Introduction
Thyroid masses are common abnormalities, many of
which are classified as benign diseases, such as cystic mass,
adenomatous goiter, and follicular tumor, and are rarely subjected
to surgical treatment. One of the still unsolved challenges for
endocrine surgeons is the diagnosis of follicular thyroid carcinoma
(FTC). Preoperative diagnosis of FTC without distant metastasis is
impossible, and there is no indication of the types of follicular
tumors (FTs) that should be operated on to obtain evidence of FTC.
Various diagnostic tools have been reported in the past, but none
have been applied clinically to date. If follicular cells without
nuclear morphology are diagnosed as benign by cytology, fewer
tumors are indicated for surgery and FTC cannot be diagnosed
(1,2). Therefore, only a retrospective study
has been conducted. The aim of this study was to examine patients
with FT or FTC who were treated in our hospital to devise
appropriate treatment strategies and to evaluate the various
treatment outcomes of FTC.
Patients and methods
Patient selection. At our hospital, Kanagawa
Cancer Center in Japan, several patients with advanced DTC and
distant metastasis have been treated. During the 5 years from April
2015 to March 2020, 797 patients with DTC and 128 patients with FT
were referred to our hospital for the treatment of distant
metastases and their recurrence. Of these 925 patients, 73 patients
were diagnosed with FTC and were retrospectively followed up. This
study was approved by the Institutional Review Board of Kanagawa
Cancer Center (IRB approval no. 27-61). All patients provided
comprehensive consent for using their samples from medical
examination for medical investigation and clinical research.
Table I presents a list of these 73
patients, including a comparison of MIFTC with WIFTC. The median
follow-up period was 6.8 years (0.5-29.7 years). WIFTC had
significant differences compared with MIFTC, with strong local
invasion and high frequency of lymph node and pulmonary metastases,
but no significant difference in the frequency of bone
metastases.
 | Table IComparison of patients with MIFTC and
WIFTC. |
Table I
Comparison of patients with MIFTC and
WIFTC.
| Pathology | MIFTC | WIFTC | P-value |
|---|
| N | 48 | 25 | |
| Age, years | 66.5 [20,
84]b | 65.0 [11,
78]b | 0.770 |
| Sex, n (%) | | | 0.021a |
|
Female | 27 (56.2) | 21 (84.0) | |
|
Male | 21 (43.8) | 4 (16.0) | |
| Stage | | | 0.009a |
|
T1 (%) | 9 (18.8) | 0 (0.0) | |
|
T2 (%) | 18 (37.5) | 7 (28.0) | |
|
T3 (%) | 21(43.8) | 16 (64.0) | |
|
T4 (%) | 0 (0.0) | 2 (8.0) | |
| Capsular
invasion | 27 (56.2) | 19 (76.0) | 0.128 |
| Vascular
invasion | 19 (39.6) | 15 (60.0) | 0.138 |
| Nodal metastasis
(%) | 2 (4.2) | 8 (32.0) | 0.002a |
| Total thyroidectomy
(%) | 23 (47.9) | 19 (76.0) | 0.026a |
| Nodal dissection
(%) | 5 (10.4) | 10 (40.0) | 0.005a |
| Initial metastasis
(%) | 13 (27.1) | 14 (56.0) | 0.022a |
| Pulmonary metastasis
(%) | 8 (16.7) | 15 (60.0) |
<0.001a |
| Bone metastasis
(%) | 13 (27.1) | 12 (48.0) | 0.118 |
| TKI therapy (%) | 4 (8.3) | 10 (40.0) | 0.003a |
| Death (%) | 0 (0.0) | 5 (20.0) | 0.004a |
| Thyroglobulin
(ng/ml) | 494 [6.80,
93,000]b | 549 [5.00,
250,000]b | 0.206 |
| TSH (µIU/ml) | 0.26 [0.01,
4.55]b | 0.09 [0.01,
4.01]b | 0.035a |
Diagnostic algorithm and
treatment
An algorithm was created to completely follow the
prognosis of FTC in the 73 patients with different diagnostic
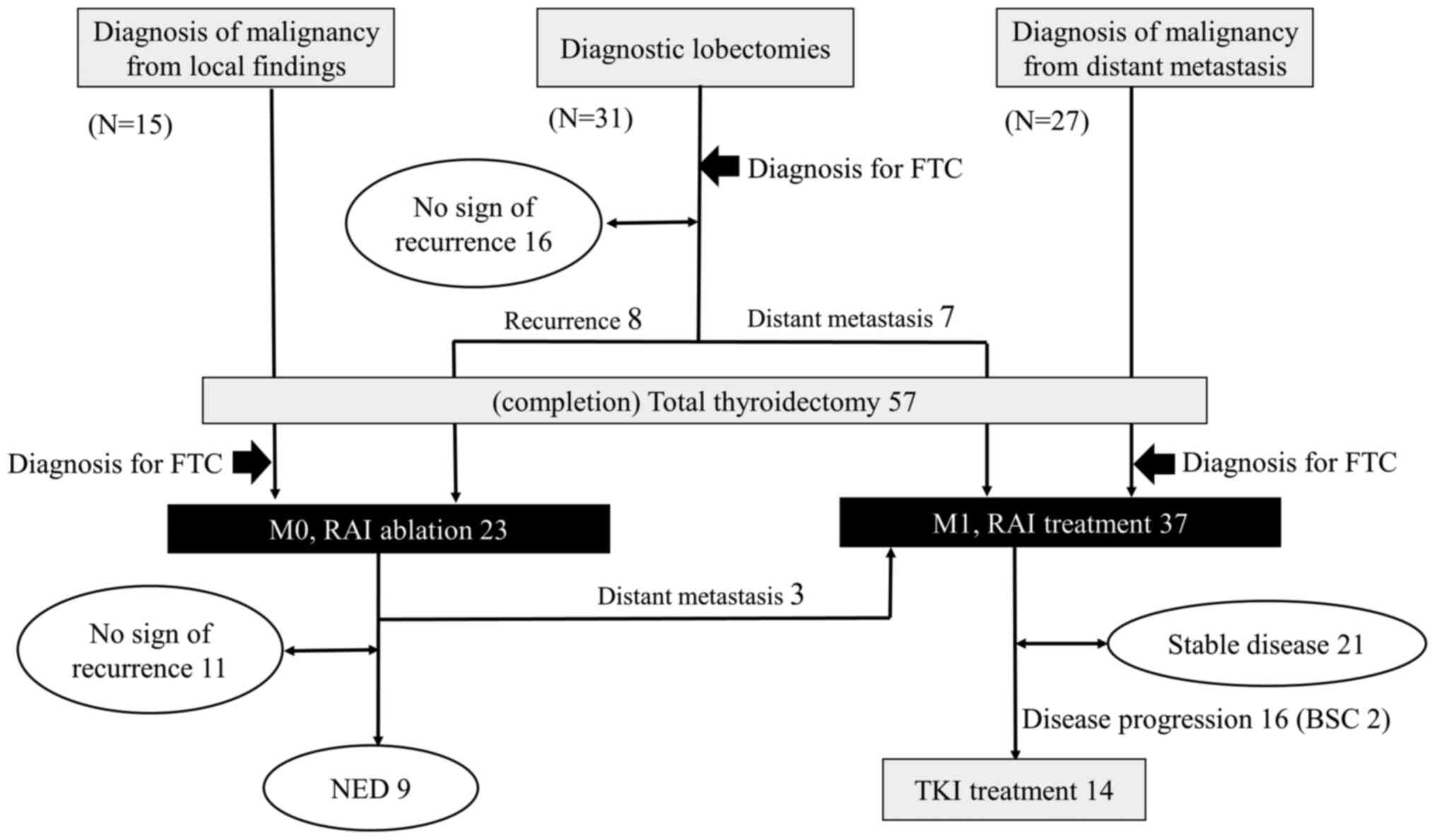
methods (Fig. 1). There are three
different methods based on which FTCs can be diagnosed:
Postoperative histological diagnosis, distant metastasis from FTC,
and surgery to assess local invasion that is suspected to be a
malignant tumor. When FTC is diagnosed based on biopsy of distant
metastasis, it is natural to perform total thyroidectomy.
Radioactive iodine (RAI) treatment is the gold standard, followed
by metastatic lesion treatment, if necessary. Patients with
thyroglobulin (Tg) levels that returned to a normal value after
diagnostic thyroidectomy were followed up. Distant metastasis was
suspected in patients with persistently high Tg and in those with
increased Tg levels after surgery; consequently, completion
thyroidectomy was performed, followed by RAI treatment. Moreover,
when follicular cells without nuclear morphology are identified by
local invasion, they may be diagnosed as FTC based on local
findings, and total thyroidectomy may be selected at the initial
surgery.
Parameters and statistical
analysis
We retrospectively reviewed the preoperative serum
Tg levels, treatment courses, and treatment results in 73 patients
with FTC treated at our hospital. The median values between two
groups were compared using Fisher's test for nominal variables and
Student's t test for continuous variables. The statistically
significant difference was set at P<0.05. All statistical data
were analyzed using EZR software version 2.4(3). We compared OS between MIFTC and WIFTC
and between the initial M0 patients and initial M1 patients. OS and
10-year survival rates were calculated using the Kaplan-Meier
method with SPSS software (version 24; IBM Corp.), and a log-rank
test was applied. P<0.05 was considered statistically
significant.
Results
Diagnostic process
Seventy-three patients with FTC were diagnosed using
three routes of the algorithm (Fig.
1). Fifteen patients were preoperatively diagnosed with
malignant lesions due to local invasion or tumor embolization to
blood vessels, and total thyroidectomy was initially performed.
Twenty-seven patients were diagnosed with FTC based on the presence
of distant metastasis. Nine patients were diagnosed with FTC based
on histological concordance between the thyroid nodule and distant
metastasis without capsular or vascular invasion. There were eight
patients with sychronicity, and only one patient was previously
diagnosed with a benign nodule. Of the 31 patients who underwent
diagnostic lobectomy, 15 patients underwent surgery for suspicious
malignancy based on ultrasound findings and 16 of 128 patients
underwent prophylactic surgery for an FT of ≥4 cm and a Tg level of
≥1,000 ng/dl.
Comparison of WIFTC and MIFTC
WIFTC showed a significantly higher degree of local
invasion (T, N) than MIFTC; therefore, total thyroidectomy was more
common at the initial surgery. Initial metastasis was noted in
14/25 (56.0%) patients with WIFTC, and postoperative metastasis was
noted in 4 patients. Vascular invasion was observed in 34 patients
and capsular invasion in 46 patients. Both invasions were not noted
in 9 patients, and they were diagnosed with FTC based on the
matching of the metastatic lesion and the thyroid mass. These
results are summarized in Table I.
There were 37 patients with distant metastases, 11 with pulmonary
metastasis, 13 with bone metastasis, 13 with pulmonary and bone
metastases, and 1 with renal metastasis. There were 5 deaths owing
to both metastases. TKI treatment was applied in 40.0% patients
with WIFTC; the prognosis was poor, and 5 (20.0%) patients died.
Conversely, none of the patients with MIFTC died in the follow-up
period (Table I). Fig. 2 depicts the clinical course of MIFTC
and WIFTC with or without distant metastasis. In the 14 patients
treated with TKI, OS was for a median of 3.1 (range, 0.2-4.6
years), and PFS was for a median of 1.6 (range, 0.2-3.3 years).
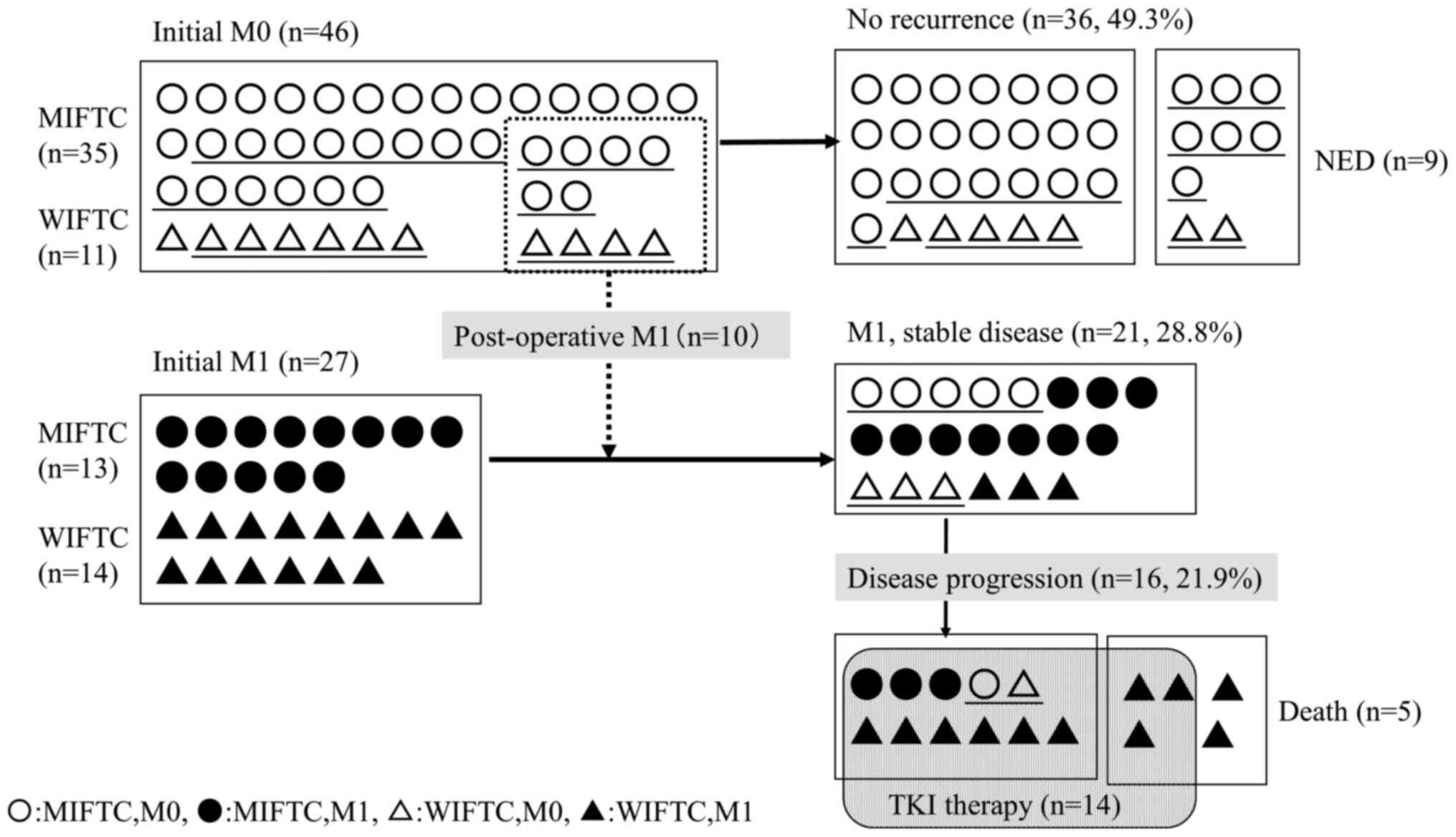 | Figure 2Initial diagnosis and treatment course
of 73 patients with follicular thyroid carcinoma. Of the 46
patients without distant metastasis at the first consultation, the
prognosis was good for both MIFTC and WIFTC; 10 (21.7%) patients
had postoperative distant metastasis and 36 (78.2%) had neither
recurrence nor distant metastasis. Conversely, among 27 patients
with distant metastasis at the first consultation, WIFTC had a poor
prognosis, with disease progression in 11 (78.6%) of 14 patients, 5
(45.5%) of which died. Only 3 (23.1%) of the 13 patients with MIFTC
exhibited disease progression, none of whom died. MIFTC, minimally
invasive follicular thyroid carcinoma; WIFTC, widely invasive
follicular thyroid carcinoma; M0, no distant metastasis; M, distant
metastasis; NED, no evidence of disease, biological complete
status; TKI, tyrosine kinase inhibitor. |
Clinical course of patients with
initial M0
No distant metastasis was noted, and FTC was
postoperatively diagnosed (n=46). Among the 35 patients with MIFTC
and 11 patients with WIFTC, total thyroidectomy was initially
performed in 15 patients based on local findings; and lobectomies
were performed in the remaining 31 patients. Fifteen patients
underwent completion total thyroidectomy because of recurrent mass
in the residual thyroid or elevated Tg after lobectomy; 10 patients
exhibited distant metastases after surgery. Twelve patients who
underwent total thyroidectomy, 16 who underwent lobectomies, and 8
who underwent completion total thyroidectomy for a total of 36
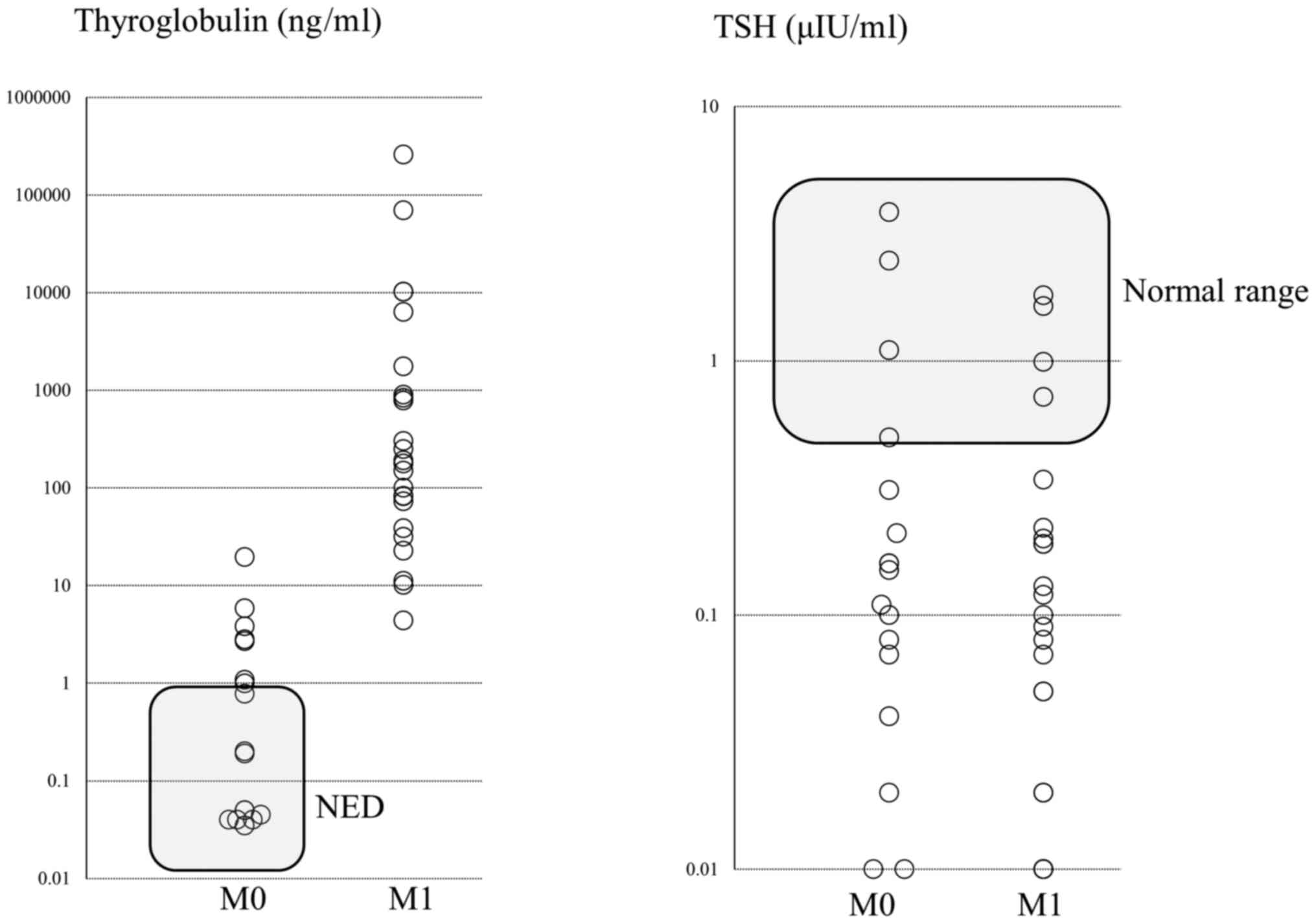
patients showed no recurrence during follow-up (Fig. 2). The Tg level was <1 ng/ml in 9
of the 19 patients who underwent total thyroidectomy, indicating no
evidence of disease (NED). The distribution of Tg levels and TSH
values after total thyroidectomy are shown in Fig. 3.
At our hospital, total thyroidectomy was initially
performed in five of 11 patients; among these 11, distant
metastasis was observed in 4 (36.4%) patients. Among the 35
patients with MIFTC at our hospital, 6 (18.2%) patients developed
distant metastases after the initial surgery, and five of the six
surgeries were lobectomies.
Clinical course of patients with
initial M1
Distant metastasis was recognized at the first
consultation, and FTC was diagnosed on the basis of the matching
tissues (n=27). There were 13 patients with MIFTC, 14 with WIFTC, 9
with lung metastasis, 6 with bone metastasis, and 12 with both
metastases. The prognosis of patients with WIFTC was poor, and 11
(78.6%) of the 14 patients showed disease progression. Nine
patients were treated with TKI drugs, and 5 (35.7%) patients died
of FTC. Conversely, because MIFTC progresses slowly even with
distant metastasis, the treatment is considered successful with
stable disease. Currently, TKI treatment for the progressive
disease in 3 (23.1%) of 13 patients, and the disease has been kept
under control to date in 10 of these 13 patients (Fig. 2).
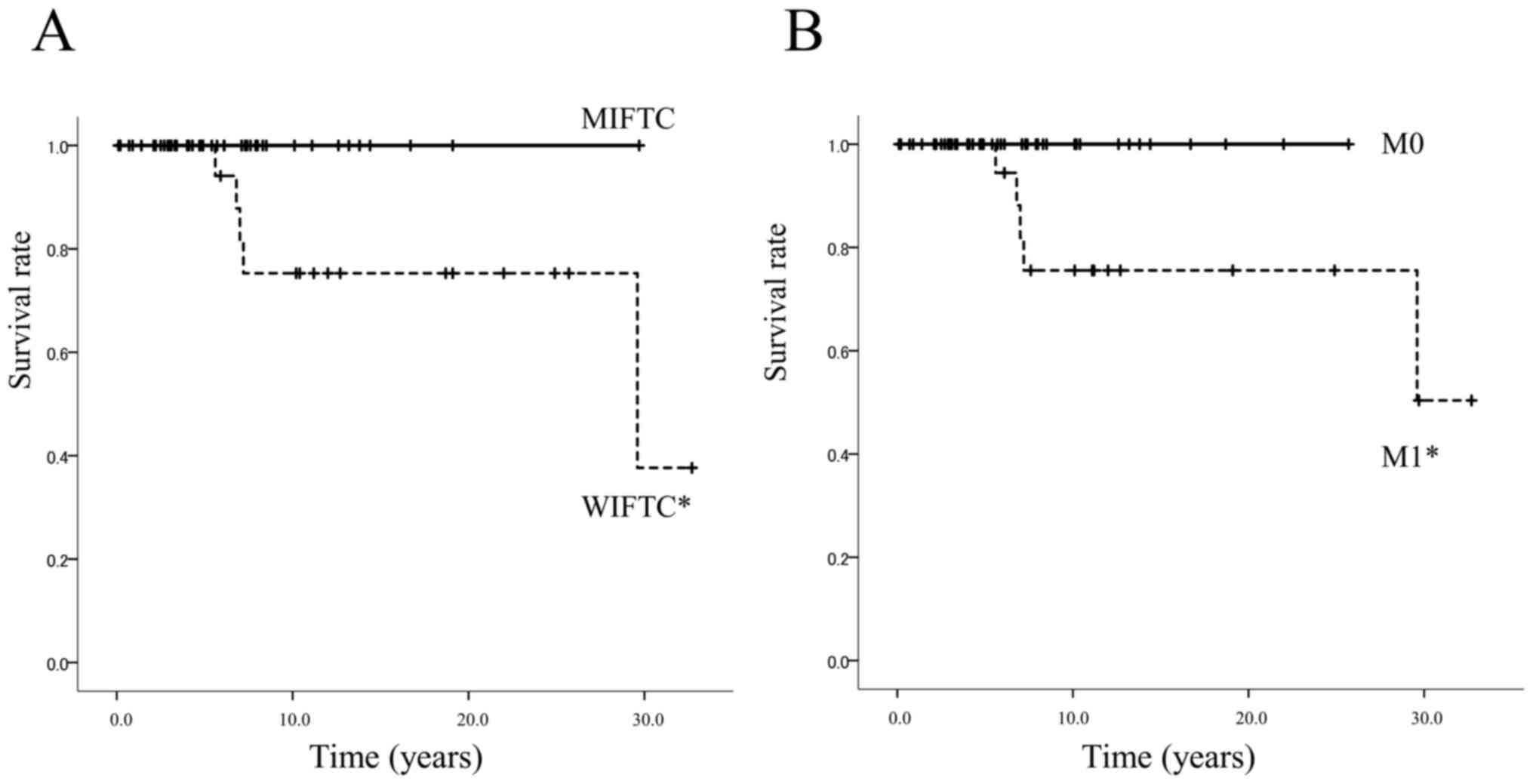
OS curves
OS was significantly better for patients with MIFTC
than for those with WIFTC (log-rank test, P=0.017), and OS for
initial M0 patients was significantly better than for initial M1
patients (log-rank test, P=0.023), as shown in Fig. 4. Although all initial M0 patients
and those with MIFTC are alive, the 10-year survival rates were
75.3% for patients with WIFTC and 75.6% for the initial M1
patients.
Discussion
Unfortunately, despite certain unique aspects of
presentation and prognosis, there are currently no specific
recommendations for the management of FTC in evidence-based
guidelines (4). We used an
algorithm to get a complete picture of FTs and FTCs in a single
institution by examining the entire clinical course of the patients
in detail. Using the algorithm, we were able to clearly show the
approach used for the diagnosis and outcomes of all patients
(Figs. 1 and 2). The major advantage of our approach was
the ability to trace the course of similar cases with an algorithm.
In our hospital, the presence of an FT of ≥4 cm (T3) or a serum Tg
level of ≥1000 ng/ml indicated the need for surgery, and 16/128
(12.5%) of the FTs were FTCs based on the surgical results. The
current American Thyroid Association guidelines recommend surgery
for growing large nodules >4 cm that are considered benign based
on repeat fine needle aspiration cytology (4). Based on reports showing that the
growth rate of FTs is not different from that of adenomas (5), we recommend that T3 FTs and those
accompanied with a serum Tg level of ≥1,000 ng/ml should be removed
surgically, as followed in our hospital. One study reported
mutational testing for BRAF or the seven-gene mutation
marker panel (BRAF, RAS, RET/PTV,
PAX8/PPARγ) for indeterminate thyroid lesions (6), a condition that is not covered by
insurance in Japan. Even benign thyroid FTs without histological
evidence of carcinoma can metastasize (7). Additionally, we followed-up patients
with the FTs diagnosed as benign after surgery, and noted that none
of the study patients with FTs developed distant metastasis at the
final follow-up examination. Nine (18.8%) patients with MIFTC could
not be operated upon without distant metastases were initially T1.
We must then decide what kind of FT should be suspected as cancer
and what type of surgery is needed.
The effect of surgical choice on FTC outcomes has
been analyzed only by retrospective observational studies. Goffredo
et al (8) reported that MIFC
is associated with survival that is comparable to that of the
normative US general population. Thyroid lobectomy alone may be
considered adequate treatment in these patients.
Lin et al (9)
reported that total thyroidectomy and postoperative RAI therapy are
mandatory in moderate- and high-risk groups. More than one-fourth
of the patients with follicular thyroid cancer in the high-risk
group died due to thyroid cancer despite aggressive treatment.
Although various studies have reported risks, the prognosis of FTC
appears to be broadly affected by two factors: i) The histological
type of MIFTC or WIFTC and ii) the presence or absence of distant
metastasis. However, various methods were used in these studies,
hindering the assessment of true prognostic factors. Clearly, WIFTC
has a poor prognosis (10). Our
analyses revealed that the prognosis of WIFTC was worse than that
of MIFTC. Our results also demonstrated that the prognosis of
patients with initial M1 was worse than that of the patients with
initial M0 (Fig. 4). In other
words, distant metastasis and WIFTC were risk factors. Even in
MIFTCs, age at diagnosis and the existence of combined capsular and
vascular invasion have been identified as important prognostic
factors (11). Naturally, age is a
risk factor for survival in statistical analyses along with
vascular invasion and distant metastasis (10,12,13).
Other prognostic factors include capsular and vascular invasion
(14), distant metastasis, tumor
size (15,16), and TERT promoter mutations;
however (17), RAS mutations
have also reportedly been associated with distant metastasis
(18).
In this study, there was no significant difference
in the frequency of distant metastasis between MIFTC and WIFTC, and
it was necessary to carefully examine the levels of serum Tg and
thyroglobulin antibody (TgAb) and to perform various imaging (e.g.,
radioiodine whole-body scans, CT scans, chest radiography, cervical
ultrasound, and 18F-fluorodeoxyglucose positron emission
tomography) studies after surgery. In other words, the frequency of
distant metastasis in M0 diagnosed as postoperative FTC is not very
high at 10/46 (21.7%), and 36/46 (78.3%) of these patients had a
favorable prognosis. Even if WIFTC is diagnosed, we believe that it
is acceptable to follow-up carefully and to perform completion
total thyroidectomy if necessary. In summary, the prognosis of
WIFTC is the worst among FTCs with distant metastasis; surgery
(total thyroidectomy) and RAI treatment are essential; and
metastatic lesions should be treated simultaneously with the
primary tumor. If the disease progresses, it is necessary to
promptly include TKI therapy.
TSH suppression therapy has been recommended in many
articles (19,20), but others have found that it had no
significant effect (11).
Clinicians should be aware of the potential adverse effects of TSH
suppression therapy, which include increased risks for atrial
fibrillation, osteoporosis, and ischemic heart disease (21,22).
As shown in Table I, postoperative
TSH levels were significantly higher for MIFTC than for WIFTC. TSH
suppression therapy is strictly applied to patients with high
levels of Tg or those with distant metastasis (Fig. 3).
RAI treatment was considered refractory if there is
a lesion without uptake of I-131 or if a new lesion appears after
treatment or if the disease progresses. Unless otherwise noted, we
considered it to be effective and continued with the treatment
every 6-12 months till a total treatment dose of 600 mci.
In most FTC patients, local invasion is rare, and
resection of the primary lesion is almost possible without any
complications. Thus, the primary prognostic factor is distant
metastasis. Details of TKI treatment are provided in other reports,
but the therapeutic effect varies depending on the target lesion
(23). The best response to TKI
treatment is noted for pulmonary metastases with poor local
involvement. Bone metastases can be expected to prolong survival if
pathological fractures or paralysis are avoided.
To better understand the current treatment of FTCs,
we reviewed our experience by presenting an algorithm of the
treatment strategies. In conclusion, the prognosis of WIFTC is the
worst among patients with FTCs having distant metastasis, and
surgery (total thyroidectomy) and RAI treatment are essential. If
the disease progresses, prompt addition of TKI is necessary.
This retrospective study had certain limitations.
The median follow-up period was relatively short. Due to the low
frequency of FTCs, the variation of the period was large. However,
the prognosis could be predicted to a certain extent by using an
algorithm to follow the clinical course of similar cases.
Acknowledgements
The authors would like to thank Dr Hiroyuki Hayashi
(Department of Pathology, Yokohama City Hospital) for assistance in
pathological diagnoses.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HI and ST designed the study. HI, ST, SK and DM
designed the study and were involved in data
analysis/investigation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The chemotherapy committee of Kanagawa Cancer Center
Hospital approved the regimen of lenvatinib for use in patients
with ATC. The cancer board of the same hospital also approved
lenvatinib treatment, including surgery, in patients with ATC. The
present study was approved by the Institutional Review Board of
Kanagawa Cancer Center.
Patient consent for publication
All patients provided a comprehensive signed consent
form before receiving the treatment, stating that personal data
could be used for academic purpose or paper presentation while
ensuring complete anonymity.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
HI is an endocrine surgeon working at the Kanagawa
Cancer Center and has extensive experience of various surgeries for
advanced DTC, as well as TKI treatment.
References
|
1
|
Deandrea M, Motta M, Divito L, Mormile A,
Gallone G, Grassi A, Pellerito R, Nasi P, Torchio B, Garberoglio R
and Fonzo D: Thyroid cytology and risk of thyroid cancer:
Differences among indeterminate specimens. Endocr Pract.
10:330–334. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Renshaw AA and Gould EW: Impact of
specific patterns on the sensitivity for follicular and Hurthle
cell carcinoma in thyroid fine-needle aspiration. Cancer
Cytopathol. 124:729–736. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim M, Han M, Lee JH, Song DE, Kim K, Baek
JH, Shong YK and Kim WG: Tumour growth rate of follicular thyroid
carcinoma is not different from that of follicular adenoma. Clin
Endocrinol (Oxf). 88:936–942. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nikiforov YE, Ohori NP, Hodak SP, Carty
SE, LeBeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT,
et al: Impact of mutational testing on the diagnosis and management
of patients with cytologically indeterminate thyroid nodules: A
prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab.
96:3390–3397. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Correction to Diagnostic performance of
ultrasound-based risk-stratification systems for thyroid nodules:
Comparison of the 2015 American thyroid association guidelines with
the 2016 Korean thyroid association/korean society of thyroid
radiology and 2017 American college of radiology guidelines Ha EJ,
Na DG, Moon W-J, Lee YH, and Choi N Thyroid 2018; 28: 1532-1537.
DOI: 10.1089/thy.2018.0094. Thyroid 29: 159, 2019.
|
|
8
|
Goffredo P, Cheung K, Roman SA and Sosa
JA: Can minimally invasive follicular thyroid cancer be approached
as a benign lesion?: A population-level analysis of survival among
1,200 patients. Ann Surg Oncol. 20:767–772. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin JD, Chao TC, Chen ST, Huang YY, Liou
MJ and Hsueh C: Operative strategy for follicular thyroid cancer in
risk groups stratified by pTNM staging. Surg Oncol. 16:107–113.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grani G, Lamartina L, Durante C, Filetti S
and Cooper DS: Follicular thyroid cancer and Hurthle cell
carcinoma: Challenges in diagnosis, treatment, and clinical
management. Lancet Diabetes Endocrinol. 6:500–514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stenson G, Nilsson IL, Mu N, Larsson C,
Lundgren CI, Juhlin CC, Höög A and Zedenius J: Minimally invasive
follicular thyroid carcinomas: Prognostic factors. Endocrine.
53:505–511. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee YM, Song DE, Kim TY, Sung TY, Yoon JH,
Chung KW and Hong SJ: Risk factors for distant metastasis in
patients with minimally invasive follicular thyroid carcinoma. PLoS
One. 11(e0155489)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Iwasaki H, Matsumoto A, Ito K, Kure Y,
Suzuki A, Sugino K, Ozaki O and Noh J: Prediction of distant
metastasis in follicular adenocarcinoma of the thyroid. World J
Surg. 14:425–430. 1990.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gulcelik MA, Gulcelik NE, Kuru B, Camlibel
M and Alagol H: Prognostic factors determining survival in
differentiated thyroid cancer. J Surg Oncol. 96:598–604.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Passler C, Scheuba C, Prager G, Kaczirek
K, Kaserer K, Zettinig G and Niederle B: Prognostic factors of
papillary and follicular thyroid cancer: Differences in an
iodine-replete endemic goiter region. Endocr Relat Cancer.
11:131–139. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Verburg FA, Mäder U, Luster M and Reiners
C: Primary tumour diameter as a risk factor for advanced disease
features of differentiated thyroid carcinoma. Clin Endocrinol
(Oxf). 71:291–297. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Melo M, da Rocha AG, Vinagre J, Batista R,
Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, et
al: TERT promoter mutations are a major indicator of poor outcome
in differentiated thyroid carcinomas. J Clin Endocrinol Metab.
99:E754–E765. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jang EK, Song DE, Sim SY, Kwon H, Choi YM,
Jeon MJ, Han JM, Kim WG, Kim TY, Shong YK and Kim WB: NRAS codon 61
mutation is associated with distant metastasis in patients with
follicular thyroid carcinoma. Thyroid. 24:1275–1281.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de Melo TG, Zantut-Wittmann DE, Ficher E
and da Assumpcao LV: Factors related to mortality in patients with
papillary and follicular thyroid cancer in long-term follow-up. J
Endocrinol Invest. 37:1195–1200. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee YM, Lee YH, Song DE, Kim WB, Sung TY,
Yoon JH, Chung KW and Hong SJ: Prognostic impact of further
treatments on distant metastasis in patients with minimally
invasive follicular thyroid carcinoma: Verification using inverse
probability of treatment weighting. World J Surg.
41(1144)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Biondi B and Cooper DS: Benefits of
thyrotropin suppression versus the risks of adverse effects in
differentiated thyroid cancer. Thyroid. 20:135–146. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vera L, Gay S, Campomenosi C, Paolino S,
Pera G, Monti E, Mortara L, Seriolo B and Giusti M: Ten-year
estimated risk of bone fracture in women with differentiated
thyroid cancer under TSH-suppressive levothyroxine therapy.
Endokrynol Pol. 67:350–358. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Sakai R, Nakayama H, Hatori S, Toda S and Masudo K:
Treatment outcomes of differentiated thyroid cancer with distant
metastasis improve by tyrosine kinase inhibitors. Oncol Lett.
17:5292–5300. 2019.PubMed/NCBI View Article : Google Scholar
|