Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal
disease with an extremely low (<6%) overall survival (OS) rate
(1,2). Recently, 5-fluorouracil, leucovorin,
irinotecan, and oxaliplatin (FOLFIRINOX) therapy and gemcitabine
(GEM) plus nab-paclitaxel (PTX; GnP) therapy were revealed
to improve treatment outcomes among patients with unresectable PDAC
(3,4); however, surgery remains the only
method for achieving cure or long-term survival. Previously, we
attempted radical surgery for patients with localized disease,
including wide lymph node dissection and complete removal of the
extrapancreatic nerve plexus around the superior mesenteric artery
or celiac axis, in an effort to extend survival (5,6).
Moreover, in some cases, en bloc resection of the pancreatic head
with the superior mesenteric artery was performed (7). However, even in patients who underwent
R0 resection, the 5-year survival rate was poor (7-24%), and the
median survival was approximately 1 year in most series. These
unfortunate results indicating that surgery alone is inadequate are
likely attributable to early distant metastasis before surgery
(8). Therefore, postoperative
adjuvant chemotherapy is important (9). Recently, it was demonstrated that
adjuvant chemotherapy for PDAC with S-1, an oral fluoropyrimidine
analog, significantly improved OS and recurrence-free survival
after PDAC resection compared with the effects of GEM (10).
An important limitation of adjuvant chemotherapy for
PDAC is that a certain proportion of patients cannot receive the
designated therapy because of postoperative complications or early
disease recurrence (11-13).
For this reason, preoperative neoadjuvant chemotherapy (NAC) has
attracted attention. The theoretical advantages of NAC include the
early treatment of occult metastases, reduction of the risk of
tumor seeding during surgery, and improved tolerance compared with
postoperative therapy (14).
Meanwhile, the potential disadvantages include problems related to
biliary drainage during chemotherapy, potential progression to an
unresectable stage in patients whose disease does not respond to
therapy, and an increasing risk of postoperative complications
(14).
Recently, several important studies of preoperative
chemotherapy for unresectable or borderline resectable PDAC have
been reported (15-18).
However, few studies have assessed NAC in the treatment of
resectable PDAC. We previously used NAC for resectable PDAC and
found that NAC with GEM-based regimens is useful for pancreatic
head cancer with lymph node metastases (19). However, the effectiveness of GnP
therapy for patients with resectable PDAC has not been
investigated. Therefore, we conducted a phase I study of NAC using
GnP for resectable PDAC to determine the maximum tolerated dose
(MTD) of each drug.
Materials and methods
Patient selection. Patients with
radiologically proven resectable pancreatic cancer were eligible
for this study. Other eligible criteria included an age of 20-79
years; Eastern Cooperative Oncology Group performance status of 0
or 1; and adequate renal function (normal serum creatinine and
blood urea nitrogen levels), liver function [total bilirubin level,
<2.5x the upper normal limit (UNL) or <3x the UNL after
biliary drainage if the patient had jaundice and serum transaminase
(GOT, GPT) levels, and <2.5x the UNL or <3x the UNL after
biliary drainage if the patient had jaundice], bone marrow reserve
(white blood cell count, 4,000-12,000 mm3; neutrophil
count, >2,000 mm3; platelet count, >100,000
mm3; and hemoglobin level, >9.5 g/dl), and pulmonary
function (PaO2, >70 mmHg). All enrolled patients were
required to have completed or discontinued prior treatment (tumor
resection, chemotherapy, immunotherapy, or radiotherapy) at least 4
weeks prior to study enrollment.
The exclusion criteria were as follows: Pulmonary
fibrosis or interstitial pneumonia, marked pleural or pericardial
effusion or marked peripheral edema, severe heart disease,
difficult-to-control diabetes mellitus, active infection, pregnancy
or lactation, childbearing age in women who did not use effective
contraception, severe drug hypersensitivity, appearance of distant
metastases during preoperative chemotherapy, severe neurological
impairment or mental disorder, active concomitant malignancy, and
other serious medical conditions.
This clinical trial was approved by the
Institutional Review Board of Kanazawa University Hospital (no.
5849) and registered with the UMIN Clinical Trials Registry (ID:
UMIN000011062).
Study design
This was an open-label, single-center,
nonrandomized, dose-escalation phase I study. The primary endpoints
of this study were the optimal dose and safety of GnP therapy as
preoperative chemotherapy. The secondary endpoints were
disease-free survival (DFS), treatment response, and adverse events
including postoperative complications. All laboratory tests to
assess eligibility were completed within 7 days prior to the start
of treatment. Nab-PTX was administered for 60 min followed
by an infusion of GEM for 30 min intravenously on days 1, 8 and 15
in two 28-day cycles. Surgery was performed >14 days after
chemotherapy ended. The dose of each drug in this study was planned
as follows: Level 1, 75 mg/m2/day for nab-PTX and
600 mg/m2/day for GEM; level 2, 100 mg/m2/day
for nab-PTX and 800 mg/m2/day for GEM; and level
3, 125 mg/m2/day for nab-PTX and 1,000
mg/m2/day for GEM.
Definition of dose-limiting toxicities
(DLTs) and MTD
DLTs were assessed during both treatment cycles. DLT
was defined using the National Cancer Institute Common Toxicity
Criteria for Adverse Events version 4.0(20) as one or more of the following
effects attributable to the study drug: i) Grade 3/4 neutropenia
complicated by fever; ii) grade 4 neutropenia lasting longer than 4
days; iii) grade 4 thrombocytopenia; iv) any other grade 3/4
non-hematologic toxicity excluding anorexia, nausea, and vomiting
in the absence of an appropriate antiemetic; and v) delayed
recovery from treatment-related toxicity for more than 2 weeks. At
least 10 patients were enrolled at each dose level. If DLTs were
observed or treatment could not be completed in <50% of
patients, dose escalation was continued. If DLTs were observed or
treatment could not be completed in >50% of patients, treatment
at that dose was discontinued. The MTD was then set at the
preceding dose level.
Assessment of efficacy
Treatment responses were evaluated according to the
Response Evaluation Criteria in Solid Tumors (RECIST) version
1.1(21). Complete response was
defined as the disappearance of all clinical evidence of a
measurable tumor. Partial response was defined as a ≥30% reduction
in the sum of the products of two perpendicular diameters of all
measurable lesions compared with the baseline values with no
evidence of new lesions. Stable disease (SD) was defined as a
<30% reduction or <20% increase in the sum of the products of
two perpendicular diameters of all measurable lesions compared with
the baseline values with no evidence of new lesions. Progressive
disease (PD) was defined as an increase of ≥20% in the sum of the
products of two perpendicular diameters of all measurable lesions
compared with the baseline values, the appearance of any new
lesion, or deterioration of clinical status consistent with disease
progression. To assess objective responses, patients were evaluated
after two cycles of preoperative chemotherapy.
Postoperative complications
The global morbidity rate and types of complications
were evaluated according to the Clavien-Dindo classification
(22). Mortality was defined as any
deaths related to surgery.
Pathological diagnosis
All surgically resected specimens were immediately
fixed in 10% neutral-buffered formaldehyde solution. As described
previously, after the specimens had been cut horizontally into 5-mm
tissue blocks (23), they were
dehydrated and embedded in paraffin. Finally, 5-mm sections were
cut and stained with hematoxylin and eosin. Each section was
examined by pathologists using light microscopy. The tumors,
including the effect of preoperative chemotherapy, were evaluated
according to the General Rules for the Clinical and Pathological
Study of Pancreatic Cancer proposed by the Japanese Pancreatic
Cancer Group (24). The degree of
tumor destruction was graded on a scale of 1 to 4 as follows: Grade
1, poor or no response; grade 1a, estimated residual tumor rate
≥90%; grade 1b, residual tumor rate of 50-90%; grade 2 (moderate
response), residual tumor rate of 10-50%; grade 3 (marked
response), estimated residual tumor rate of <10%; and grade 4
(complete response), presence of no viable tumor cells.
Statistical analyses
Categorical variables were compared using the
Chi-squared test, Student's t-test, and the paired t-test.
P<0.05 indicated statistical significance. All analyses were
performed using SPSS II statistical software (version 23.0; SPSS,
Inc.).
Results
Patient characteristics
From May 2015 to March 2019, 39 patients (20 males
and 19 females) diagnosed with resectable pancreatic cancer were
enrolled in this study, as presented in Table I. Preoperative pathological
examination was attempted in all cases, but a definitive diagnosis
was obtained in only 29 of 39 cases (74.4%). The median age of
patients at the time of study entry was 68 years (range, 46-79).
All patients had an Eastern Cooperative Oncology Group performance
status of 0. The tumor location was the pancreatic head in 21
patients and body/tail in 18 patients. Finally, tumor resection was
performed for 33 (pancreatoduodenectomy in 17 patients and distal
pancreatectomy in 16 patients) of 39 patients entered in this phase
I trial (84.6%). Among the study patients, six (15.4%) could not
undergo tumor resection because of tumor progression.
 | Table ICharacteristics of the enrolled
patients. |
Table I
Characteristics of the enrolled
patients.
|
Characteristics | Level 1 | Level 2 | Total |
|---|
| Patients, n | 21 | 18 | 39 |
| Sex, n |
|
Male | 13 | 7 | 20 |
|
Female | 8 | 11 | 19 |
| Age, years |
|
Median | 68.5 | 68.0 | 68.3 |
|
Range | 46-78 | 57-79 | 46-79 |
| Location, n |
|
Head | 9 | 12 | 21 |
|
Body/tail | 12 | 6 | 18 |
| Surgery, n |
|
PD | 7 | 10 | 17 |
|
DP | 11 | 5 | 16 |
| Resection rate,
% | 85.7 | 83.3 | 84.6 |
Toxicity and postoperative
complications
As shown in the patient flow chart (Fig. 1), treatment was started at dose
level 1 in 21 patients. However, because of non-hematologic adverse
events in three patients (two cases of skin rash and one case of
biliary tract infection), treatment could not be continued, and
these patients withdrew from protocol treatment. The remaining 18
patients were able to complete two treatment cycles at this dose
level. During treatment, grade 3 adverse events were observed in
eight (44.4%) patients (neutropenia, anemia, thrombocytopenia,
and/or liver injury), necessitating the skipping of at least one
dose (Table II). Afterward, a
separate group of 18 patients started treatment at dose level 2;
however, three patients withdrew from protocol treatment because of
skin rash (two cases) and repeated acute pancreatitis (one case).
The remaining 15 patients were able to complete two treatment
cycles at this dose level (Fig. 1).
During the two cycles, grade 3 or 4 adverse events were observed in
9 of 15 patients (60.0%; neutropenia, anemia, thrombocytopenia, or
liver injury), and administration was skipped for at least one dose
(Table II). Therefore, further
dose escalation was not performed based on the aforementioned
criteria, and the MTD of NAC was set as dose level 1
(nab-PTX 75 mg/m2/day and GEM 600
mg/m2) for resectable PDAC. At both dose levels,
relatively mild non-hematological toxicities other than skin rash
including malaise, nausea, numbness, and anorexia occurred. Hair
loss was observed in all patients. Conversely, severe peripheral
neuropathies were not observed.
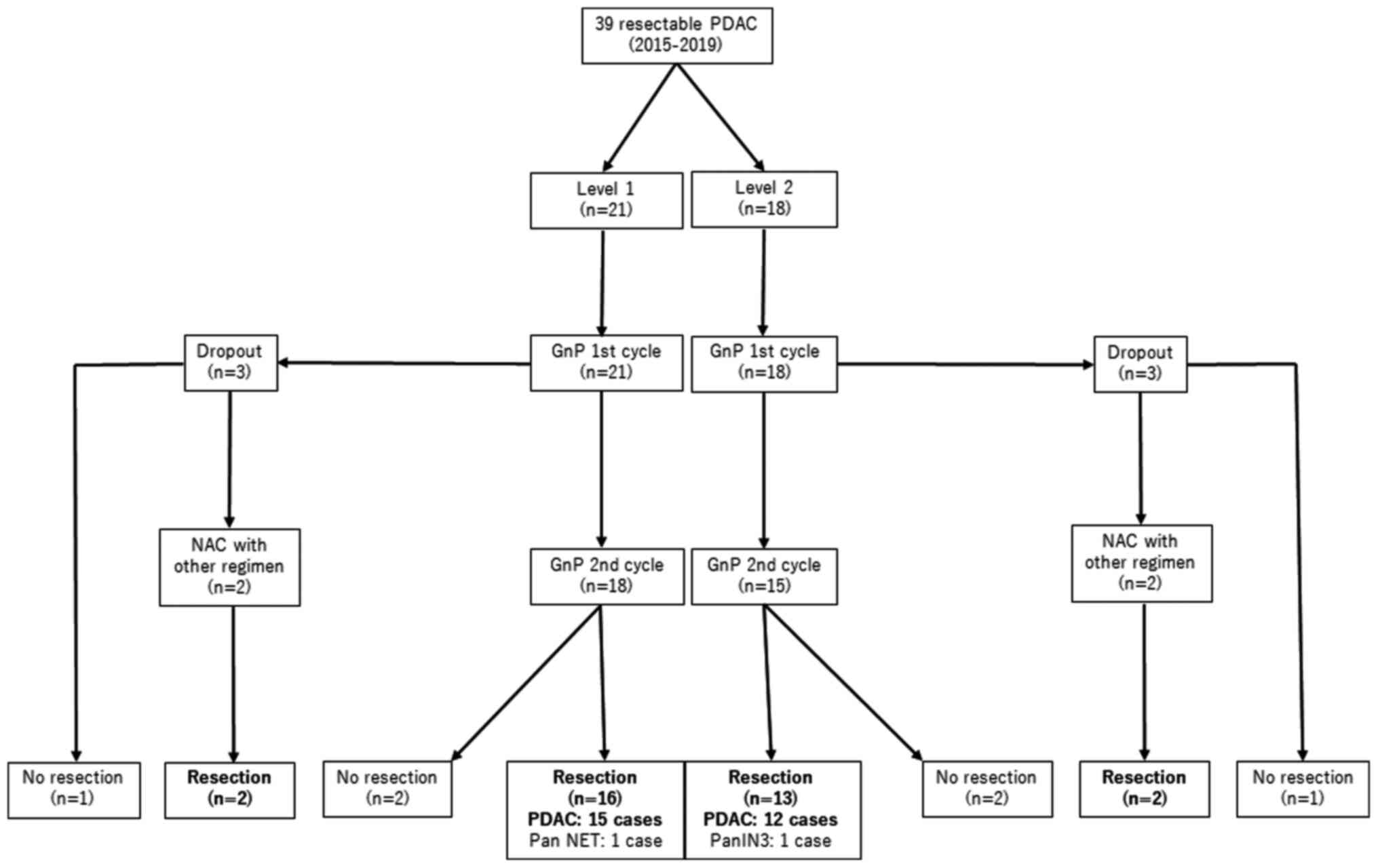 | Figure 1Patient flow chart. A total of 39
patients clinically diagnosed with PDAC were assigned to treatment
at dose level 1 (21 patients) or 2 (18 patients). At both levels,
three patients withdrew during the first cycle of GnP therapy,
events precluding resection occurred in two patients after the
completion of chemotherapy. Finally, 16 patients at dose level 1
and 13 patients at dose level 2 underwent tumor resection. Among
the six patients who withdrew from study treatment, NAC was
continued with other regimens, and tumor resection was subsequently
performed. A total of two patients who underwent resection were
determined to have PanNET and PanIN-3, and they were excluded from
the evaluation of therapeutic efficacy. GnP, gemcitabine plus
nab-paclitaxel; NAC, neoadjuvant chemotherapy; PDAC, pancreatic
ductal adenocarcinoma; PanNET, pancreatic neuroendocrine tumor;
PanIN, pancreatic intraepithelial neoplasia. |
 | Table IIGrade 3 or 4 adverse events in
patients who completed chemotherapy. |
Table II
Grade 3 or 4 adverse events in
patients who completed chemotherapy.
| Adverse event | Level 1, n (%)
(n=18) | Level 2, n (%)
(n=15) | Total, n (%)
(n=33) |
|---|
| Anemia | 1 (5.6) | 0 (0.0) | 1 (3.0) |
| Neutropenia | 8 (44.4) | 9 (60.0) | 17 (51.5) |
|
Thrombocytopenia | 1 (5.6) | 1 (6.7) | 2 (5.1) |
| Liver
dysfunction | 1 (5.6) | 0 (0.0) | 1 (3.0) |
Postoperative complications are listed in Table III. According to the International
Study Group on Pancreatic Fistula classification (25), grade B pancreatic fistula,
classified as grade IIIa according to the Clavien-Dindo
classification, was observed in 4 of 16 patients treated at dose
level 1 and 7 of 13 patients treated at dose level 2. Other
complications at dose level 1 included intestinal obstruction and
diarrhea, and delayed gastric empty, diarrhea, infectious
complications (deep surgical site infection), acute appendicitis,
and pyogenic spondylitis), and ischemic colitis were observed at
dose level 2.
 | Table IIIPostoperative complications. |
Table III
Postoperative complications.
| Clavien-Dindo's
classification grade | Level 1, n
(n=16) | Level 2, n
(n=13) | Total, n
(n=29) |
|---|
| Grade I/II |
|
Diarrhea | 2 | 1 | 3 |
|
Pyogenic
spondylitis | 0 | 1 | 1 |
| Grade IIIa |
|
ISGPF Grade
B | 4 | 7 | 13 |
|
Ileus | 1 | 0 | 1 |
|
Deep
SSI | 0 | 1 | 1 |
|
Ischemic
colitis | 0 | 1 | 1 |
| Grade IIIb |
|
Acute
appendicitis | 0 | 1 | 1 |
| Grade IV/V | 0 | 0 | 0 |
Efficacy
Two patients who underwent resection were determined
to not have PDAC (neuroendocrine tumor and PanIN-3), and they were
excluded from the evaluation of therapeutic efficacy. Concerning
the remaining 31 patients with histologically confirmed PDAC,
partial response, SD, and PD were recorded in 4 (12.9%), 24
(77.4%), and 3 patients (9.7%), respectively, on computed
tomography (CT) according to RECIST. Three cases of PD involved
distant metastases (liver or peritoneal metastasis), and a patient
initially judged to have SD was diagnosed with peritoneal
metastasis during surgery. Table
IV shows the efficacy data for NAC. Tumor size on CT was
significantly decreased from 24.7 to 23.1 mm (P=0.049). The CA-19-9
level before treatment was elevated (>37 IU/ml) in 18 of 31
(58.1%) patients. Of these 31 patients, the average CA19-9 level
was significantly decreased from 166.1 to 82.5 IU/ml after
preoperative chemotherapy (P=0.003). Significant reductions of CEA
and DUPAN-2 levels were not observed after preoperative treatment.
Positron emission tomography-CT was performed using
18F-fluorodeoxyglucose (FDG) in all patients, before and
after preoperative chemotherapy. A significant decrease in the FDG
maximum standardized uptake value from 6.0 to 3.3 was documented
after preoperative therapy (P=0.001).
 | Table IVChanges in clinical factors after
NAC. |
Table IV
Changes in clinical factors after
NAC.
| Factor | Before NAC | After NAC | P-value |
|---|
| Tumor size on CT,
mm | 24.7 | 23.1 | 0.049 |
|
18FDG-PET SUVmax | 6.0 | 3.3 | 0.001 |
| CA19-9, U/ml | 166.1 | 82.5 | 0.003 |
| CEA, ng/ml | 3.8 | 4.1 | 0.512 |
| DUPAN-2, U/ml | 649.5 | 2,600.1 | 0.355 |
In the 27 patients who underwent resection as
planned, the pathological treatment effect was judged as grade Ia
in 21 patients (77.8%), grade Ib in four patients (14.8%), and
grade II in two patients (7.4%) patients. There was no case judged
as grade 3 or 4. The pathological tumor size was 32.8 mm (range,
7-65), and lymph node metastasis was detected in 16 of 27 patients
(59.3%). R0 resection was performed in 24 of 27 patients as planned
(88.9%). Although the observation period was short and the data are
immature, OS and DFS among these patients were 25.7 and 20.2
months, respectively. Adjuvant chemotherapy with oral S-1 was
performed for 21 of 27 patients (77.8%).
Four patients who withdrew from protocol treatment
because of skin rash continued preoperative chemotherapy with other
regimens (GEM alone or modified FOLFIRINOX), and R0 resection was
performed for all four patients. Including these patients, R0
resection was performed in 28 of 37 enrolled patients (75.7%).
Discussion
The National Comprehensive Cancer Network guidelines
recommend that NAC with GnP or (modified) FOLFIRINOX should be
considered for patients with borderline resectable PDAC (26). Conversely, for resectable PDAC,
frontline surgery is recommended for all but high-risk patients.
However, patients with pancreatic head cancer in particular require
a long postoperative recovery period before adjuvant chemotherapy
because of surgical stress. Therefore, preoperative therapy could
be expected to reduce the risk of distant metastasis. We introduced
NAC for resectable PDAC using GEM plus S-1 in 2007 (14,27).
Recently, the effectiveness of NAC with GEM plus S-1 for resectable
PDAC was demonstrated in a randomized phase II/III clinical trial
(28), and this regimen has become
the standard treatment in Japan.
GnP and FOLFIRINOX are currently in use as NAC for
patients with borderline resectable or locally advanced PDAC
(18,29,30).
Okada et al (31) reported a
phase I study of GnP as NAC for borderline resectable PDAC at doses
equivalent to level 3 in the current study (nab-PTX 125
mg/m2/day and GEM 1,000 mg/m2). The mean
frequency of administration in their study was 4.6 of 6 possible
doses during two treatment cycles, compared with 5.4 at level 1 and
4.8 at level 2 in our series. From these results, our results are
considered valid, but because <50% of planned doses were
administered at level 2, we concluded that the MTD was dose level
1. The most frequent DLT was neutropenia, as described previously
(4,31). Whether the dose of the drug should
be increased in combination with granulocyte colony-stimulating
factor to prevent neutropenia is a future issue. There were no
serious adverse events such as sepsis, febrile neutropenia,
interstitial pneumonia, and severe peripheral sensory neuropathy in
the current study, probably because of the short-term treatment
duration. Skin rash caused by nab-PTX, occurring in four
patients (10.0%), was not a severe adverse event, but this event
precluded further protocol treatment. Fortunately, these four
patients were able to continue chemotherapy with another regimen
(GEM alone or modified FOLFIRINOX therapy) and finally undergo
surgical resection.
Postoperative complications attributable to NAC were
not recognized. Although infectious complications were observed in
patients treated at dose level 2, these events were considered
accidental phenomena. Acute appendicitis developed immediately
before discharge, and infectious spondylitis occurred in a patient
with steroid-treated autoimmune disease. The most common
complication was grade B pancreatic fistula. There was no severe
complication classified as grade IV or V in this study. Two cycles
of GnP therapy as NAC are not considered to have safety issues
based on these findings.
The most important purposes of preoperative
chemotherapy are preventing metastasis from the primary site and
treating occult metastasis. Recently, it was reported that
epithelial-mesenchymal transition (EMT) of cancer stem cells plays
an important role in tumor invasion, metastasis, and
chemoradioresistance in PDAC (32).
PTX suppresses the EMT of cancer stem cells and activation of
cancer-associated fibroblasts (33-36).
We previously reported that NAC with GnP reduced the numbers of
cancer-associated fibroblasts (37). Therefore, we determined that GnP
therapy is most suitable for preoperative chemotherapy
theoretically (19).
However, it was recently demonstrated that
FOLFIRINOX was associated with longer OS than GnP in patients with
unresectable locally advanced or metastatic PDAC (38). Moreover, FOLFIRINOX treatment was
reported to decrease the frequency of lymph node metastasis
(30,39), consistent with our results
supporting the effectiveness of NAC in patients with node-positive
pancreatic head cancer (19). In
addition, in a study limited to NAC for resectable and borderline
resectable pancreatic head cancer, the OS of the FOLFIRINOX group
was slightly higher than that of the GnP group, although the
difference was not significant because of the limited frequency of
treatment in the preoperative period (39).
Recently, many studies on preoperative
chemoradiotherapy (CRT) for locally advanced or borderline PDAC
have been published and these data demonstrate that CRT improves
resectability, R0 resection rate, OS and DFS rate, and reveal a
survival advantage compared with NAC cases (40,41).
On the other hand, few authors have prospectively evaluated the
role of CRT for preoperative treatment for resectable PDAC
(42). Theoretically, radiotherapy
may have a marked impact on microscopic tumor spread and local
disease management. However, it is reported that radiotherapy could
induce EMT in cancer cells (43)
and increase distant metastasis in patients with locally advanced
PDAC (44). In recent years,
progression of image diagnosis made possible accurate understand of
the tumor spreading (45), for that
reason we have adopted optimal resection rather than radiation for
local spread of resectable PDAC.
In pancreatic head cancer treatment in particular,
postoperative chemotherapy may often be limited by surgical stress,
and thus, the concept that more powerful regimens should be used
before surgery was also affirmed. Uesaka et al (10) obtained good results for
postoperative adjuvant chemotherapy with oral S-1, which has become
the standard treatment in Japan, in patients with PDAC. In this
study, adjuvant chemotherapy with S-1 was administered to
approximately 80% of patients with PDAC who underwent resection. In
our previous study (19), it was
revealed that NAC with GEM-based regimens improve prognosis of
pancreatic head cancer with lymph node metastases compared with
surgery first cases. That study included three regimens of
preoperative chemotherapy, GEM plus S-1, GEM alone and GnP therapy.
Therefore, we will clarify the long term outcomes of NAC with
single regimen of GnP in the future.
In conclusion, NAC with two cycles of GnP for
resectable PDAC was safe and feasible at dose level 1
(nab-PTX 75 mg/m2/day and GEM 600
mg/m2/day). In the future, it will be necessary to
verify the long-term results of this regimen, and a phase II
clinical trial based on the results of this study is
anticipated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT, TM and TO designed the present study. IN and SF
performed the statistical and pharmacokinetic analyses. RG, YO,
HSh, SN, JK, HSa and MS explained the content of chemotherapy in
this clinical trial to patients, obtained their consent,
administered chemotherapy and performed pharmacokinetic analyses.
TY, KO, HM and KN explained the content of surgery in this clinical
trial to patients, obtained their consent, contributed to the
clinical trial operation, and were also involved in the
pathological diagnosis and statistical analysis. IM, MO and HI
created pathological specimens, and made all pathological final
diagnoses. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The clinical trial was approved by the Institutional
Review Board of Kanazawa University Hospital (approval no. 5849)
and registered with the UMIN Clinical Trials Registry (ID:
UMIN000011062), and written informed consent was obtained from each
patient at enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cancer Research UK: Pancreatic Cancer
Mortality Statistics. Available from: urihttp://www.cancerresearchuk.org/cancer-info/cancerstats/types/pancreas/mortality/simplehttp://www.cancerresearchuk.org/cancer-info/cancerstats/types/pancreas/mortality/.
|
|
3
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Eng J Med. 364:1817–1825.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nagakawa T, Kurachi M, Konishi K and
Miyazaki I: Translateral retroperitoneal approach in radical
surgery for pancreatic carcinoma. Jpn J Surg. 12:229–233.
1982.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nagakawa T, Nagamori M, Futakami F,
Tsukioka Y, Kayahara M, Ohta T, Ueno K and Miyazaki I: Result of
extensive surgery for pancreatic carcinoma. Cancer. 77:640–645.
1996.PubMed/NCBI
|
|
7
|
Kitagawa H, Tajima H, Nakagawara H, Makino
I, Miyashita T, Shoji M, Nakanuma S, Hayashi N, Takamura H, Ohta T
and Ohtake H: En bloc vascular resection for the treatment of
borderline resectable pancreatic head carcinoma. Mol Clin Oncol.
2:369–374. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Evans DB, Abbruzzese JL and Willett CG:
Cancer of the pancreas. In: Cancer: Priciples and Practice of
Oncology (6th edition). DeVita VT, Hellman S and Rosenberg SA
(eds). Lippincott Williams and Wilkins, Philadelphia, PA,
pp1126-1161, 2001.
|
|
9
|
Ottle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomized,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Klinlenbijl JH, Jeekel J, Sahmoud T, van
Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT,
Hennipman A and Wils J: Adjuvant radiotherapy and 5-fluorouracil
after curative resection of cancer of the pancreas and
periampullary region: Phase III trial of the EORTC gastrointestinal
tract cancer cooperative group. Ann Surg. 230:776–784.
1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Spitz FR, Abbruzzese JL, Lee JE, Pisters
PW, Lowy AM, Fenoglio CJ, Cleary KR, Janjan NA, Goswitz MS, Rich TA
and Evans DB: Preoperative and postoperative chemoradiation
strategies in patients treated with pancreaticoduodenectomy for
adenocarcinoma of the pancreas. J Clin Oncol. 15:928–937.
1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yeo CJ, Abrams RA, Grochow LB, Sohn TA,
Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, et
al: Pancreaticoduodenectomy for pancreatic adenocarcinoma:
Postoperative adjuvant chemoradiation improves survival. A
prospective, single-institution experience. Ann Surg. 225:621–636.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tajima H, Ohta T, Kitagawa H, Okamoto K,
Sakai S, Makino I, Kinoshita J, Furukawa H, Nakamura K, Hayashi H,
et al: Pilot study of neoadjuvant chemotherapy with gemcitabine and
oral S-1 for resectable pancreatic cancer. Exp Therap Med.
3:787–792. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh
Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, et
al: Preoperative modified FOLFIRINOX treatment followed by
capecitabine-based chemoradiation for borderline resectable
pancreatic cancer: Alliance for clinical trials in oncology Trial
A021101. JAMA Surg. 151(e161137)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shubert CR, Bergquist JR, Groeschl RT,
Habermann EB, Wilson PM, Truty MJ, Smoot RL, Kendrick ML, Nagorney
DM and Farnell MB: Overall survival is increased among stage III
pancreatic adenocarcinoma patients receiving neoadjuvant
chemotherapy compared to surgery first and adjuvant chemotherapy:
An intention to treat analysis of the National cancer database.
Surgery. 160:1080–1096. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nagakawa Y, Hosokawa Y, Nakayama H, Sahara
Y, Takishita C, Nakajima T, Hijikata Y, Kasuya K, Katsumata K,
Tokuuye K and Tsuchida A: A phase II trial of neoadjuvant
chemoradiotherapy with intensity-modulated radiotherapy combined
with gemcitabine and S-1 for borderline-resectable pancreatic
cancer with arterial involvement. Cancer Chemother Pharmacol.
79:951–957. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ettrich TJ, Berger AW, Perkhofer L, Daum
S, König A, Dickhut A, Wittel U, Wille K, Geissler M, Algül H, et
al: Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus
gemcitabine for resectable pancreatic cancer-the NEONAX trial
(AIO-PAK-0313), a prospective, randomized, controlled, phase II
study of the AIO pancreatic cancer group. BMC Cancer.
18(1298)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tajima H, Ohta T, Okazaki M, Yamaguchi T,
Ohbatake Y, Okamoto K, Nakanuma S, Kinoshita J, Makino I, Nakamura
K, et al: Neoadjuvant chemotherapy with gemcitabine-based regimens
improves prognosis of node positive resectable pancreatic head
cancer. Mol Clinic Oncol. 11:157–166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Common Terminology Criteria for Adverse
Event (CTCAE) Version 4.0. Department of Health and Human Services,
National Institutes of Health, National Cancer Institute, May 28,
2009.
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumors:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dino D, Demartines N and Clavian PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results lf a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Makino I, Kitagawa H, Ohta T, Nakagawara
H, Tajima H, Ohnishi I, Takamura H, Tani T and Kayahara M: Nerve
plexus invasion in pancreatic cancer: Spread patterns on
histopathologic and embryological analysis. Pancreas. 37:358–365.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Classification of Pancreatic Carcinoma
(Fourth English Edition). Japan Pancreas Society, Kanehara &
Co., Ltd., 2017.
|
|
25
|
Bassi C, Marchegiani G, Dervenis C, Sarr
M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink
MG, et al: The 2016 update of the international study group (ISGPS)
definition and grading of postoperative pancreatic fistula: 11
years after. Surgery. 161:584–591. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
National Comprehensive Cancer Network:
Practice guidelines in oncology for pancreatic adenocarcinoma
version 1.2020, 2019.
|
|
27
|
Tajima H, Kitagawa H, Tsukada T, Nakanuma
S, Okamoto K, Sakai S, Makino I, Furukawa H, Nakamura K, Hayashi H,
et al: A phase I study of neoadjuvant chemotherapy with gemcitabine
plus oral S-1 for resectable pancreatic cancer. Mol Clinic Oncol.
1:768–772. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi
S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, et al: Randomized
phase II/III trial of neoadjuvant chemotherapy with gemcitabine and
S-1 versus upfront surgery for resectable pancreatic cancer
(Prep-02/JSAP05). Jpn J Clin Oncol. 49:190–194. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miyasaka Y, Ohtsuka T, Kimura R, Matsuda
R, Mori Y, Nakata K, Kakihara D, Fujimori N, Ohno T, Oda Y and
Nakamura M: Neoadjuvant chemotherapy with gemcitabine plus
nab-paclitaxel for borderline resectable pancreatic cancer
potentially improves survival and facilitates surgery. Ann Surg
Oncol. 26:1528–1534. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Macedo FI, Ryon E, Maithel SK, Lee RM,
Kooby DA, Fields RC, Hawkins WG, Williams G, Maduekwe U, Kim HJ, et
al: Survival outcomes associated with clinical and pathological
response following neoadjuvant FOLFIRINOX or
gemcitabine/nab-paclitaxel chemotherapy in resected pancreatic
cancer. Ann Surg. 270:400–413. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okada K, Hirono S, Kawai M, Miyazawa M,
Shimizu A, Kitahata Y, Ueno M, Hayami S and Yamaue H: Phase I study
of Nab-Paclitaxel plus gemcitabine as neoadjuvant therapy for
borderline resectable pancreatic cancer. Anticancer Res.
37:853–858. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Valle S, Martin-Hijano L, Alcalá S,
Alonso-Nocelo M and Sainz B Jr: The ever-evolving concept of the
cancer stem cell in pancreatic cancer. Cancers (Basel).
10(33)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang D, Sun L, Xian W, Liu F, Ling G,
Xiao L, Liu Y, Peng Y, Haruna Y and Kanwar YS: Low-dose paclitaxel
ameliorates renal fibrosis in rat UUO model by inhibition of
TGF-beta/Smad activity. Lab Invest. 90:436–447. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou J, Zhong DW, Wang QW, Miao XY and Xu
XD: Paclitaxel ameliorates fibrosis in hepatic stellate cells via
inhibition of TGF-beta/Smad activity. World J Gastroenterol.
16:3330–3334. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Choi HS, Savard CE, Choi JW, Kuver R and
Lee SP: Paclitaxel interrupts TGF-beta1 signaling between
gallbladder epithelial cells and myofibroblasts. J Surg Res.
141:183–191. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hirose A, Tajima H, Ohta T, Tsukada T,
Okamoto K, Nakanuma S, Sakai S, Kinoshita J, Makino I, Furukawa H,
et al: Low-dose paclitaxel inhibits the induction of
epidermal-mesenchymal transition in the human cholangiocarcinoma
CCKS-1 cell line. Oncol Lett. 6:915–920. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Miyashita T, Tajima H, Makino I, Okazaki
M, Yamaguchi T, Ohbatake Y, Nakanuma S, Hayashi H, Takamura H,
Ninomiya I, et al: Neoadjuvant chemotherapy with gemcitabine plus
Nab-paclitaxel reduces the number of cancer-associated fibroblasts
through depletion of pancreatic stroma. Anticancer Res. 38:337–343.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chan KKW, Guo H, Cheng S, Beca JM,
Redmond-Misner R, Isaranuwatchai W, Qiao L, Earle C, Berry SR,
Biagi JJ, et al: Real-world outcomes of FOLFIRINOX vs gemcitabine
and nab-paclitaxel in advanced pancreatic cancer: A
population-based propensity score-weighted analysis. Cancer Med.
9:160–169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dhir M, Zenati MS, Hamad A, Singhi AD,
Bahary N, Hogg ME, Zeh HJ III and Zureikat AH: FOLFIRINOX versus
gemcitabine/nab-paclitaxel for neoadjuvant treatment of resectable
and borderline resectable pancreatic head adenocarcinoma. Ann Surg
Oncol. 25:1896–1903. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Polistina F, Di Natale G, Bonciarelli G,
Ambfosino G and Frego M: Neoadjuvant strategies for pancreatic
cancer. World J Gastroenterol. 20:9374–9383. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gillen S, Schuster T, Meyer Zum
Büschenfelde C, Friess H and Kleeff J: Preoperative/neoadjuvant
therapy in pancreatic cancer: A systemic review and meta-analysis
of response and resection percentages. PLoS Med.
7(e1000267)2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Silvestris N, Brunetti O, Vasile E,
Cellini F, Cataldo I, Pusceddu V, Cattaneo M, Partelli S, Scartozzi
M, Aprile G, et al: Multimodal treatment of resectable pancreatic
ductal adenocarcinoma. Crit Rev Oncol Hematol. 111:152–165.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nakayama A, Ninomiya I, Harada S, Tsukada
T, Okamoto K, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi
H, et al: Metformin inhibits the radiation-induced invasive
phenotype of esophageal squamous cell carcinoma. Int J Oncol.
49:1890–1898. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Van Laethem JL, Hammel P, Mornex F, Azria
D, Van Tienhoven G, Vergauwe P, Peeters M, Polus M, Praet M, Mauer
M, et al: Adjuvant gemcitabine alone versus gemcitabine-based
chemoradiotherapy after curative resection for pancreatic cancer: A
randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J
Clin Oncol. 28:4450–4456. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mochizuki K, Gabata T, Kozaka K, Hattori
Y, Zen Y, Kitagawa H, Kayahara M, Ohta T and Matsui O: MDCT
findings of extrapancreatic nerve plexus invasion by pancreas head
carcinoma: Correlation with en bloc pathological specimens and
diagnostic accuracy. Eur Radiol. 20:1757–1767. 2010.PubMed/NCBI View Article : Google Scholar
|















