1. Introduction
Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) is the virus strain responsible for coronavirus
disease 2019 (COVID-19) which started in December 2019 in Wuhan
(China). The disease became a pandemic in a brief period and the
number of infected cases still keep growing worldwide. Up to the
25th of June, the WHO (World Health Organization) released a report
counting 9,296,202 cases total confirmed cases globally and
1,145,906 cases in Brazil (1).
COVID-19 is caused by a virus belonging to the
coronaviruses family of single-stranded RNA which can infect both
humans and some animals (2,3). Upon infection, patients' symptoms are
variable. Huang and colleagues published the clinical features of
41 infected patients and observed that the most common clinical
symptoms were fever, dry cough, dyspnoea, and bilateral
ground-glass opacities on chest computed tomography (CT) scans
(4). Patients also had pneumonia
and other complications including acute respiratory distress
syndrome followed by RNAaemia, acute cardiac injury and secondary
infection (4). The level of disease
severity could be attributed to different factors such as age,
presence of comorbid health conditions and also the viral load of
SARS-CoV-2, which was indicated as a potential marker for assessing
disease severity and prognosis (5).
The higher the viral load, the severe were the clinical outcomes in
patients, which also presented a long virus-shedding period
(5). The time of symptom's onset
varies, however most patients will develop symptoms within 11.5
days with a median incubation period of approximately 5.1 days
(6). Most patients who recovered
from SARS-CoV-2 infection showed the greatest severity of lung
disease on CT-scans at around 10 days after the initial onset of
symptoms and the chest CT image improvements appeared at
approximately 14 days (7). Current
diagnostic tests involve reverse transcription-polymerase chain
reaction (RT-PCR) using nasal swab (8), tracheal aspirate or bronchoalveolar
lavage samples which detect active infections (9) and serological testing that can detect
an active and also past infection (10). A Cochrane database systematic review
recently addressed the diagnostic accuracy of different SARS-CoV-2
antibody tests (11). This study
collected, and analysed, results from different reports for IgG,
IgM, IgA, total antibodies and IgG/IgM and overall they presented
low sensitivity during the first week since onset of symptoms (all
less than 30.1%) and the sensitivity increased in the second week,
presenting higher values in the third week. Besides, they mentioned
that there were not enough studies to evaluate sensitivity of tests
beyond 35 days post-symptom onset (11).
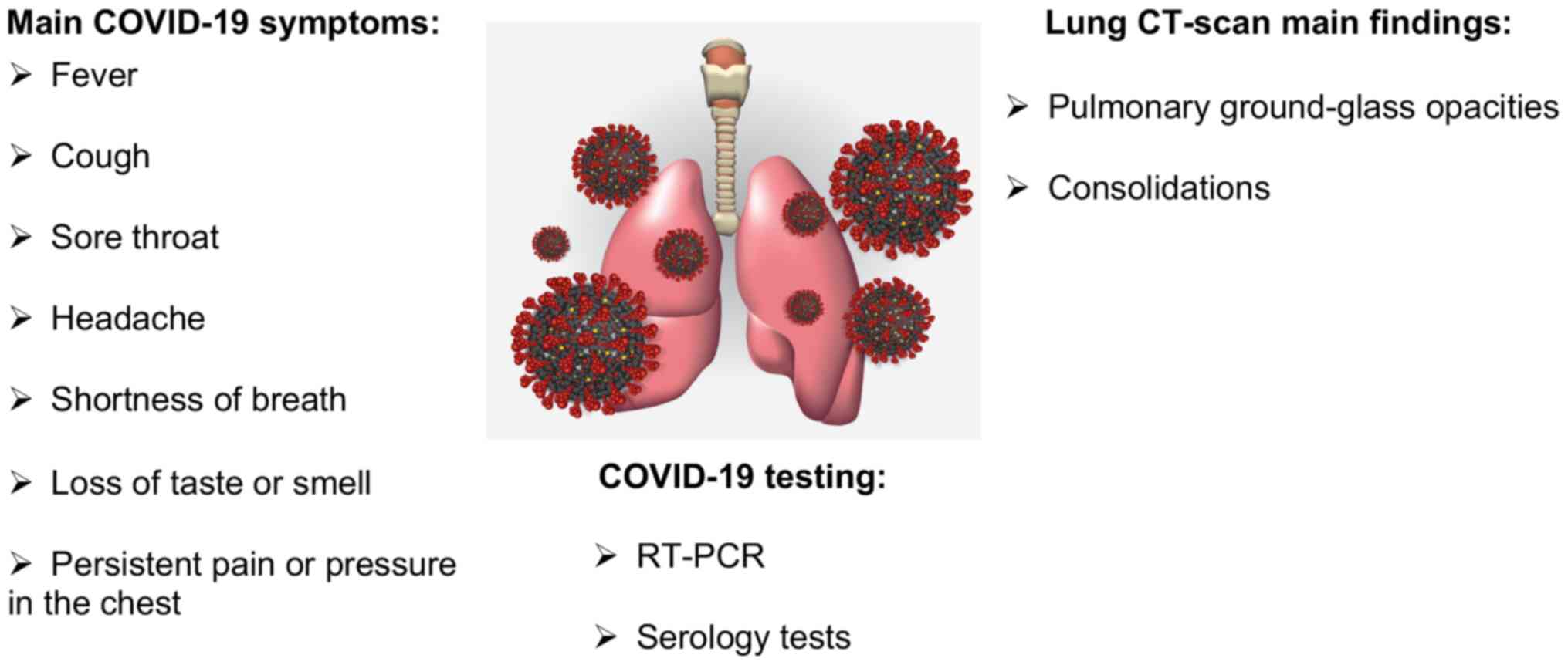
Fig. 1 summarises
the main symptoms, diagnostic methods and CT-scan findings upon
SARS-CoV-2 infection.
2. Cancer and COVID-19
The routine diagnoses and treatments for different
diseases including cancer were largely affected by the pandemic
outbreak. It is well known that cancer patients are often
immunocompromised, which make them more vulnerable than others to
infections. For example, when the impact of other infections on
cancer patients were analysed, such as the retrospective study
analysing influenza A (H1N1), these patients were shown to be more
prone to suffer severe symptoms and worse outcomes than the general
population (12). Very few studies
to date have addressed this issue concerning COVID-19. A recent
article published by The Lancet Oncology stated that patients with
cancer had a higher risk of severe symptoms and poor outcomes upon
COVID-19 infection than patients without cancer (13). Another Chinese study reported that
in 1,524 cancer patients, a 2-fold increased risk of COVID-19
infection was observed when compared with the general population
(14). Besides, the China Centres
for Disease Control and Prevention, after analysing 44,672
laboratory-confirmed cases nationwide, found 2.3% case fatality
rate of COVID-19 in the overall population, whereas for cancer
patients alone the rate was higher, 5.6% (15). Consequently, the WHO and CDC
websites recommend that confirmed COVID-19 patients should be
assessed for holding their cancer treatment regimens until they are
clear of the infection (16,17).
The mechanism of viral entry into cells and the
molecular machinery involved in this process could also explain the
disease severity and pathophysiological response of the human host.
In the following section, we will discuss briefly one of the
mechanisms described for this viral-host interaction.
3. SARS-CoV-2 entry into host cells
There are currently seven different coronaviruses
known to infect humans: MERS-CoV, SARS-CoV, SARS-CoV-2, NL63, HKU1,
OC43 and 229E. The first three are known to be more severe than the
last four (18). They are a large
family of single-stranded enveloped RNA viruses which have an
envelope-anchored spike protein (the transmembrane spike
glycoprotein or S-protein) responsible to mediate its entry into
host cells via membrane fusion (19). SARS-CoV-2 genome shows variability
in the region called the receptor-binding domain (RBD) in the spike
protein and this variability can influence the host range of the
SARS-CoV viruses (20,21). The RBD domain of SARS-CoV-2
S-protein has an affinity and recognizes angiotensin-converting
enzyme 2 (ACE2) receptors from humans and other species (22). Interestingly, experiments showed
that ACE2 knockout diminishes viral infection and replication in
mice infected with another type of coronavirus, the SARS-CoV
(23) that has a S-protein 80%
homologous with the one from SARS-CoV-2(24). These infected mice were shown to
present acute respiratory failure and lung parenchymal injury
(23). Regarding structural
analysis, the crystal structure of the C-terminal domain of
SARS-CoV-2 S-protein in complex with human ACE2 revealed an
ACE2-binding mode similar to that observed for SARS-CoV (25). This information could shed a light
in the pursuit of effective treatments.
Coronaviruses entry into host cells involve several
pathways, including endosomal and non-endosomal, in the presence of
different proteases (26-29).
In addition to ACE2 receptor, the viral entry was also shown to
rely on transmembrane protease serine 2 (TMPRSS2) protease activity
which is involved in the priming step of the S-protein (30). In addition, the SARS-CoV-2 cell
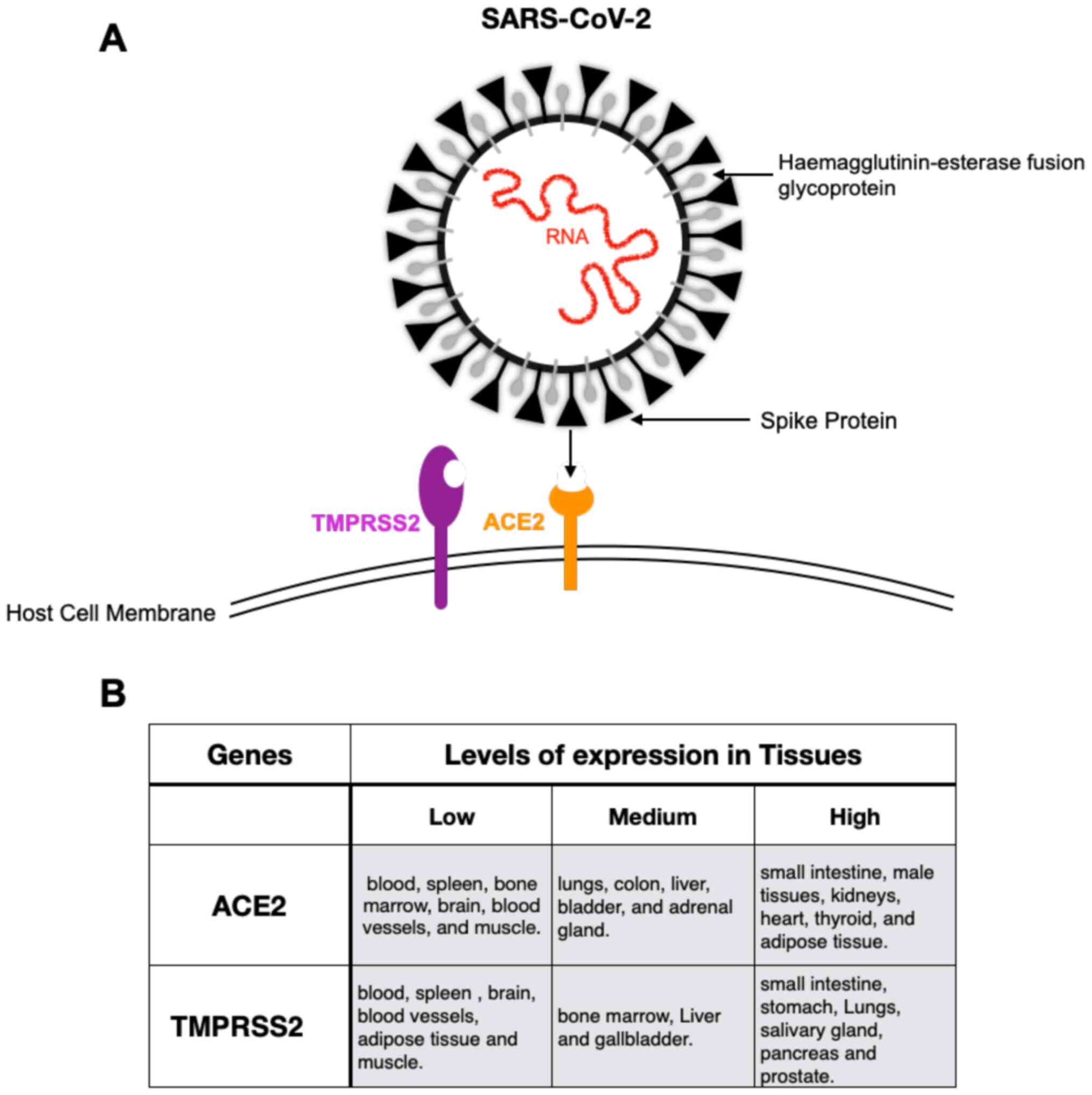
entrance was shown to be blocked by a TMPRSS2 inhibitor (30). Fig.
2A shows a simplified illustration of SARS-CoV-2 coming closer
to the host membrane where ACE2 and TMPRSS2 are membrane-bound.
Also, Fig. 2B shows a summary of
how ACE2 and TMPRSS2 expression varies in different tissues
according to different published data. For example, both genes are
expressed in the lung, specially at the alveolar epithelial type II
cells. Furthermore, ACE2 is also expressed in other organs such as
colon, kidney, blood vessels, nasal epithelium, the mucosa of oral
cavity and cornea, which are considered a high risk of infection
route (26,31-33).
Interestingly, a recent report identified a higher expression of
ACE2 in samples of severe COVID-19 patients with comorbidities than
those of control individuals (34).
Noteworthy, scientists have suggested analysing circulating blood
levels of ACE2 as a prognostic indicator for monitoring
COVID-infection (35).
Both SARS-CoV and SARS-CoV-2 use ACE2 as a cell
surface receptor and TMPRSS2 as the most important protease that
facilitates their entry into the host cell (30,24).
However, SARS-CoV-2, but not SARS-CoV, also contains a FURIN
cleavage site in its S- protein, potentially increasing its priming
upon ACE2 receptor binding (36).
Therefore, the S- protein cleavage at the FURIN protease site could
be responsible for the increased binding affinity of SARS-CoV-2 to
the ACE2 receptor. Interestingly, FURIN was also shown to be highly
expressed in the lung tissue, being also found co-expressed with
ACE2 and TMPRSS2(37). The
involvement of these genes in the viral infection process and their
confirmed expression in the lungs might contribute to the severe
pulmonary injury observed in some SARS-CoV-2 infected patients.
Possibly, alterations in their expression patterns, or even
mutations, could be one of the causes for the variability seen in
terms of disease severity among patients. Hence, these same genes
could be considered as potential therapeutic candidates for the
disease treatment. In addition, structural biology experiments on
the SARS-CoV-2 S-Proteins binding to ACE-2 and TMPRSS2, could allow
the development of targeted therapies aimed to block these
interactions (38).
The immune system also plays a vital role in
SARS-CoV-2 infection and disease response. The haemophagocytic
lymphohistiocytosis (sHLH) is an inflammatory syndrome known to
cause rapid and fatal hypercytokinaemia with multiorgan failure,
and this syndrome can be prompted by viral infections (4). Interestingly, COVID19 patients show a
similar cytokine profile with elevated interleukins (IL-2 and
IL-7), granulocyte- colony stimulating factor (G-CSF), interferon-γ
inducible protein 10kD (IP-10), monocyte chemoattractant protein 1
(MCP-1), macrophage inflammatory protein 1-α (MIP-1α), and tumour
necrosis factor-α (TNF-α) (39).
Patients will respond differently to treatment depending also on
their immunosuppression status, with therapeutic options including
steroids, intravenous immunoglobulin, selective cytokine blockade
(e.g., anakinra or tocilizumab) and JAK inhibition (39). Therefore, a proper investigation on
the patients' immune status is important, especially for cancer
patients.
4. COVID-19 and Lung Cancer
Lung cancer is one of the deadliest cancers and data
from the Brazilian National Cancer Institute (INCA) indicates it as
the third most frequent cancer type in men and the fourth in women,
whereas in the world it occupies the first position among men and
third among women (40). Tobacco
smoking is one of the greatest lung cancer risk factors (41). This cancer is divided into two major
cell types: small cell lung cancer (SCLC) and non-small cell lung
cancer (NSCLC), comprising 15 and 85% of all lung cancer,
respectively (42). In addition,
NSCLC is further separated into the three subtypes: Adenocarcinoma,
squamous cell carcinoma, and large cell carcinoma. The histology
and genetic profile of lung cancer are important factors for
treatment choices and preventive strategies. Current treatment
options involve surgery, adjuvant and neoadjuvant therapies,
chemotherapy, radiotherapy, targeted therapy and immunotherapy.
COVID-19 frequently causes respiratory distress that
may result in progressive respiratory failure, rendering the lung a
key player in the disease process. Hence, the presence of lung
tumours could decrease the patient's capacity to recover as faster
as other patients with healthy lungs. Interestingly, lung cancer
was shown to be the most frequent cancer type in SARS-CoV-2
infected patients when 2,007 cases from 575 Chinese hospitals were
investigated (13). Furthermore, a
retrospective case study including cancer patients from three
different Chinese hospitals, with laboratory confirmation of
COVID-19, also identified lung cancer as the most frequent cancer
type (43).
The frequent pneumonia observed in COVID-19 patients
could be a consequence of the expression of ACE2 by epithelial
cells of the lung as aforementioned. Regarding the molecular
aspects of lung cancer in relation to COVID-19, the ACE2 and
TMPRSS2 gene expression levels were investigated and correlated to
prognosis in lung adenocarcinoma and lung squamous cell carcinoma,
and surprisingly, TMPRSS2 was downregulated in lung adenocarcinoma
compared to normal tissues (44).
ACE2 gene expression was shown to be higher in lung adenocarcinoma
than in normal lung tissues, whereas for lung squamous cell
carcinoma they were similar (44).
Inflammatory pathways in cancer patients could be
associated with decreased immune surveillance (45). Hence, SARS-CoV-2 infection could
lead to an inflammation scenario that may even help tumour growth,
a process called pro-tumour inflammation (PTI) (45). PTI could lead to worse outcomes
among patients with NSCLC suggesting the stratification of lung
cancer patients according to their immunosuppression profile. This
results in a more personalized treatment to fight both inflammation
and tumour growth in order to improve mortality rates.
In the face of that, it has become increasingly
important to suggest clinical recommendations for lung cancer
management during the pandemic. Some Chinese doctors have addressed
this issue and indicated that for patients with lung occupying
lesions, the whole process of diagnosis and treatment should not be
carried out as usual (46). They
also proposed that the timing of surgical intervention should be
very carefully analysed (46). The
European Institute of Oncology, IRCCS from Milan (Italy) proposed
to take in consideration the following factors: lung cancer stage,
histology, treatment type, presence of comorbidities, performance
status (PS) and recent pneumonitis (47).
Not such a report has been published in South
America, therefore we aim to draw attention to the hard task that
oncologists and cancer translational teams are facing to properly
advice their patients in a critical situation as this, since
infections put the already vulnerable cancer patients at further
risks.
5. Recommendations to manage lung cancer
patients from a Brazilian perspective
In a large and developing country such as Brazil, it
is highly important to critically implement effective primary
preventive measures to avoid unwanted exposure of cancer patients
to infections such as SARS-CoV-2 and to deal with cancer patients
routine during pandemic times. Accordingly, three questions stand
out: Are the patients currently receiving immune-suppressive
treatments at higher risk of bad outcomes or this also depends on
their clinicopathologic characteristics? Do patients receiving
anti-programmed cell death protein 1 (PD-1)/programmed death ligand
1 (PD-L1) immunotherapy face additional risks? Should postponing
surgery and neoadjuvant chemotherapy be considered in certain
cases? Patients undergoing different treatments such as radiation,
surgery or chemotherapy are probably more immunosuppressed and need
to be extra careful to avoid unwanted infections, therefore visits
to hospitals and surgical procedures could be reduced as much as
possible by limiting them to the most urgent cases. This was also
mentioned by a recent study from Kumar et al, which
suggested a reduction in the number of visits to a health care
facility in order to reduce chances of contracting the virus while
keep providing effective oncologic therapy (48). For patients with concerning
symptoms, the ideal would be testing for COVID-19 at a
drive-through facility or at home (lab home-service). Whereas, in
the case of severe symptoms the test should be performed upon
arrival at the hospital emergency area. Doctors should also pay
attention to their patients and consider which drug therapies
should be applied, limited or decreased to lower levels in the case
of positive SARS-CoV-2 infection.
At INCA, patients are being evaluated following the
Brazilian Ministry of health advice. For patients with tumours
requiring resection, the idea is to keep the schedule unless the
patients, with symptoms, test positive for COVID-19. Since some
patients are asymptomatic, having them tested before surgery would
be recommendable. For the infected patients, the recommendation
would be to wait the necessary time until the patients are free
from the virus. In the case of suspicion of infection, and no
immediate molecular test available, pneumologists recommend that
the CT-scan should be the preferred exam since the X-ray might not
give all the resolution needed do distinguish infected from
non-infected cases before molecular testing. Although there is not
enough evidence of strict association between CT-scan results and
COVID-19 infection, it was shown that the CT-scans from COVID-19
patients exhibit different patterns when compared to CT-scans from
bacterial pneumonia, the first showing multiple ground-glass
opacities with consolidations (4,49). If
the images exhibit a total lung involvement greater than 50% plus
abnormal O2 saturation, the patient should be considered
as high risk when compared to total lung involvement below 50% with
normal O2 saturation. Regarding pulmonary sequelae after
coronavirus pneumonia, we still need more time to evaluate this
issue, although there are some discussions concerning the
possibility that certain cases may evolve early to pulmonary
fibrosis.
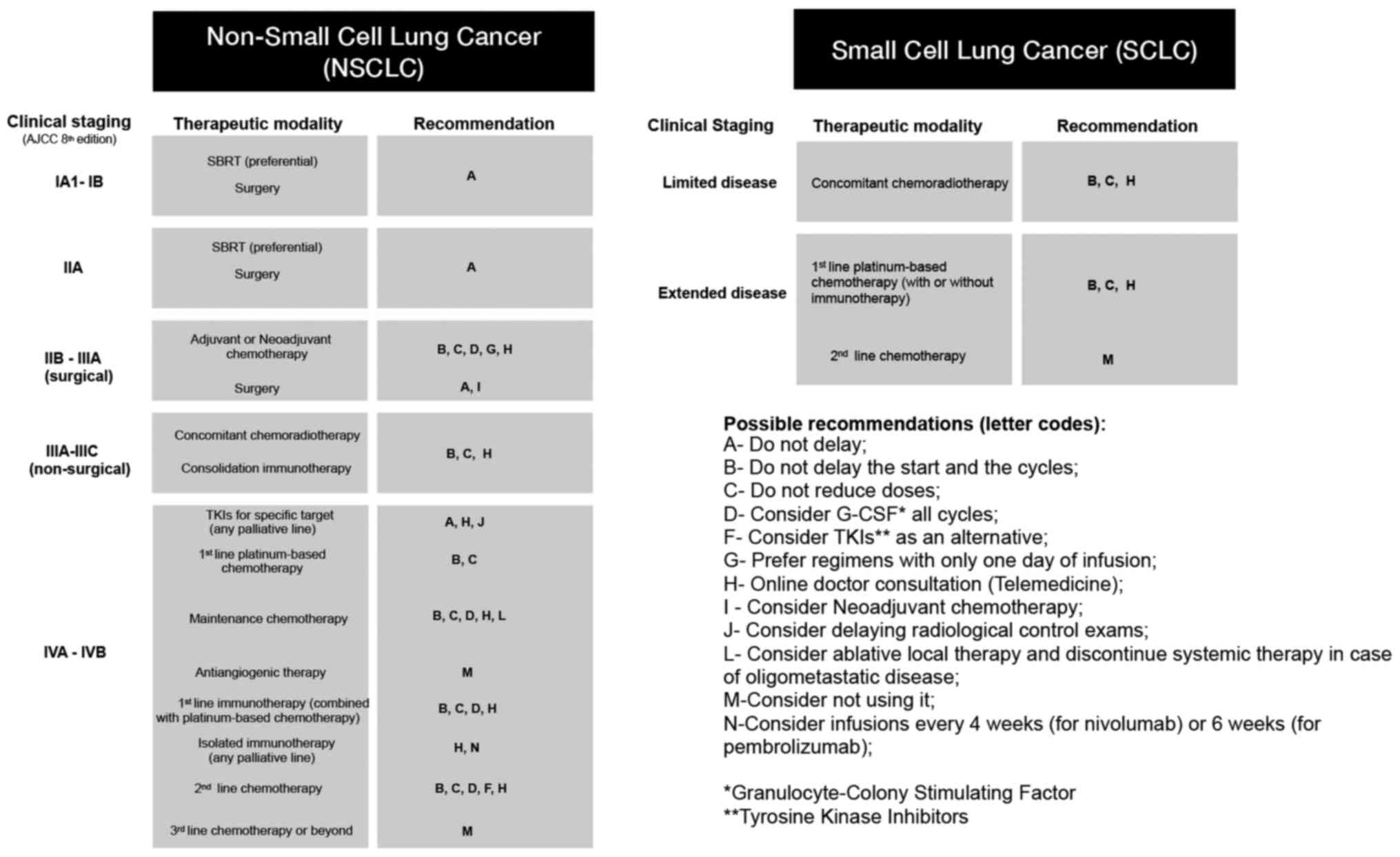
After covering information available from the
Chinese and local experts, we summarise in Fig. 3 what could be a feasible
recommendation during the coronavirus pandemic, for the management
of both, NSCLC and SCLC patients. It is important to note that all
recommendations are based on expert opinions and are primarily
based on seeking therapeutic alternatives that aim to reduce the
time of these patients within hospitals and clinics during the
pandemic period. These alternatives, however, are strategies
already used in clinical practice and all proposals aim to ensure
that proven effective treatments are offered. While the
presentation of COVID-19 in a more severe way is a possibility for
these patients, lung cancer and its aggressiveness are a certainty.
Thus, for patients with early stage disease, we suggest a
preference for stereotactic body radiotherapy (SBRT) over surgery.
For those patients who need surgery and complementary systemic
treatment, we suggest performing neoadjuvant chemotherapy with the
intention of extending the surgery to a second moment, possibly
post pandemic. Regarding platinum-based chemotherapy, we recommend
a reduction in the threshold for G-CSF prophylactic use, that no
random dose reductions or infusion delays are performed and that
the combination with antiangiogenics should be discouraged,
considering a borderline benefit and increased toxicity. For
oligometastatic disease, the use of local ablative therapy for all
lesions seems to be a better alternative than the continuation of
maintenance chemotherapy. Infusions of immunotherapies every 4
(nivolumab) or 6 (pembrolizumab) weeks are desirable instead of
every 2 or 3 weeks. When available, oral drugs are preferable and
online consultations should be encouraged.
Although more variables could be considered such as
age and other comorbidities, there is no universal solution and
each case should still be analysed in a personalized manner by the
oncologists in charge. Thereby, we based our recommendations mainly
on the lung cancer type and stage. Whenever possible, the
treatments with a survival benefit should be carried on and the
overall risk/benefit ratio should be taken in consideration. In
addition, proper information guidance should be undertaken to
instruct patients, along with their family members, to increase
awareness concerning personal protective measures such as social
distancing, constant hand hygiene, the use of masks, and close
surveillance by their doctors via telemedicine, for example. In
fact, an increasing number of studies are being published pointing
out the importance of telemedicine in COVID-19 patient screening
and follow-up (47,50,51).
These organised and efficient COVID-19 related measures were shown
to significantly benefit patients by identifying in advance the
ones with suspected symptoms, limiting the need of face-to-face
contact at cancer centres (47). It
is also important to point out that it is hard to predict how long
this pandemic will last, a fact that could interfere with current
clinical recommendations. Hopefully, our point of view will be
helpful for the local and international community which are
fighting non-stop to beat COVID-19.
6. Current Brazilian experience: What have
we learned so far?
While the pandemic was spreading across the country,
there was an expected increase in the fear of cancer patients,
including those with lung cancer. The main concern was to leave
home for medical appointments, diagnostic tests or therapeutic
procedures. Nevertheless, even at the beginning of the pandemic,
two important measures were taken to potentially relieve the
negative impacts of the pandemic on the patients' treatment: The
first was a very early position by the Brazilian Society of
Clinical Oncology (SBOC) (52)
emphasizing not only the importance of continuing oncological
treatment, but also the discussion with the attending oncologist
regarding possible adjustments in therapeutic planning, measures of
social distance, the use of masks, and hand hygiene (52); The second was the approval of a
governmental bill authorizing telemedicine in the country (53), which was regulated by the Federal
Council of Medicine (CFM). These initiatives guaranteed lung cancer
patients a safer access to their oncologist, generating greater
confidence regarding the continuity of their disease
monitoring.
It is important to point out that although the
pandemic initially generated great concerns related to treatment
losses, reductions and delays in diagnosis in the lung cancer
patient population, after few months what many Brazilian
oncologists have noticed is quite the opposite. This occurred
probably due to a lower threshold for indicating chest CT scans in
the country's emergency services resulting in different
consequences such as some patients obtaining early COVID-19
diagnosis, while others were incidentally diagnosed with other lung
diseases such as lung cancer in the early stages. Therefore, it
will be interesting to compare, in the near future, lung cancer
data in the year 2020 with historical data.
7. Concluding remarks and future
perspectives
Management of patients with cancer, and specially
lung cancer, is not an easy task in the everyday medical routine,
thus it is reasonable to imagine the tremendous amount of effort
the multidisciplinary oncology teams have to cope with during the
troubled times such as the one we are facing with the COVID-19
pandemic. The challenge of not having a vaccine or effective
therapy yet, only makes things more complicated. Therefore, the
implementation of organised and structured interventions in
healthcare practice is crucial to enhance effectiveness and improve
patient care. Planning and building strategies such as
recommendation guidelines for the particularly vulnerable can
optimise time and lives in pandemic emergencies. Under stressful
and unprecedented times, cancer patients are facing an extra burden
which can be softened by personalising their treatment and
maintaining them in close surveillance.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
VA conceived the idea for the review and contributed
with the molecular and translational aspects discussed, whereas
PDM, MZ and CGF contributed with their vast clinical expertise. VA,
PDM, MZ and CGF wrote the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): Novel
coronavirus (2019-nCoV) situation report-1. urihttps://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4simplehttps://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4.
Accessed January 21, 2020.
|
|
2
|
He F, Deng Y and Li W: Coronavirus disease
2019: What we know? J Med Virol. 92:719–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cui J, Li F and Shi ZL: Origin and
evolution of pathogenic coronaviruses. Nat Rev Microbiol.
17:181–192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu
JM, Peiris M, Poon LLM and Zhang W: Viral dynamics in mild and
severe cases of COVID-19. Lancet Infect Dis. 20:656–657.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng
Q, Meredith HR, Azman AS, Reich NG and Lessler J: The incubation
period of coronavirus disease 2019 (CoVID-19) from publicly
reported confirmed cases: Estimation and application. Ann Intern
Med. 172:577–582. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Pan F, Ye T, Sun P, Gui S, Liang B, Li L,
Zheng D, Wang J, Hesketh RL, Yang L and Zheng C: Time course of
lung changes on chest CT during recovery from 2019 novel
coronavirus (covid-19) pneumonia. Radiology. 295:715–721.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takeuchi Y, Furuchi M, Kamimoto A, Honda
K, Matsumura H and Kobayashi R: Saliva-based PCR tests for
SARS-CoV-2 detection. J Oral Sci. 62:350–351. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pascarella G, Strumia A, Piliego C, Bruno
F, Del Buono R, Costa F, Scarlata S and Agrò FE: COVID-19 diagnosis
and management: A comprehensive review. J Intern Med. 288:192–206.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Winter AK and Hegde ST: The important role
of serology for COVID-19 control. Lancet Infect Dis. 20:758–759.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deeks JJ, Dinnes J, Takwoingi Y, Davenport
C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J,
Ferrante di Ruffano L, et al: Antibody tests for identification of
current and past infection with SARS-CoV-2. Cochrane database Syst
Rev. 6(CD013652)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dignani MC, Costantini P, Salgueira C,
Jordán R, Guerrini G, Valledor A, Herrera F, Nenna A, Mora C,
Roccia-Rossi I, et al: Pandemic 2009 influenza A (H1N1) virus
infection in cancer and hematopoietic stem cell transplant
recipients; a multicenter observational study. F1000Res.
3(221)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liang W, Guan W, Chen R, Wang W, Li J, Xu
K, Li C, Ai Q, Lu W, Liang H, et al: Cancer patients in SARS-CoV-2
infection: A nationwide analysis in China. Lancet Oncol.
21:335–337. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu J, Ouyang W, Chua MLK and Xie C:
SARS-CoV-2 transmission in patients with cancer at a tertiary care
hospital in Wuhan, China. JAMA Oncol. 6:1108–1110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu Z and McGoogan JM: Characteristics of
and important lessons from the coronavirus disease 2019 (COVID-19)
outbreak in China: Summary of a report of 72 314 cases from the
Chinese center for disease control and prevention. JAMA.
323:1239–1242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
World Health Organization (WHO):
Coronavirus disease (2019-COVID-19) technical guidance: Patient
management. urihttps://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-managementsimplehttps://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management.
|
|
17
|
Centers for Disease Control And
Prevention. Interim clinical guidance for management of patients
with confirmed coronavirus disease (COVID-19).
|
|
18
|
Andersen KG, Rambaut A, Lipkin WI, Holmes
EC and Garry RF: The proximal origin of SARS-CoV-2. Nat Med.
26:450–452. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li F: Structure, function, and evolution
of coronavirus spike proteins. Annu Rev Virol. 3:237–261.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song
ZG, Hu Y, Tao ZW, Tian JH, Pei YY, et al: A new coronavirus
associated with human respiratory disease in China. Nature.
579:265–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wan Y, Shang J, Graham R, Baric RS and Li
F: Receptor recognition by the novel coronavirus from Wuhan: An
analysis based on decade-long structural studies of SARS
coronavirus. J Virol. 94:e00127–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan
B, Huan Y, Yang P, Zhang Y, Deng W, et al: A crucial role of
angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced
lung injury. Nat Med. 11:875–879. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Walls AC, Park YJ, Tortorici MA, Wall A,
McGuire AT and Veesler D: Structure, function, and antigenicity of
the SARS-CoV-2 spike glycoprotein. Cell. 181:281–292.e6.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Q, Zhang Y, Wu L, Niu S, Song C,
Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, et al: Structural and
functional basis of SARS-CoV-2 entry by using human ACE2. Cell.
181:894–904.e9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hamming I, Timens W, Bulthuis MLC, Lely
AT, Navis GJ and van Goor H: Tissue distribution of ACE2 protein,
the functional receptor for SARS coronavirus. A first step in
understanding SARS pathogenesis. J Pathol. 203:631–637.
2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
White JM and Whittaker GR: Fusion of
enveloped viruses in endosomes. Traffic. 17:593–614.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang H, Yang P, Liu K, Guo F, Zhang Y,
Zhang G and Jiang C: SARS coronavirus entry into host cells through
a novel clathrin- and caveolae-independent endocytic pathway. Cell
Res. 18:290–301. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matsuyama S, Ujike M, Morikawa S, Tashiro
M and Taguchi F: Protease-mediated enhancement of severe acute
respiratory syndrome coronavirus infection. Proc Natl Acad Sci USA.
102:12543–12547. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sungnak W, Huang N, Bécavin C, Berg M,
Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D,
Sampaziotis F, et al: SARS-CoV-2 entry factors are highly expressed
in nasal epithelial cells together with innate immune genes. Nat
Med. 26:681–687. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng
X, Li T and Chen Q: High expression of ACE2 receptor of 2019-nCoV
on the epithelial cells of oral mucosa. Int J Oral Sci.
12(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zou X, Chen K, Zou J, Han P, Hao J and Han
Z: Single-cell RNA-seq data analysis on the receptor ACE2
expression reveals the potential risk of different human organs
vulnerable to 2019-nCoV infection. Front Med. 14:185–192.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pinto BGG, Oliveira AER, Singh Y, Jimenez
L, Gonçalves ANA, Ogava RLT, Creighton R, Schatzmann Peron JP and
Nakaya HI: ACE2 expression is increased in the lungs of patients
with comorbidities associated with severe COVID-19. J Infect Dis.
222:556–563. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ciaglia E, Vecchione C and Puca AA:
COVID-19 infection and circulating ACE2 levels: Protective role in
women and children. Front Pediatr. 8(206)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Coutard B, Valle C, de Lamballerie X,
Canard B, Seidah NG and Decroly E: The spike glycoprotein of the
new coronavirus 2019-nCoV contains a furin-like cleavage site
absent in CoV of the same clade. Antiviral Res.
176(104742)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lukassen S, Chua RL, Trefzer T, Kahn NC,
Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, et
al: SARS -CoV-2 receptor ACE 2 and TMPRSS 2 are primarily expressed
in bronchial transient secretory cells. EMBO J.
39(e105114)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gupta I, Rizeq B, Elkord E, Vranic S and
Moustafa AE: Sars-cov-2 infection and lung cancer: Potential
therapeutic modalities. Cancers (Basel). 12(2186)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mehta P, McAuley DF, Brown M, Sanchez E,
Tattersall RS and Manson JJ: HLH Across Speciality Collaboration,
UK. COVID-19: Consider cytokine storm syndromes and
immunosuppression. Lancet. 395:1033–1034. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
José Alencar Gomes da Silva National
Cancer Institute/Ministry of Health: Estimate/2020-Cancer Incidence
in Brazil. urihttps://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/estimativa-2020-incidencia-de-cancer-no-brasil.pdfsimplehttps://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/estimativa-2020-incidencia-de-cancer-no-brasil.pdf.
|
|
41
|
de Groot PM, Wu CC, Carter BW and Munden
RF: The epidemiology of lung cancer. Transl Lung Cancer Res.
7:220–233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang L, Zhu F, Xie L, Wang C, Wang J,
Chen R, Jia P, Guan HQ, Peng L, Chen Y, et al: Clinical
characteristics of COVID-19-infected cancer patients: A
retrospective case study in three hospitals within Wuhan, China.
Ann Oncol. 31:894–901. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kong Q, Xiang Z, Wu Y, Gu Y, Guo J and
Geng F: Analysis of the susceptibility of lung cancer patients to
SARS-CoV-2 infection. Mol Cancer. 19(80)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li X, Liu M, Zhao Q, Liu R, Zhang H, Dong
M, Xu S, Zhao H, Wei S, Song Z, et al: Preliminary recommendations
for lung surgery during SARS-CoV-2 novel coronavirus pneumonia
epidemic period. Zhongguo Fei Ai Za Zhi. 23:133–135.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
47
|
de Marinis F, Attili I, Morganti S, Stati
V, Spitaleri G, Gianoncelli L, Del Signore E, Catania C, Rampinelli
C, Omodeo Salè E, et al: Results of multilevel containment measures
to better protect lung cancer patients from COVID-19: The IEO
model. Front Oncol. 10(665)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kumar S, Chmura S, Robinson C, Lin SH,
Gadgeel SM, Donington J, Feliciano J, Stinchcombe TE, Werner-Wasik
M, Edelman MJ and Moghanaki D: Alternative multidisciplinary
management options for locally advanced nsclc during the
coronavirus disease 2019 global pandemic. J Thorac Oncol.
15:1137–1146. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li X, Zeng W, Li X, Chen H, Shi L, Li X,
Xiang H, Cao Y, Chen H, Liu C and Wang J: CT imaging changes of
corona virus disease 2019(COVID-19): A multi-center study in
Southwest China. J Transl Med. 18(154)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Moazzami B, Razavi-Khorasani N, Dooghaie
Moghadam A, Farokhi E and Rezaei N: COVID-19 and telemedicine:
Immediate action required for maintaining healthcare providers
well-being. J Clin Virol. 126(104345)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Calton B, Abedini N and Fratkin M:
Telemedicine in the time of coronavirus. J Pain Symptom Manage.
60:e12–e14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Brazilian Society of Clinical Oncology
(SBOC): SBOC Positioning - Coronavirus (COVID-19). urihttps://sboc.org.br/noticias/item/1797-posicionamesimplehttps://sboc.org.br/noticias/item/1797-posicioname.
Accessed March 12, 2020.
|
|
53
|
Poder Legislativo: Diário Oficial da
União. urihttps://www.in.gov.br/web/dou/-/lei-n-13.989-de-15simplehttps://www.in.gov.br/web/dou/-/lei-n-13.989-de-15.
2020 (In Portuguese).
|
|
54
|
Li MY, Li L, Zhang Y and Wang XS:
Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide
variety of human tissues. Infect Dis Poverty. 9(45)2020.PubMed/NCBI View Article : Google Scholar
|