Introduction
Basal cell carcinoma (BCC) is the most common skin
malignancy worldwide (1). The
primary treatment for BCC includes surgical resection followed by
radiation therapy (RT) if indicated based on pathologic risk
factors including perineural involvement (PNI) or positive margins
(2). In cases where functional and
cosmetic outcomes are compromised with a surgical approach or in
patients with a poor performance status, RT offers similar disease
control rates in small BCCs (3).
For larger tumors however, the efficacy of RT decreases, as 5-year
local control rates of 95% for early stage disease decrease to 56%
in advanced stages (3).
Consequently, patients with advanced BCC who are not candidates for
surgery have limited local control options.
Implication of the Sonic hedgehog (Shh) pathway in
the development of BCC has led to the development of novel systemic
Shh pathway inhibitors, providing patients with advanced BCCs new
treatment options and improved survival. The primary target is
through inhibition of the smoothened (SMO) protein, and both
vismodegib and sonidegib are FDA approved for locally advanced or
metastatic BCC (4). Vismodegib is
approved for BCC patients based on data from the ERIVANCE trial,
study, in which 104 patients with measurable advanced BCC received
oral vismodegib 150 mg once daily until disease progression or
intolerable toxicity. The primary end point was independent
review-assessed ORR (5). Objective
response rates were 48 and 33% for locally advanced and metastatic
BCC, with a median response duration of 9.5 and 7.6 months,
respectively (6).
In locally advanced BCC patients who are not
surgical candidates and where RT alone would offer lower control
rates, the combination of vismodegib and RT delivered concurrently
may potentially improve outcomes. There are limited case reports on
combined modality therapy, although data suggest there is a
cytotoxic synergy when RT and Shh pathway inhibition are combined
(7). With this in mind and in the
setting of limited data, we chose to offer concurrent vismodegib
and RT for a patient with very advanced, multifocal BCC.
Case report
A 65-year-old female with no prior significant
history aside from a remote history of BCC removed from her scalp
20 years prior presented to our center with multiple fungating
lesions that had been neglected for the past year. At the time of
presentation, she had masses on the left preauricular region, right
shoulder, mid-upper chest (periclavicular and parasternal), and
right lateral ankle. Biopsies confirmed BCC (BER-EP4 positive),
nodular type. She was evaluated by surgical oncology and not felt
to be a surgical candidate due to the size and extent of her
lesions, and was referred to Medical Oncology to discuss systemic
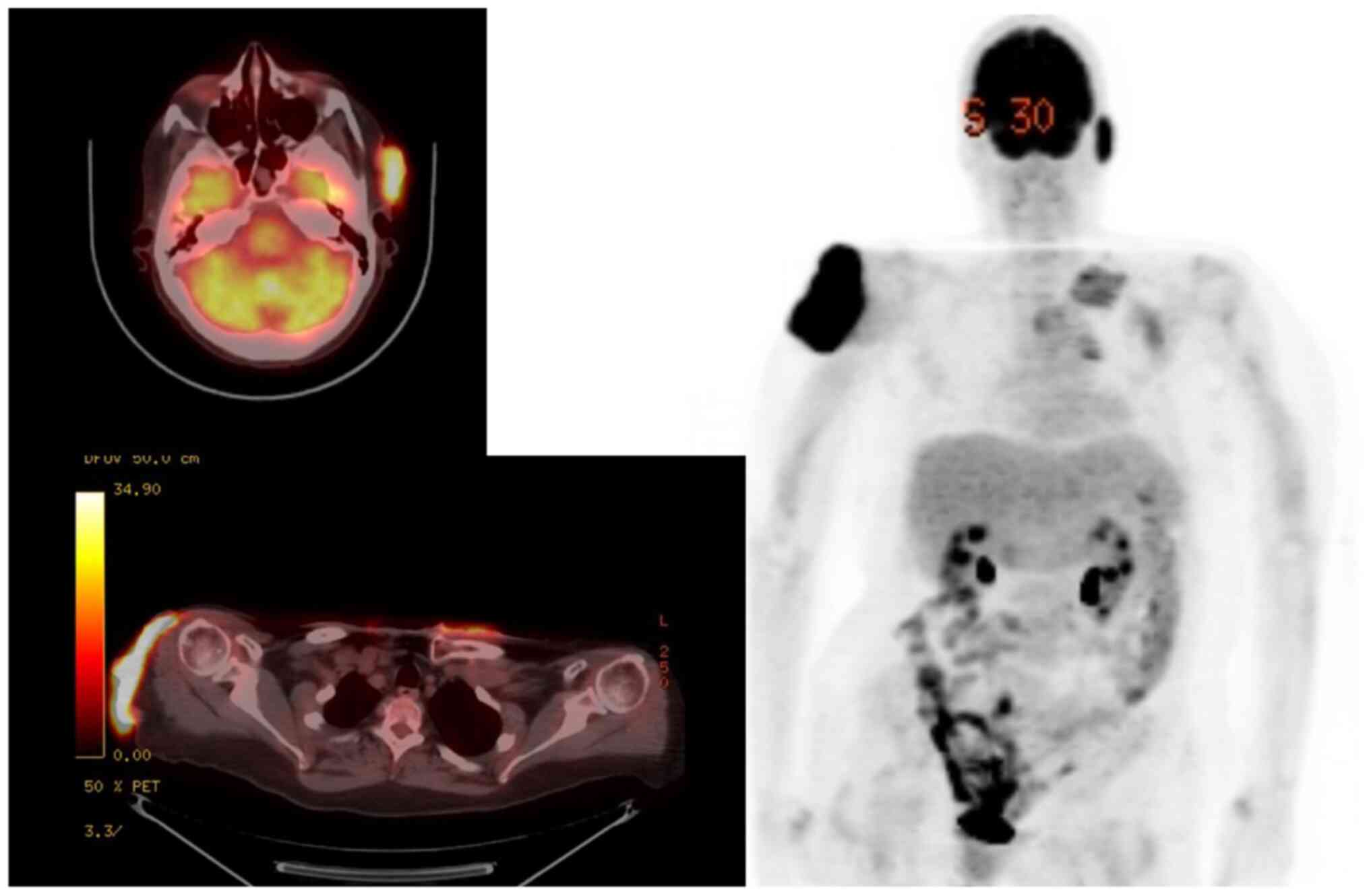
therapy options. To rule out distant metastases, positron-emission
tomography (PET) imaging was obtained, confirming
fluorodeoxyglucose (FDG) avidity at the left preauricular lesion
[standardized uptake value (SUV) 10.1; maximum dimensions of
35x13x42 mm], right shoulder (SUV 19.7; 81x19x97 mm), upper chest
wall including the parasternal (SUV 5.8; 66x37 mm) and
periclavicular regions (SUV 7.1; 65x11x39 mm), and right lateral
ankle (SUV 7.1; 29 mm in length) (Figs.
1 and 2). Her case was
discussed at our multidisciplinary skin tumor board and given the
extent of her disease, the recommendation was to proceed with
concurrent vismodegib 150 mg daily and RT to each of the involved
sites. RT was initially directed at the largest symptomatic areas
which included the left preauricular region and the right shoulder.
She received 55 Gy in 20 fractions (2.75 Gy per fraction) with
electrons and was referred to Wound Care for local home health
services. In addition to fatigue, she developed grade 2
desquamation at both treatment sites [Common Terminology Criteria
for Adverse Events (CTCAE_v5)], which resolved within 4 weeks of RT
completion. Following treatment, she continued vismodegib but
required dose interruption and dose reductions due to grade 2
fatigue, grade 2 alopecia, grade 2 loss of appetite, grade 1
diarrhea, and grade 1 muscle spasms. At 6 months post-RT
evaluation, she had clinically suspected residual disease at the
right shoulder and the decision was made to proceed with wide local
excision. Final pathology returned negative for BCC, confirming a
pathologic complete response. She continued to have a clinical
complete response in the left preauricular region, and similarly
had a complete clinical response in the R lateral ankle with
systemic treatment alone.
The two chest wall areas showed a suboptimal
clinical response, with only a 20% reduction in size after 6 months
of vismodegib, likely due to the extensive involvement of the
chest. Given persistent bleeding at the site, the patient opted for
consolidative RT (55 Gy in 20 fractions), which lead to a clinical
complete response. Grade 3 moist desquamation was observed, and in
addition to Wound Care she was referred to Pain Management due to
escalating analgesic requirements. At the time of completion of RT
to her chest wall (9 months from the initial start of systemic
therapy), vismodegib was discontinued due to ongoing diarrhea,
fatigue, muscle spasms (outside the radiation field), and
persistent moist desquamation at the chest wall site.
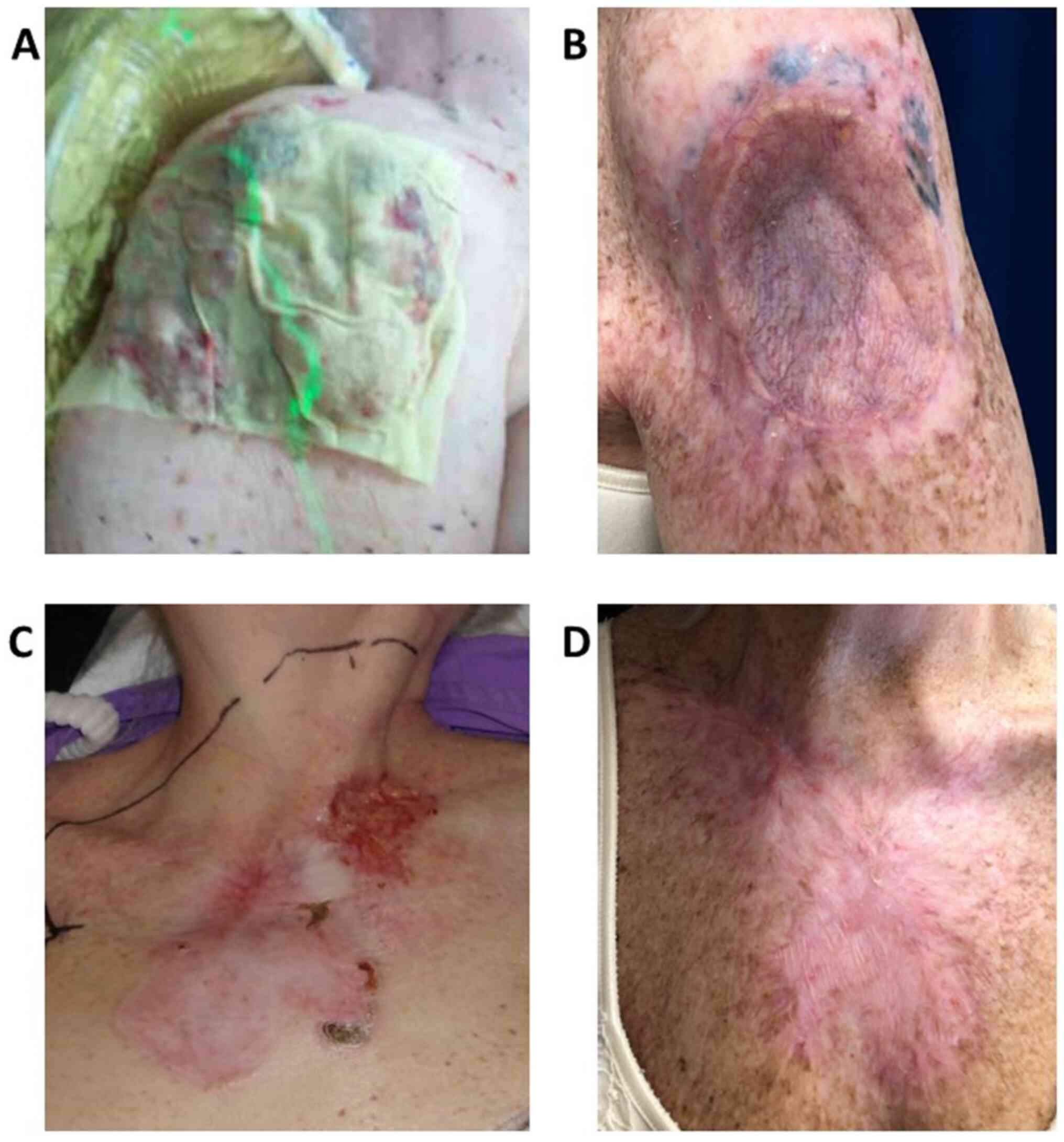
The patient continued to be followed by the
multidisciplinary team at regular clinical intervals, and her
fatigue and diarrhea resolved within 3 months of drug
discontinuation. At 18-month follow up, she continued to have a
complete clinical response at all of her treated sites, with no
further evidence of recurrence (Fig.
2).
Discussion
While surgery and RT or a combination of both are
curative options for the majority of patients presenting with BCC,
in patients with advanced disease who are not surgical candidates,
local therapy with RT alone results in suboptimal disease control
rates. Vismodegib has demonstrated clinical efficacy in locally
advanced and metastatic BCC, although the side effect profile
limits how long patients can continue on therapy. In the case
presented herein, we demonstrate the efficacy and tolerability of
combination RT and vismodegib to treat very advanced, multifocal
BCC. The patient presented with four large primary areas of disease
including the left preauricular, right shoulder, chest wall, and
right lateral ankle. All sites achieved a clinical complete
response, with a pathologic complete response at the right
shoulder. The ankle lesion did not require RT and continues to have
a clinical complete response. The findings from our case report
support several other cases with similar efficacy when vismodegib
and RT are combined (8,9).
To date, there is no evidence to suggest systemic
chemotherapy improves local control outcomes when combined with RT
for advanced BCC. In 2012, vismodegib became the first systemic
therapy approved for BCC (5),
followed by sonidegib approval in 2015, and evidence continues to
be generated as combination with RT is explored in advanced,
unresectable cases (10,11). In our patient with multifocal BCC,
all lesions exhibited some response to vismodegib, with complete
and durable responses noted in those larger lesions treated with
combination therapy and a durable complete clinical response in the
right lateral ankle lesion with vismodegib alone, a remarkable
finding. In particular, pathologic complete response was observed
in the right shoulder, where lesions measuring >10 cm would
normally be expected to have relatively low durable control rates
with RT alone. Radiosensitization with vismodegib is supported in
pre-clinical models for lung cancer and potentially appears to have
similar results in BCC based on other reported series (12). Therefore, in light of knowing some
BCC do not respond completely to vismodegib alone, based on
clinical trial data as well as what was observed in our patient's
chest wall lesion, a combination of RT and vismodegib may be an
effective option for non-surgical candidates.
There is limited data on the toxicity profile of
combining RT with vismodegib. Our patient was able to tolerate 9
months of vismodegib, at which point it had to be discontinued due
to persistent side effects including fatigue, loss of appetite,
alopecia, diarrhea and muscle spasms (outside the radiation field),
as demonstrated in other studies (8). Of note, patients in the ERIVANCE trial
(5) were on vismodegib for a median
12.9 months; this difference in duration of treatment compared to
our patient may be related to the toxicity of concurrent RT. The
fatigue associated with RT may be further compounded by the malaise
attributed to Shh inhibitors, and, as in our case, dose
interruption or reduction may be necessary to ensure continued drug
adherence. Further, local side effects due to RT also appeared to
be enhanced in our patient, who experienced grade 3 moist
desquamation after treatment to the chest wall that persistent for
1 month following treatment before resolving. The patient required
escalated analgesic therapy as well as weekly wound care visits
until her local side effects improved; the delayed tissue recovery
post-radiation may be explained by the fact that hedgehog signaling
is essential for wound healing, and Shh interfere with this process
(13).
The optimal management for multifocal, advanced BCC
can be challenging and requires a multidisciplinary discussion
between dermatologists, surgeons, medical oncologists, and
radiation oncologists in order to achieve best outcomes. In our
case, tri-modality therapy (systemic treatment, radiation and
surgery) resulted in complete clinical and pathologic responses in
multiply enlarged BCC lesions for which surgery alone would not
have been curative. Additional specialty referrals to Wound Care
and Pain Management should be considered for patients with
bleeding, fungating, or physically deformative tumors. Future
prospective trials including the ongoing phase II study combining
RT and vismodegib for advanced head and neck BCC (NCT01835626) will
provide insight into this combination approach (14), and further guide multidisciplinary
treatment decisions for patients with advanced disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AA and BM analyzed and interpreted the patient case
and reviewed the literature. VP reviewed the pathology section of
the paper. AA, MF, LM, KM, FRA, VP and BM were major contributors
in writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Consent to participate was obtained from the
patient.
Patient consent for publication
Informed consent was obtained for publication of
patient data.
Competing interests
Dr Amini is a paid consultant for RefleXion and is
on the speaker's bureau for AstraZeneca and Takeda Oncology. Dr
Modi is a paid consultant and a member of the speaker's bureau for
Regeneron and Sanofi Genzyme. Dr Abdulla has grant/research support
from Johnson & Johnson, Elorac, Trillium, Sterline, MiRagen,
Bioniz, Mallinkcrodt, is a consultant for Mallinkrodt and a member
of the speaker's bureau for Mallinkrodt. Drs. Melstrom, Margolin,
and Parekh have no competing interests.
References
|
1
|
Cameron MC, Lee E, Hibler BP, Barker CA,
Mori S, Cordova M, Nehal KS and Rossi A: Basal cell carcinoma:
Epidemiology; pathophysiology; clinical and histological subtypes;
and disease associations. J Am Acad Dermatol. 80:303–317.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Likhacheva A, Awan M, Barker CA, Bhatnagar
A, Bradfield L, Brady MS, Buzurovic I, Geiger JL, Parvathaneni U,
Zaky S and Devlin PM: Definitive and postoperative radiation
therapy for basal and squamous cell cancers of the skin: Executive
summary of an American society for radiation oncology clinical
practice guideline. Pract Radiat Oncol. 10:8–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wilder RB, Kittelson JM and Shimm DS:
Basal cell carcinoma treated with radiation therapy. Cancer.
68:2134–2137. 1991.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Armstrong P, Martin S and Lask G: Sonic
hedgehog pathway inhibition in the treatment of advanced basal cell
carcinoma. In: Biologic and Systemic Agents in Dermatology.
Springer, Cham, pp541-548, 2018.
|
|
5
|
Sekulic A, Migden MR, Oro AE, Dirix L,
Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander
PA, et al: Efficacy and safety of vismodegib in advanced basal-cell
carcinoma. N Engl J Med. 366:2171–2179. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sekulic A, Migden MR, Lewis K, Hainsworth
JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin
CM, et al: Pivotal ERIVANCE basal cell carcinoma (BCC) study:
12-month update of efficacy and safety of vismodegib in advanced
BCC. J Am Acad Dermatol. 72:1021–1026.e8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hehlgans S, Booms P, Güllülü Ö, Sader R,
Rödel C, Balermpas P, Rödel F and Ghanaati S: Radiation
sensitization of basal cell and head and neck squamous cell
carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol
Sci. 19(2485)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Franco AI, Eastwick G, Farah R, Heyboer M,
Lee M and Aridgides P: Upfront radiotherapy with concurrent and
adjuvant vismodegib is effective and well-tolerated in a patient
with advanced, multifocal basal cell carcinoma. Case Rep Dermatol
Med. 2018(2354146)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pollom EL, Bui TT, Chang AL, Colevas AD
and Hara WY: Concurrent vismodegib and radiotherapy for recurrent,
advanced basal cell carcinoma. JAMA Dermatol. 151:998–1001.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Block AM, Alite F, Diaz AZ, Borrowdale RW,
Clark JI and Choi M: Combination trimodality therapy using
vismodegib for basal cell carcinoma of the face. Case Rep Oncol
Med. 2015(827608)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gathings RM, Orscheln CS and Huang WW:
Compassionate use of vismodegib and adjuvant radiotherapy in the
treatment of multiple locally advanced and inoperable basal cell
carcinomas and squamous cell carcinomas of the skin. J Am Acad
Dermatol. 70:e88–e89. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zeng J, Aziz K, Chettiar ST, Aftab BT,
Armour M, Gajula R, Gandhi N, Salih T, Herman JM, Wong J, et al:
Hedgehog pathway inhibition radiosensitizes non-small cell lung
cancers. Int J Radiat Oncol Biol Phys. 86:143–149. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Le H, Kleinerman R, Lerman OZ, Brown D,
Galiano R, Gurtner GC, Warren SM, Levine JP and Saadeh PB: Hedgehog
signaling is essential for normal wound healing. Wound Repair
Regen. 16:768–773. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
ClinicalTrials.gov: Phase II Study of Radiation
Therapy and Vismodegib for Advanced Head/Neck Basal Cell Carcinoma.
ClinicalTrials.gov Identifier: NCT01835626. https://clinicaltrials.gov/ct2/show/NCT01835626. Last
updated October 5, 2020.
|