Introduction
The prognoses of patients with recurrent and/or
metastatic soft tissue sarcoma (STS) remain poor, with median
survival according to recent data limited to 14-20 months (1). Standard chemotherapy as a first-line
treatment for STS is generally a doxorubicin-based regimen in most
histological subtypes, but there are some systemic treatment
options, including eribulin, available to STS patients refractory
and/or intolerant to doxorubicin (2). The clinical evidence to date regarding
eribulin in STS patients in a phase III trial has, however, been
limited to L-sarcoma patients, i.e., those with leiomyosarcoma or
liposarcoma, who had received at least two previous systemic
chemotherapy treatments, including anthracyclines (3). As a result, the approval of eribulin
for STS in the U.S. and Europe has been limited to patients with
specific histological subtypes and treatment histories (4).
In Japan, eribulin has been approved for recurrent
and/or metastatic STS patients regardless of histological subtype
or treatment history, although its indication as a first-line
therapy is not recommended. However, there is still insufficient
clinical data about whether and how much differences in
histological subtype and treatment line affect its efficacy.
Differences in the efficacy of eribulin for STS in patients with
different histological subtypes have been reported in previous
phase II trials (5,6) but, to the best of our knowledge, it
has not yet been examined in different treatment lines or with
respect to the number of previous systemic chemotherapies. The
purpose of the present study, therefore, was to evaluate certain
clinical factors that might affect the efficacy and safety of
eribulin in STS patients.
Materials and methods
Patient data
We retrospectively reviewed the clinical records of
recurrent and/or metastatic STS patients who underwent eribulin
treatment and evaluated its efficacy and safety, analyzing
prognostic factors by histological subtype and treatment line,
which were defined as the number of chemotherapy regimens in a
recurrent/metastatic setting. The patients who were diagnosed as
STS by histological pathology, either obtained from biopsy or
surgical resection were included in the analysis, and patients who
were intended to receive eribulin but actually never received by
disease progression, patients' refusal or other reasons were
excluded. This retrospective study was approved by the
Institutional Review Board of Cancer Institute Hospital of Japanese
Foundation for Cancer Research and written informed consent was
obtained from each patient.
Statistical analysis
The patients' objective response and disease
progression were evaluated using the Response Evaluation Criteria
In Solid Tumours (RECIST), version 1.1. Adverse events were
documented based on the Common Terminology Criteria for Adverse
Events (CTCAE), version 5.0. To evaluate prognoses, both overall
survival (OS) and progression-free survival (PFS) were estimated by
the Kaplan-Meier method. The log-rank test was used to compare
prognoses. The Chi-square test was used to compare the differences
in the characteristics, chemotherapy treatment details and adverse
events. The SPSS software ver. 25.0 was used for all statistical
analyses, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient data
A total of 41 STS patients were treated with
eribulin between March 2016 and December 2018, 26 of whom had
L-sarcoma (12 leiomyosarcoma and 14 liposarcoma). The histological
subtypes of non-L-sarcoma patients were as follows: 8
undifferentiated pleomorphic sarcoma, 2 synovial sarcoma, 1
clear-cell sarcoma, 1 endometrial stromal sarcoma, 1 myxoid
chondrosarcoma, 1 malignant peripheral nerve sheath tumor, and 1
solitary fibrous tumor. The characteristics of the enrolled
patients are summarized in Table I.
There were no significant differences in the patients' gender, age
or primary lesion between the L-sarcoma and non-L-sarcoma groups,
but there were differences in the number of previous
chemotherapies: Nearly half of L-sarcoma patients received eribulin
as a second-line therapy, while most non-L-sarcoma patients had
received two or more chemotherapies in a recurrent/metastatic
setting. In L-sarcoma patients, higher rates of liposarcoma
patients (11 of 14 patients; 78.6%) than leiomyosarcoma patients (1
of 12; 8.3%) received eribulin as a second-line therapy. All
patients received doxorubicin as a perioperative or earlier-line
chemotherapy regardless of histological subtype (Table II). History of radiation therapy
was observed in 21 (51.2%) patients, including 8 primary lesions (4
retroperitoneal/intra-abdomen, 2 soft tissue of trunk, 1 thoracic
lesion, and 1 head and neck) and 13 metastatic lesions (7 bone
metastases, 5 lung/thoracic metastases and 1 soft tissue or
trunk).
 | Table ICharacteristics of the patients
enrolled in the present study. |
Table I
Characteristics of the patients
enrolled in the present study.
| Characteristics | All patients, n
(%) | L-sarcoma, n (%) | Non-L-sarcoma, n
(%) | P-value (Two-tailed
χ2) |
|---|
| Sex | | | | 0.097 |
|
Male | 16 (39.0) | 13 (50.0) | 3 (20.0) | |
|
Female | 25 (61.0) | 13 (50.0) | 12 (80.0) | |
| Age, years | | | | |
|
Median | 61 | 64 | 54 | |
|
Range | 23-75 | 44-74 | 23-75 | |
|
Age ≥60
years | 24 (58.5) | 17 (65.4) | - | 0.200 |
| Primary lesion | | | | 0.453 |
|
Extremities | 10 (24.4) | 5 (19.2) | 5 (33.3) | |
|
Non-extremities | 31 (75.6) | 21 (80.8) | 10 (66.7) | |
| Treatment
history | | | | |
|
Surgery | 36 (87.8) | 23 (88.5) | 13 (86.7) | >0.999 |
|
Radiation | 21 (51.2) | 12 (46.2) | 9 (60.0) | 0.520 |
|
Perioperative
chemotherapy | 13 (31.7) | 8 (30.8) | 5 (33.3) | >0.999 |
| Treatment lines | | | | 0.044 |
|
Second
line | 14 (34.1) | 12 (46.2) | 2 (13.3) | |
|
Later
lines | 27 (65.9) | 14 (53.8) | 13 (86.7) | |
| Total | 41 | 26 | 15 | |
 | Table IIDetails of the antitumor drugs used as
previous chemotherapy, including preoperative treatments. |
Table II
Details of the antitumor drugs used as
previous chemotherapy, including preoperative treatments.
| | All patients | L-sarcoma
patients | |
|---|
| Antitumor drug | 2nd line, n (%) | Later lines, n
(%) | 2nd line, n (%) | Later lines, n
(%) | P-value (Two-tailed
χ2)a |
| Doxorubicin | 14 (100.0) | 26 (96.3) | 12 (100.0) | 14 (100.0) | 0.366 |
| Ifosfamide | 11 (78.6) | 17 (63.0) | 2 (16.7) | 9 (64.3) | 0.341 |
| Gemcitabine | 1 (7.1) | 17 (63.0) | 0 (0.0) | 11 (78.6) | >0.999 |
| Docetaxel | 1 (7.1) | 13 (48.1) | 0 (0.0) | 9 (64.3) | >0.999 |
| Pazopanib | 0 (0.0) | 20 (74.1) | 0 (0.0) | 10 (71.4) | 0.111 |
| Trabectedin | 2 (14.2) | 5 (18.5) | 2 (16.7) | 2 (14.3) | 0.693 |
| Other | 2 (14.2) | 9 (33.3) | 1 (8.3) | 7 (50.0) | >0.999 |
| Total | 14 | 27 | 12 | 14 | - |
Statistical analysis
A comparison of patients' prognoses by histological
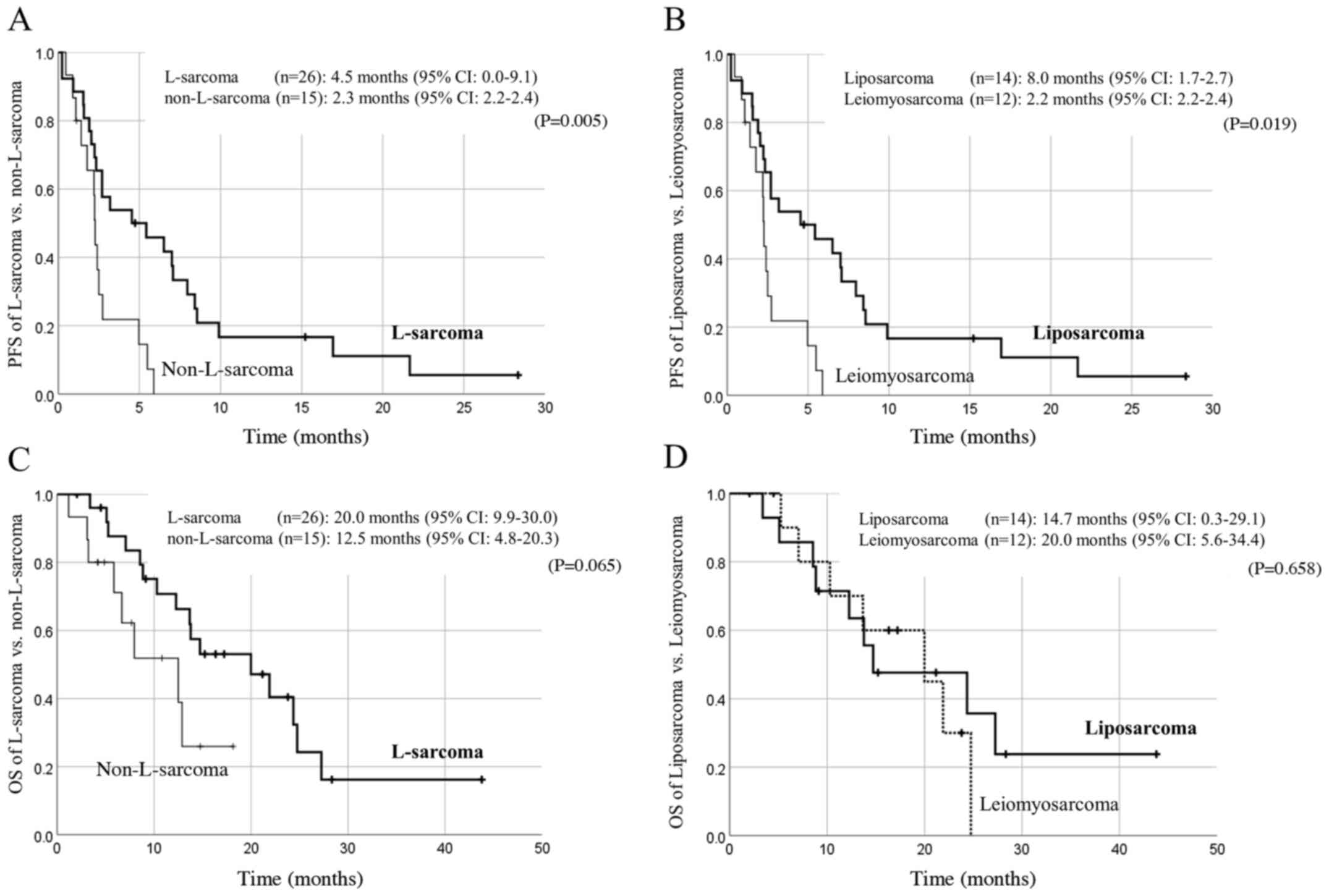
subtype is shown in Fig. 1. The
median PFS in L-sarcoma patients was statistically longer than in
non-L-sarcoma patients (4.5 months vs. 2.3 months, P=0.005;
Fig. 1A), and the median OS also
tended to be longer in L-sarcoma patients though statistical
significance was not reached (20.0 months vs. 12.5 months, P=0.065;
Fig. 1C). In L-sarcomas, there
might be differences in prognoses between leiomyosarcoma and
liposarcoma. The PFS of liposarcoma patients was statistically
longer than that of leiomyosarcoma patients (8.0 vs. 2.2 months,
P=0.019; Fig. 1B), but in terms of
OS, there were no significant differences between liposarcoma and
leiomyosarcoma (14.7 vs. 20.0 months, P=0.658).
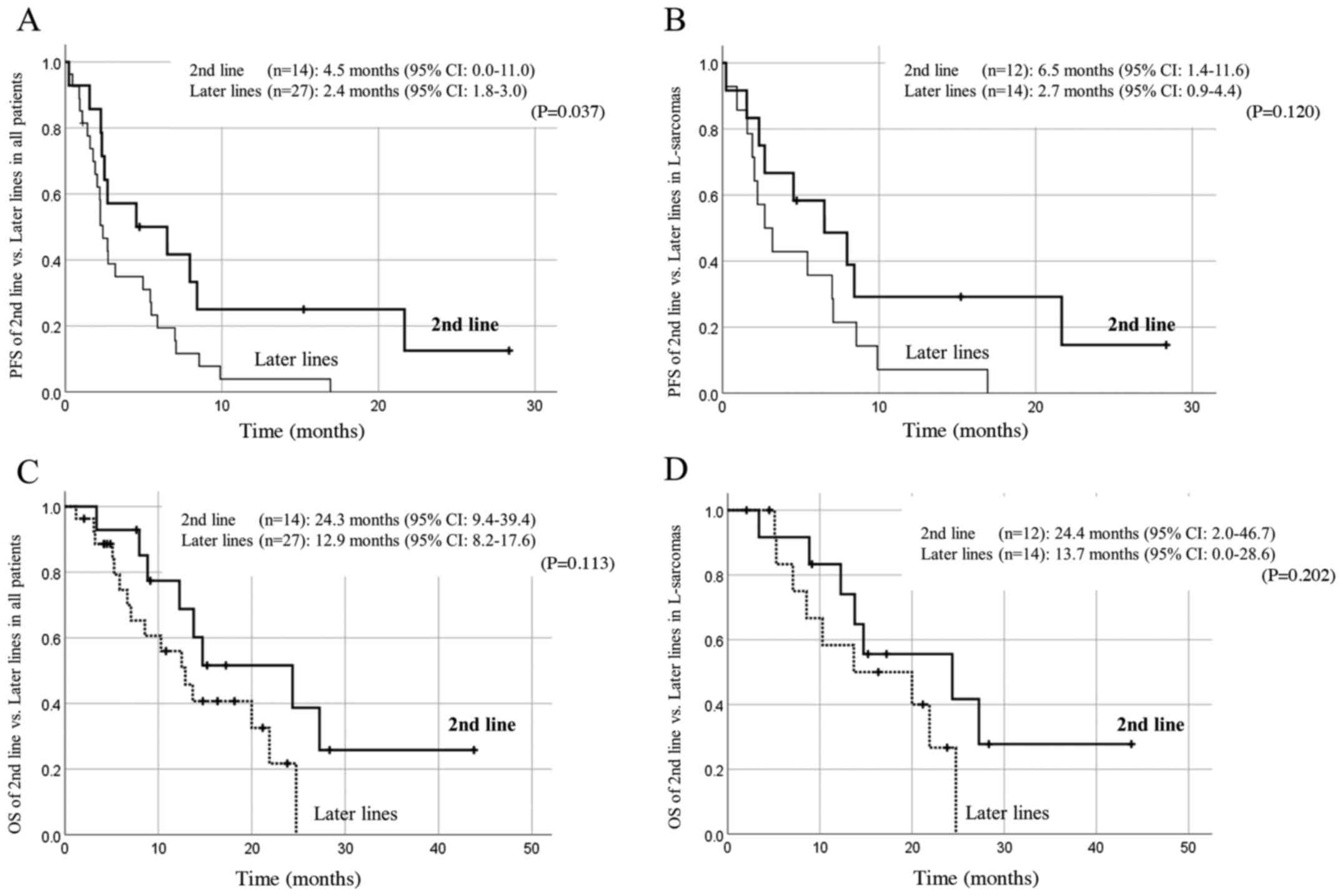
A comparison of patients' prognoses by treatment
line is shown in Fig. 2. Patients
receiving eribulin as a second-line treatment tended to have a
better prognosis, though statistical significance was observed only
in the PFS of all patients. Objective responses were observed in
three liposarcoma patients (7.3%).
History of radiation therapy did not affect the
effectiveness of eribulin; the PFS of patients with and without
history of radiation therapy were both 2.7 months (P=0.125) and the
OS were 13.8 and 14.7 months (P=0.282).
Adverse events associated with eribulin are shown in
Table III. Myelosuppresion,
especially neutropenia and anemia, was highly observed.
Non-hematological adverse events such as gastrointestinal events
such as anorexia and nausea, as well as increased transaminase and
neuropathy, were observed in relatively high incidence, but severe
non-hematological adverse events were rare. Regarding histological
subtype, leukocytopenia, neutropenia, thrombocytopenia,
constipation and neuropathy were observed in 20% or more of
L-sarcoma patients; regarding treatment line, neutropenia was
observed in 20% or more of patients with second-line treatment. No
patients terminated eribulin treatment due to adverse events, and
there were no lethal adverse events.
 | Table IIIAdverse events associated with
eribulin in patients with soft tissue sarcoma. |
Table III
Adverse events associated with
eribulin in patients with soft tissue sarcoma.
| | Comparison by
histological subtypes | | Comparison by
treatment line | |
|---|
| | All patients
(n=41) | L-sarcoma (n=26) | Non-L-sarcoma
(n=15) | | Second line
(n=14) | Later lines
(n=27) | |
|---|
| Variable | All grades, n
(%) | Grade 3-4, n
(%) | All grades, n
(%) | Grade 3-4, n
(%) | All grades, n
(%) | Grade 3-4, n
(%) | P-value (Two-tailed
χ2)a | All grades, n
(%) | Grade 3-4, n
(%) | All grades, n
(%) | Grade 3-4, n
(%) | P-value (Two-tailed
χ2)b |
|---|
| Hematologic adverse
events | | | | | | | | | | | | |
|
Leukocytopenia | 29 (70.7) | 13 (31.7) | 21 (80.8) | 10 (38.5) | 8 (53.3) | 3 (20.0) | 0.083 | 11 (78.6) | 4 (28.6) | 18 (66.7) | 9 (33.3) | 0.494 |
|
Neutropenia | 31 (75.6) | 24 (58.5) | 22 (84.6) | 18 (69.2) | 9 (60.0) | 6 (40.0) | 0.130 | 13 (92.9) | 10 (71.4) | 18 (66.7) | 14 (51.9) | 0.123 |
|
Anemia | 25 (61.0) | 3 (7.3) | 16 (61.5) | 3 (11.5) | 9 (60.0) | 0 (0.0) | >0.999 | 6 (42.9) | 1 (7.1) | 19 (70.4) | 2 (7.4) | 0.105 |
|
Thrombopcytopenia | 13 (31.7) | 1 (2.4) | 11 (42.3) | 1 (3.8) | 2 (13.3) | 0 (0.0) | 0.084 | 6 (42.9) | 1 (7.1) | 7 (25.9) | 0 (0.0) | 0.307 |
| Non-hematologic
adverse events | | | | | | | | | | | | |
|
Increased
total bilirubin | 3 (7.3) | 0 (0.0) | 3 (11.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.287 | 1 (7.1) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 0.265 |
|
Increased
AST | 25 (61.0) | 0 (0.0) | 13 (50.0) | 0 (0.0) | 9 (60.0) | 0 (0.0) | >0.999 | 7 (50.0) | 0 (0.0) | 18 (66.7) | 0 (0.0) | 0.332 |
|
Increased
ALT | 19 (46.3) | 0 (0.0) | 11 (42.3) | 0 (0.0) | 8 (53.3) | 0 (0.0) | 0.533 | 7 (50.0) | 0 (0.0) | 12 (44.4) | 0 (0.0) | 0.754 |
|
Increased
serum creatinine | 10 (24.3) | 0 (0.0) | 8 (30.8) | 0 (0.0) | 2 (13.3) | 0 (0.0) | 0.277 | 3 (21.4) | 0 (0.0) | 7 (25.9) | 0 (0.0) | >0.999 |
|
Elevated
QTc | 2 (4.8) | 0 (0.0) | 2 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.524 | 1 (7.1) | 0 (0.0) | 1 (3.7) | 0 (0.0) | >0.999 |
|
Fatigue | 22 (53.7) | 0 (0.0) | 14 (53.8) | 0 (0.0) | 8 (53.3) | 0 (0.0) | >0.999 | 8 (57.1) | 0 (0.0) | 14 (51.9) | 0 (0.0) | >0.999 |
|
Nausea | 13 (31.7) | 0 (0.0) | 8 (30.8) | 0 (0.0) | 5 (33.3) | 0 (0.0) | >0.999 | 2 (14.3) | 0 (0.0) | 11 (40.7) | 0 (0.0) | 0.156 |
|
Anorexia | 15 (36.6) | 0 (0.0) | 11 (42.3) | 0 (0.0) | 4 (26.7) | 0 (0.0) | 0.502 | 5 (35.7) | 0 (0.0) | 10 (37.0) | 0 (0.0) | >0.999 |
|
Diarrhea | 4 (9.8) | 0 (0.0) | 4 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.278 | 1 (7.1) | 0 (0.0) | 3 (11.1) | 0 (0.0) | >0.999 |
|
Constipation | 8 (19.5) | 0 (0.0) | 8 (30.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.018 | 3 (21.4) | 0 (0.0) | 5 (18.5) | 0 (0.0) | >0.999 |
|
Neuropathy | 14 (34.1) | 0 (0.0) | 13 (50.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 0.006 | 7 (50.0) | 0 (0.0) | 7 (25.9) | 0 (0.0) | 0.170 |
|
Infection | 5 (12.2) | 4 (9.8) | 4 (15.4) | 4 (15.4) | 1 (6.7) | 0 (0.0) | 0.434 | 1 (7.1) | 1 (7.1) | 4 (14.8) | 3 (11.1) | 0.227 |
|
Non-infectious
fever | 8 (19.5) | 0 (0.0) | 4 (15.4) | 0 (0.0) | 4 (26.7) | 0 (0.0) | 0.636 | 1 (7.1) | 0 (0.0) | 7 (25.9) | 0 (0.0) | 0.645 |
Discussion
Eribulin is an antitumor-drug derived from the
marine sponge Halichondria okadai, which acts as a
microtubule dynamics inhibitor. The clinical benefit of eribulin to
STS was confirmed by phase III trial (E7389-G00-309) in which
eribulin showed significant OS prolongation compared to dacarbazine
(3). This phase III trial, however,
was designed to enroll only patients with L-sarcoma who had
received at least two previous systemic chemotherapy treatments,
including anthracycline. Thus, high-level clinical evidence
regarding eribulin for STS is limited to L-sarcoma as a 3rd- or
later-line therapy.
Differences in the efficacy of eribulin by
histological subtype have been evaluated in some analyses. One
phase II trial incorporating STS patients with various STS
histological subtypes suggested that the survival benefits of
eribulin for STS was greater in L-sarcoma patients (5), and was the basis of the inclusion
criteria for the 309 phase III trial. In Japan, a similar tendency
has been observed in both prospective and retrospective data: The
prognoses of L-sarcoma patients receiving eribulin tends to be
longer than those of non-L-sarcoma patients (6-8).
Our retrospective analysis is also consistent with previous
clinical data (2), with differences
in the PFS of leiomyosarcoma and liposarcoma patients (Fig. 1B). In a subanalysis of the 309 phase
III trial, in terms of hazard ratio to control arm, the OS benefit
of eribulin was not relevant in leiomyosarcoma compared to
liposarcoma (9,10), and in the U.S. and Europe,
leiomyosarcoma is excluded from eribulin indications (4). The OS, PFS and response rates in the
309 phase III trial, however, were not so change between
leiomyosarcoma and liposarcoma. The differences in PFS between
leiomyosarcoma and liposarcoma in our analysis might have been
influenced by another confounding factor; one candidate is the
difference in treatment line.
In the 309 phase III trial, a treatment history of
two or more chemotherapies was the indication for enrollment, and
there were no apparent differences in treatment line between the
eribulin arm and the control arm, nor between leiomyosarcoma and
liposarcoma in the eribulin arm (9,10). In
our retrospective analysis, liposarcoma patients tended to receive
eribulin as a second-line therapy at high rates, which might have
led to the differences in PFS between leiomyosarcoma and
liposarcoma patients, arising from the fact that that there were
other treatment options considered to be more effective in
leiomyosarcoma patients than in liposarcoma patients, such as
gemcitabine-based therapy and pazopanib (11,12).
In fact, our retrospective analysis showed no changes in OS between
leiomyosarcoma and liposarcoma, which might be a result of
differences in treatment options other than eribulin.
Regarding safety, L-sarcoma patients with or without
a second line, who showed longer PFS, tended to have had more
cumulative doses of eribulin, so some adverse events related to
cumulative exposure to eribulin, such as myelosuppression and
peripheral neuropathy, were observed in L-sarcoma patients and/or
second-line patients (Table III),
but most of these events were considered tolerable.
Recently, personalized therapies for STS have
emerged, and biomarkers and/or patients' factors affecting the
efficacy of eribulin have been evaluated (13,14).
The histological subtype of L-sarcoma is now considered to be a
positive predictive factor of efficacy of eribulin, but there have
been non-L-sarcoma patients whose clinical data indicate a benefit
from eribulin treatment (7,8). Treatment line, however, also affects
the efficacy of eribulin and the patient's prognosis, so in
considering new predictive markers of eribulin, the possibility of
differences in treatment line should be considered.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN designed the study and wrote the initial draft of
the manuscript. KH, YF, TT, KA, SM, JT, MO, ST, MN, XW, AO, YS, NF
and TU provided clinical treatment. ST supervised the manuscript
and clinical treatment. All authors approved the final version of
the manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the
institutional review board of Cancer Institute Hospital of Japanese
Foundation for Cancer Research and written informed consent was
obtained from each patient. Written informed consent was obtained
from each participant.
Patient consent for publication
Patient consent for publication was obtained from
each participant.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeVita VT Jr, Lawrence TS and Rosenberg
SA: DeVita, Hellman, and Rosenberg's Cancer Principles &
Practice of Oncology 11th edition. Lippincott Williams &
Wilkins, Philadelphia, PA, 2018.
|
|
2
|
Kawai A, Yonemori K, Takahashi S, Araki N
and Ueda T: Systemic therapy for soft tissue sarcoma: Proposals for
the optimal use of pazopanib, trabectedin, and eribulin. Adv Ther.
34:1556–1571. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schöffski P, Chawla S, Maki RG, Italiano
A, Gelderblom H, Choy E, Grignani G, Camargo V, Bauer S, Rha SY, et
al: Eribulin versus dacarbazine in previously treated patients with
advanced liposarcoma or leiomyosarcoma: A randomised, open-label,
multicentre, phase 3 trial. Lancet. 387:1629–1637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Osgood CL, Chuk MK, Theoret MR, Huang L,
He K, Her L, Keegan P and Pazdur R: FDA approval summary: Eribulin
for patients with unresectable or metastatic liposarcoma who have
received a prior anthracycline-containing regimen. Clin Cancer Res.
23:6384–6389. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schöffski P, Ray-Coquard IL, Cioffi A, Bui
NB, Bauer S, Hartmann JT, Krarup-Hansen A, Grünwald V, Sciot R,
Dumez H, et al: European Organisation for Research and Treatment of
Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) Activity
of eribulin mesylate in patients with soft-tissue sarcoma: A phase
2 study in four independent histological subtypes. Lancet Oncol.
12:1045–1052. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kawai A, Araki N, Naito Y, Ozaki T,
Sugiura H, Yazawa Y, Morioka H, Matsumine A, Saito K, Asami S and
Isu K: Phase 2 study of eribulin in patients with previously
treated advanced or metastatic soft tissue sarcoma. Jpn J Clin
Oncol. 47:137–144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kobayashi E, Naito Y, Asano N, Maejima A,
Endo M, Takahashi S, Megumi Y and Kawai A: Interim results of a
real-world observational study of eribulin in soft tissue sarcoma
including rare subtypes. Jpn J Clin Oncol. 49:938–946.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakamura T, Tsukushi S, Asanuma K,
Katagiri H, Ikuta K, Nagano A, Kozawa E, Yamada S, Shido Y, Yamada
K, et al: The clinical outcome of eribulin treatment in Japanese
patients with advanced soft tissue sarcoma: A Tokai Musculoskeletal
oncology consortium study. Clin Exp Metastasis. 36:343–350.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Blay JY, Schöffski P, Bauer S,
Krarup-Hansen A, Benson C, D'Adamo DR, Jia Y and Maki RG: Eribulin
versus dacarbazine in patients with leiomyosarcoma: Subgroup
analysis from a phase 3, open-label, randomised study. Br J Cancer.
120:1026–1032. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Demetri GD, Schöffski P, Grignani G, Blay
JY, Maki RG, Van Tine BA, Alcindor T, Jones RL, D'Adamo DR, Guo M
and Chawla S: Activity of eribulin in patients with advanced
liposarcoma demonstrated in a subgroup analysis from a randomized
phase III study of eribulin versus dacarbazine. J Clin Oncol.
35:3433–3439. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hensley ML, Maki R, Venkatraman E, Geller
G, Lovegren M, Aghajanian C, Sabbatini P, Tong W, Barakat R and
Spriggs DR: Gemcitabine and docetaxel in patients with unresectable
leiomyosarcoma: Results of a phase II trial. J Clin Oncol.
20:2824–2831. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wiemer EAC, Wozniak A, Burger H, Smid M,
Floris G, Nzokirantevye A, Sciot R, Sleijfer S and Schöffski P:
Identification of microRNA biomarkers for response of advanced soft
tissue sarcomas to eribulin: Translational results of the EORTC
62052 trial. Eur J Cancer. 75:33–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kobayashi H, Okuma T, Oka H, Okajima K,
Ishibashi Y, Zhang L, Hirai T, Ohki T, Tsuda Y, Ikegami M, et al:
Body composition as a predictor of toxicity after treatment with
eribulin for advanced soft tissue sarcoma. Int J Clin Oncol.
24:437–444. 2019.PubMed/NCBI View Article : Google Scholar
|