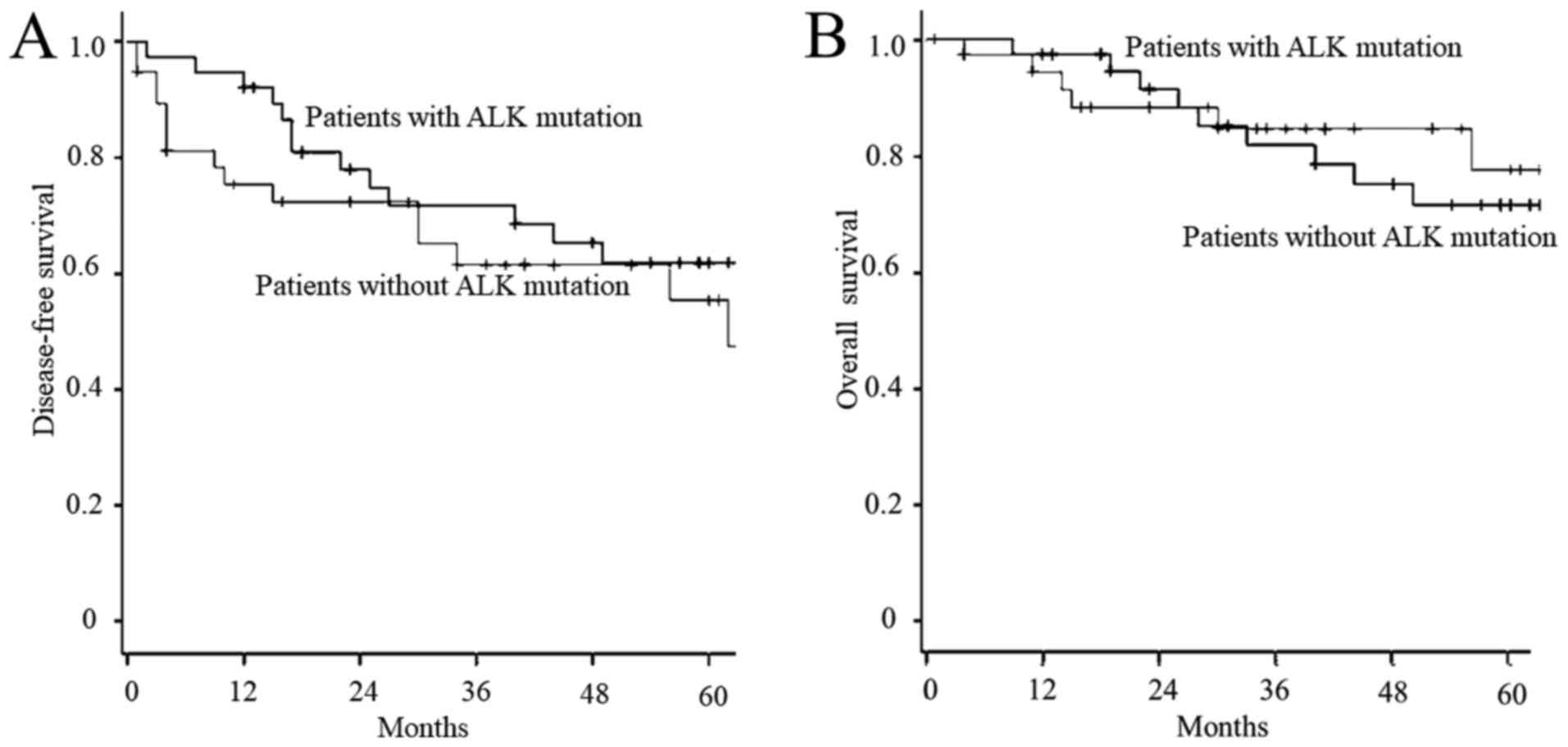
surgery in these two groups of patients are shown in Fig. 1. There was no significant difference
between these two groups in either DFS (P=0.303) or OS
(P=0.820).
 | Table IIClinicopathological characteristics
of patients matched according to clinical characteristics: 38
patients with ALK-rearranged mutation and 38 patients with neither
ALK nor EGFR mutation. |
Table II
Clinicopathological characteristics
of patients matched according to clinical characteristics: 38
patients with ALK-rearranged mutation and 38 patients with neither
ALK nor EGFR mutation.
|
Characteristics | Resected patients
with ALK mutation | Resected controls
with neither ALK nor EGFR | P-value |
|---|
| Age (year), median,
range | 65, 40-82 | 67, 46-82 | 0.763 |
| Sex | | | 0.807 |
|
Male | 13 | 12 | |
|
Female | 25 | 26 | |
| Performance
status | | | 0.361 |
|
0 | 30 | 33 | |
|
1 | 8 | 5 | |
| Smoking status | | | 0.622 |
|
Never | 13 | 11 | |
|
Past or
current | 25 | 27 | |
| P-stage | | | 0.841 |
|
IA-IB | 20 | 22 | |
|
IIA-IIB | 9 | 9 | |
|
IIIA | 9 | 7 | |
| Recurrence during
the study period | | | 0.803 |
|
Present | 11 | 12 | |
|
Absent | 27 | 26 | |
| 5-year
survival | 77.5 | 71.5 | 0.820 |
Post-recurrence survival
Table III shows
the time to recurrence, the administration of ALK-TKI after
recurrence, and PRS in the two groups of patients. As for these,
there were no differences between the two groups. As shown in
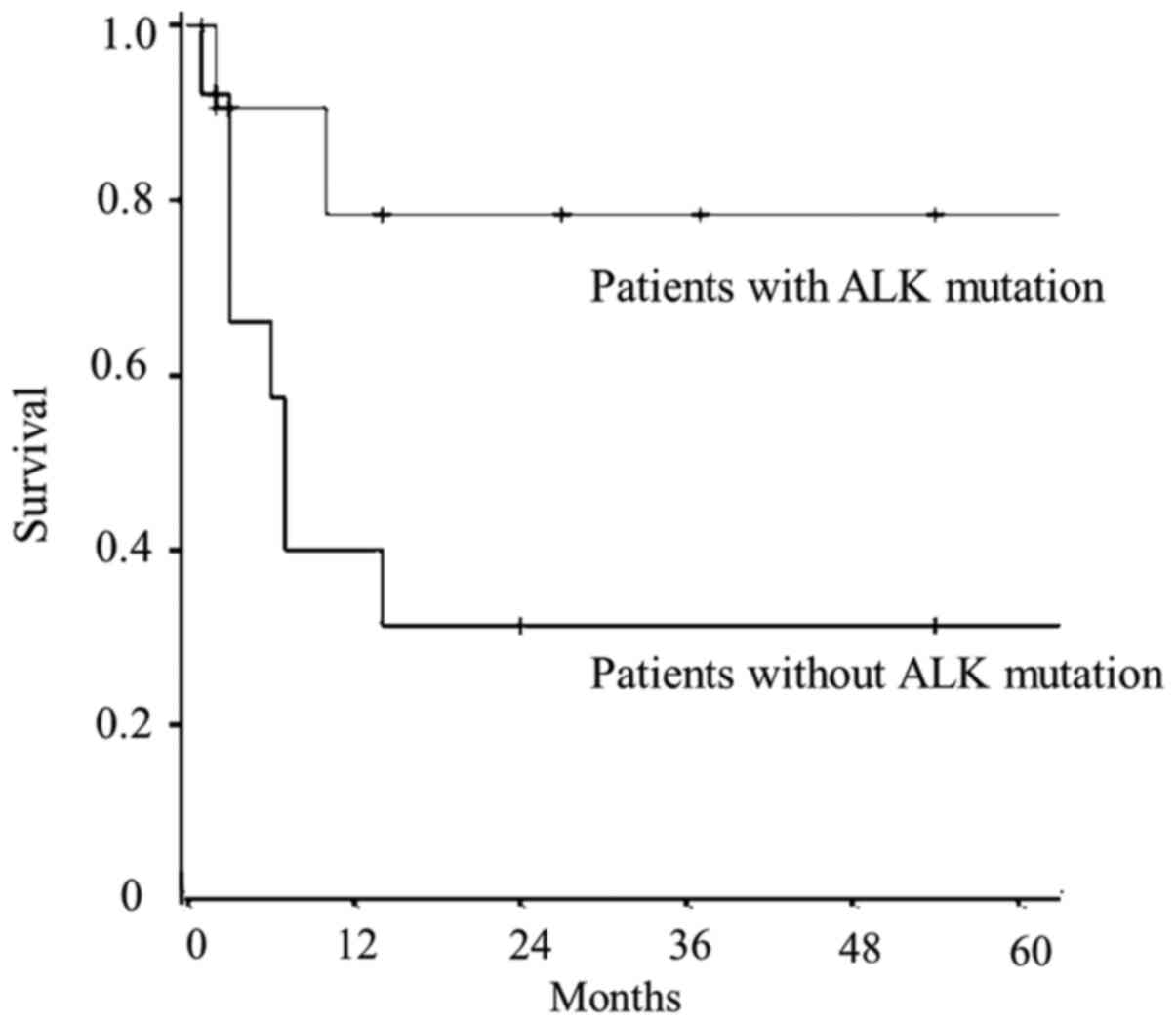
Fig. 2, patients with
ALK-rearranged mutations had longer PRS compared with those who had
neither ALK nor EGFR mutations (P=0.042).
 | Table IIIClinicopathological characteristics
of pair-matched recurrent patients: Patients with ALK-rearranged
mutation and patients with neither ALK-rearranged mutation nor EGFR
mutation. |
Table III
Clinicopathological characteristics
of pair-matched recurrent patients: Patients with ALK-rearranged
mutation and patients with neither ALK-rearranged mutation nor EGFR
mutation.
| Characteristic | Patients with
ALK | Patients with
neither ALK nor EGFR | P-value |
|---|
| Number of
patients | 11 | 12 | |
| ALK-TKI therapy
after recurrence | | | |
|
Present | 9 | 0 | |
|
Absent | 2 | 10 | |
| Period until
recurrence, median (range) | 4 (1-62)
months | 17 (2-94)
months | 0.104 |
| Survival
status | | | 0.059 |
|
Dead | 3 | 8 | |
|
Alive | 8 | 4 | |
| Survival from
recurrence | 14 (1-91)
months | 7 (1-72)
months | 0.487 |
Discussion
It is well known that the proportion of patients
with ALK-rearranged mutations is higher among young patients,
female patients, and non-smokers (6,13-15,24-26).
The characteristics of younger patients were comparable to previous
studies (13-15,24,25).
The percentage of non-smokers was higher among patients with
ALK-rearranged mutations than among patients without ALK mutations
(8,12-14,16,20,21,23).
The high percentage of non-smokers was also comparable among
patients with ALK mutations who underwent resections (13,14,16,21).
Only a study by Blackhall et al reported a high percentage
of smokers (9). In the present
study, the proportion of female patients and non-smokers was 68.6
and 29.4%, respectively, consistent with previous studies (10,12-14,16,20,21,23).
However, the median age in our patients was 65 years, which was
older than that of patients in previous studies (13-15,24,25).
Our data suggested that there might be more patients with
surgically resectable ALK-rearranged NSCLC over the age of 60 years
and those with a smoking habit. Therefore, evaluation of ALK fusion
events might need to be proactively pursued in elderly NSCLC
patients. It has been controversial whether the prognosis of
patients with ALK-rearranged NSCLC is better than that of patients
without ALK-rearranged mutations (9-23).
Previous studies on the survival of ALK-mutated
patients focused on one of three groups: All ALK-mutated patients;
patients with advanced or recurrent disease; and patients who
underwent resections (9-23).
Survival results in these studies were inconsistent among the three
patient groups (9-23).
Particularly among studies of patients who underwent resections
(9,13-17,19,21,22),
some reports indicated a good prognosis in patients with
ALK-rearranged mutations (9) while
other reports indicated poor prognosis (13-17).
Yet other reports indicated that the ALK-rearranged mutations was
not associated with prognosis (19,21,22).
Except for a study Blackhall et al, all were small with up
to 50 patients, and control patients in these studies were those
without ALK-rearranged mutations resected at the same study period
(9,13-17,19,21,22).
As described above, the proportion of young, female, and
non-smoking patients was higher in patients with ALK-rearranged
mutations than in those without (6,13-17,24-26).
As a result, it might be that patients with ALK-rearranged
mutations and those without ALK mutations had significantly
different backgrounds that might account for the different
prognosis. Therefore, we consider a simple comparison of patient
groups with different background factors an inappropriate
methodology. Matched-pair analysis, which matches factors known to
be important prognostic factors, was considered one of the suitable
statistical analyses to be applied. However, there have been only
two studies analyzed using this statistical method (9,23). One
was a study of only 28 patients with clinical stages III and IV
(23). Although this report showed
that there was no difference in the survival of patients with and
without ALK mutations, this result only applied to patients with
advanced disease (23). Another
report was by Blackhall et al in 80 patients who underwent
resections with pathological stages I through III (9). They reported that patients with
ALK-rearranged mutations were associated with better OS (9). Our matched-pair analysis, which
matched some clinical characteristics, however, showed that DFS and
OS in patients with ALK mutations were not significantly different
from those with neither ALK nor EGFR mutations. EGFR mutations are
the most frequent driver gene recognized at present (6,8).
However, Blackhall et al did not mention the exclusion of
patients with EGFR mutations (9).
In addition, their cohort had a very high percentage of patients
with a smoking history, with 81.7% of ALK-mutated patients being
current or past smokers (9). In our
study, 70.6% of ALK-mutated patients had a smoking history. The
inconsistency between the study by Blackhall et al and ours
might be related to a difference in the background of the
ALK-rearranged patients studied.
In the present study, we obtained another important
finding. PRS of patients with ALK-rearranged mutations was
significantly longer than that of patients without the mutations.
Clinical trials in ALK-TKIs included patients with advanced, as
well as those with recurrent disease (6-8),
and retrospective clinical studies also included these patients
(6-8).
However, there have been no reports comparing PRS of patients with
ALK mutations and that of patients without the mutations. This was
the first study to compare PRS of patients with or without ALK
rearranged mutations using matched-pair analysis. We matched
clinical factors considered as major prognostic factors. ALK-TKI
treatment seemed to be the reason why the survival of patients with
ALK-rearranged mutations was longer than that of patients without
the mutations. Whether the presence of the ALK fusion gene itself
is associated with prognosis, or whether administration of TKI is
associated with prognosis, is not a major problem in actual
practice. If the patient with the ALK fusion gene mutations
relapses, administering TKI is a standard treatment. Clarification
of these reasons should be left to future biological studies. Our
findings were derived from a small number of patients treated with
ALK-TKIs, and need to be confirmed by future studies.
In this research, we chose the matched-pair analysis
because we wanted to make the clinical and pathological variables
as even as possible in comparison the survival with ALK rearranged
mutations. We matched background characteristics that were
previously associated with prognosis, such as age, gender,
performance status, pathological stage, and smoking habit.
Regarding OS, no difference could be confirmed between the two
patients with or without ALK rearrangement mutations. This might
relate to the short observation period and the small number of
patients. Alternatively, it might suggest that ‘curative
resections’ had overwhelmingly larger impact on contributing to
overall survival than ‘ALK rearrangement mutations and presence of
ALK-TKI treatment’ did.
The present study had several limitations. Firstly,
it was a retrospective study with patients from miscellaneous
backgrounds. Secondly, the methods for examining ALK-fusion gene
mutations were not unified. For ALK examination, FISH and
immunostaining were performed. Patients who confirmed the presence
of the ALK fusion gene by both examinations were also included. The
effects of the difference in detection sensitivity of these
measurements on our results could not be fully investigated.
Comprehensive screening with next generation sequencer was not used
in this study population. Thirdly, the limited number of patients
and the short period of investigation were also limitations.
Fourthly, it was a matched-pair study with a one-to-one patient
ratio, but it would be favorable to include more control patients
with matched clinical characteristics. Fifthly, as for the
survival, our study could not analyze the presence of the ALK
mutations and the use of ALK-TKI separately. At present,
administration of ALK-TKI is the standard treatment in recurrent
patients with ALK mutations, so it is not realistic to consider
these separately in clinical practice. Despite these limitations,
we suppose that our data from ALK-rearranged patients collected by
multiple institutions will provide clinically meaningful
information.
In summary, DFS and OS of patients with
ALK-rearranged mutations were not significantly different from
those with neither ALK nor EGFR mutations. PRS of patients with
ALK-rearranged mutations was significantly longer than that of
patients without the mutations. ALK-TKI treatment after recurrence
might play an important role. It is important to treat patients
with resected ALK-rearranged NSCLC with consideration of driver
gene mutations. Successful treatment with ALK-TKI might be
required, even for patients who experience recurrence after
surgery. In order to fully elucidate these relationships, it is
necessary to accumulate information obtained, not only by clinical
trials, but also from clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MI, NH, KF, HSa and YS designed the study. MI, HIc,
SU, KI, OI, RN, YI, HS, MaK, KK, MoK, SY, MS, NK, KF collected the
data. MI, KM and HSa analyzed the data and prepared the article.
All authors approved the final version of the article.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Mito Kyodo General Hospital-Mito Medical
Center, University of Tsukuba (approval no. 16-66, 18-15).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Daniels MG, Bowman RV, Yang IA, Govindan R
and Fong KM: An emerging place for lung cancer genomics in 2013. J
Thorac Dis. 5 (Suppl 5):S491–S497. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Singhal S, Miller D, Ramalingam S and Sun
SY: Gene expression profiling of non-small cell lung cancer. Lung
Cancer. 60:313–324. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bronte G, Rizzo S, La Paglia L, Adamo V,
Siragusa S, Ficorella C, Santini D, Bazan V, Colucci G, Gebbia N
and Russo A: Driver mutations and differential sensitivity to
targeted therapies: A new approach to the treatment of lung
adenocarcinoma. Cancer Treat Rev. 36 (Suppl 3):S21–S29.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Delmonte A, Burgio MA, Verlicchi A, Bronte
G, Cravero P, Ulivi P, Martinelli G and Crinò L: New generation
anaplastic lymphoma kinase inhibitors. Transl Lung Cancer Res. 8
(Suppl 3):S280–S289. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stinchcombe TE: Targeted therapies for
lung cancer. Cancer Treat Res. 170:165–182. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Blackhall FH, Peters S, Bubendorf L, Dafni
U, Kerr KM, Hager H, Soltermann A, O'Byrne KJ, Dooms C, Sejda A, et
al: Prevalence and clinical outcomes for patients with ALK-positive
resected stage I to III adenocarcinoma: Results from the European
Thoracic Oncology Platform Lungscape Project. J Clin Oncol.
32:2780–2787. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu SG, Kuo YW, Chang YL, Shih JY, Chen YH,
Tsai MF, Yu CJ, Yang CH and Yang PC: EML4-ALK translocation
predicts better outcome in lung adenocarcinoma patients with
wild-type EGFR. J Thorac Oncol. 7:98–104. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim HR, Shim HS, Chung JH, Lee YJ, Hong
YK, Rha SY, Kim SH, Ha SJ, Kim SK, Chung KY, et al: Distinct
clinical features and outcomes in never-smokers with nonsmall cell
lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement.
Cancer. 118:729–739. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou JX, Yang H, Deng Q, Gu X, He P, Lin
Y, Zhao M, Jiang J, Chen H, Lin Y, et al: Oncogenic driver
mutations in patients with non-small-cell lung cancer at various
clinical stages. Ann Oncol. 24:1319–1325. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao Q, Li P, Jiang X, Zhan Z, Yan Q, Zhang
B and Huang C: Worse disease-free, tumor-specific, and overall
survival in surgically-resected lung adenocarcinoma patients with
ALK rearrangement. Oncotarget. 8:86066–86081. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shin SH, Lee H, Jeong BH, Choi YS, Shin
MH, Kim S, Han J, Lee KS, Shim YM, Kwon OJ and Kim H: Anaplastic
lymphoma kinase rearrangement in surgically resected stage IA lung
adenocarcinoma. J Thorac Dis. 10:3460–3467. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim MH, Shim HS, Kang DR, Jung JY, Lee CY,
Kim DJ, Lee JG, Bae MK, Kim HR, Lim SM, et al: Clinical and
prognostic implications of ALK and ROS1 rearrangements in
never-smokers with surgically resected lung adenocarcinoma. Lung
Cancer. 83:389–395. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li P, Gao Q, Jiang X, Zhan Z, Yan Q, Li Z
and Huang C: Comparison of clinicopathological features and
prognosis between ALK rearrangements and EGFR mutations in
surgically resected early-stage lung adenocarcinoma. J Cancer.
10:61–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Seto K, Kuroda H, Yoshida T, Sakata S,
Mizuno T, Sakakura N, Hida T, Yatabe Y and Sakao Y: Higher
frequency of occult lymph node metastasis in clinical N0 pulmonary
adenocarcinoma with ALK rearrangement. Cancer Manag Res.
10:2117–2124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang P, Kulig K, Boland JM,
Erickson-Johnson MR, Oliveira AM, Wampfler J, Jatoi A, Deschamps C,
Marks R, Fortner C, et al: Worse disease-free survival in
never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol.
7:90–97. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun JM, Lira M, Pandya K, Choi YL, Ahn JS,
Mao M, Han J, Park K, Ahn MJ and Kim J: Clinical characteristics
associated with ALK rearrangements in never-smokers with pulmonary
adenocarcinoma. Lung Cancer. 83:259–264. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fukui T, Yatabe Y, Kobayashi Y, Tomizawa
K, Ito S, Hatooka S, Matsuo K and Mitsudomi T: Clinicoradiologic
characteristics of patients with lung adenocarcinoma harboring
EML4-ALK fusion oncogene. Lung Cancer. 77:319–325. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Paik JH, Choi CM, Kim H, Jang SJ, Choe G,
Kim DK, Kim HJ, Yoon H, Lee CT, Jheon S, et al: Clinicopathologic
implication of ALK rearrangement in surgically resected lung
cancer: A proposal of diagnostic algorithm for ALK rearranged
adenocarcinoma. Lung Cancer. 76:403–409. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mizuno T, Arimura T, Kuroda H, Sakakura N,
Yatabe Y and Sakao Y: Current outcomes of postrecurrence survival
in patients after resection of non-small cell lung cancer. J Thorac
Dis. 10:1788–1796. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee JK, Park HS, Kim DW, Kulig K, Kim TM,
Lee SH, Jeon YK, Chung DH, Heo DS, Kim WH and Bang YJ: Comparative
analyses of overall survival in patients with anaplastic lymphoma
kinase-positive and matched wild-type advanced nonsmall cell lung
cancer. Cancer. 118:3579–3586. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tantraworasin A, Lertprasertsuke N,
Kongkarnka S, Euathrongchit J, Wannasopha Y and Saeteng S:
Retrospective study of ALK rearrangement and clinicopathological
implications in completely resected non- small cell lung cancer
patients in Northern Thailand: Role of screening with D5F3
antibodies. Asian Pac J Cancer Prev. 15:3057–3063. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tao H, Cai Y, Shi L, Tang J and Liu Z,
Wang Z, Bai L and Liu Z: Analysis of clinical characteristics and
prognosis of patients with anaplastic lymphoma kinase-positive and
surgically resected lung adenocarcinoma. Thorac Cancer. 8:8–15.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jin Y, Chen Y, Yu X and Shi X: A
real-world study of treatment patterns and survival outcome in
advanced anaplastic lymphoma kinase-positive non-small-cell lung
cancer. Oncol Lett. 15:8703–8710. 2018.PubMed/NCBI View Article : Google Scholar
|