Introduction
Gastric cancer is the fourth most common cancer
worldwide, and >50% of cases occur in Eastern Asia with nearly
one million new cases and >720,000 deaths per year (1,2). The
risk factors of gastric cancer include race, sex, tobacco, alcohol,
infection, and family history (3).
The etiology of gastric cancer is still not fully known. While a
battery of somatic mutations in driver genes have been identified,
little is known about predisposing genes for gastric cancer. Aside
from rare familial forms of gastric cancers, recent genetic
analysis suggests that ~10% of the sporadic cases may have rare
variants with pathogenic implication (1,4). The
close digit of the incidence and mortality of gastric cancer
reflects that only a limited fraction is detected at early stages
for efficient intervention, which highlights the need to the
detection of high risk population. Even more rare variants are
likely to be involved in the pathogenesis, but due to the paucity
of such variants, the detection of them is not straightforward.
Candidate gene analysis may be worthwhile for the identification of
predisposing genes and variants.
Hydrogen sulfide (H2S) is a gas signaling
molecule with various physiological activities in multiple organs
(5). One of the major actions of
H2S is tissue protective activity in the
gastrointestinal (GI) tract (6).
Recently, D-amino oxidase (DAO) was found to be involved in a novel
pathway for H2S production by catalyzing D-cysteine
(7,8). D-cysteine is changed from L-cysteine
by alkaline treatment and mostly provided from food (9). D-cysteine is easily absorbed through
GI tract and enters the blood stream. Administration of D-cysteine
has protective effect against ethanol-induced gastric mucosal
damage through DAO-H2S signaling (10). To this end we hypothesized that DAO
gene may be a candidate for susceptibility to gastric cancer.
Materials and methods
Study population
We employed the subjects registered in the Internet
Database of Japanese single nucleotide polymorphisms for Geriatric
Research (JG-SNP) (11). The study
subjects comprised of consecutive autopsy cases collected at Tokyo
Metropolitan Geriatric Hospital between 1995 and 2012. Autopsy
procedures were performed on ~29% of patients who died in the
hospital. There were a total of 2,343 subjects, where 1,298 were
men and 1,045 were women; the mean age at the time of death was 80
years. The presence or absence of any disease was determined by a
thorough examination on autopsy. The detail of JG-SNP database can
be seen elsewhere (11).
Cancer-bearing subjects include those with any type of cancer,
including occult cancer, found on autopsy. Smoking habit included
both current smoking and ex-smoking. The distribution of any
disease of the study group was not largely apart from those
reported in a survey by the Ministry of Health, Labor, and Welfare
of Japan (12).
Genotyping and statistical
analysis
Genomic DNA was extracted from the renal cortex by a
standard procedure using phenol and chloroform. All samples were
genotyped with Illumina Infinium Human Exome Bead Chip Version 1.1
(Illumina, Inc.) by iScan in accordance with the Illumina
protocols. Genotype calling was performed for all samples as a
single project using the Genotyping Module (version 1.9) of the
Genome Studio data analysis software package. Initial genotype
clustering was performed using the default Illumina cluster file
(Human Exome 12v1-1_A.egt) and manifest file
(HumanExome-12v1-1_A.bpm) using the GenTrain2 clustering algorithm.
We considered a per sample call rate of >98% as eligible, and 15
samples were excluded from the analysis. A total of 2,328 (99.4%)
out of the initial 2,343 subjects were successfully genotyped.
Association of the rare variants with cancer state of the patients
and P-value were completed via a Fisher's exact test using IBM SPSS
Statistics software 25.0 (IBM Corp.).
The pathological assessment, genotyping and
statistical analysis were performed in different institutions in a
double-blind fashion to minimize bias.
Ethical statement
This study was approved by the Tokyo Medical and
Dental University Ethics Committee (approval no. 2016-011-02), and
the Tokyo Metropolitan Geriatric Hospital Ethics Committee
(approval no. 230405).
Results
DAO variants
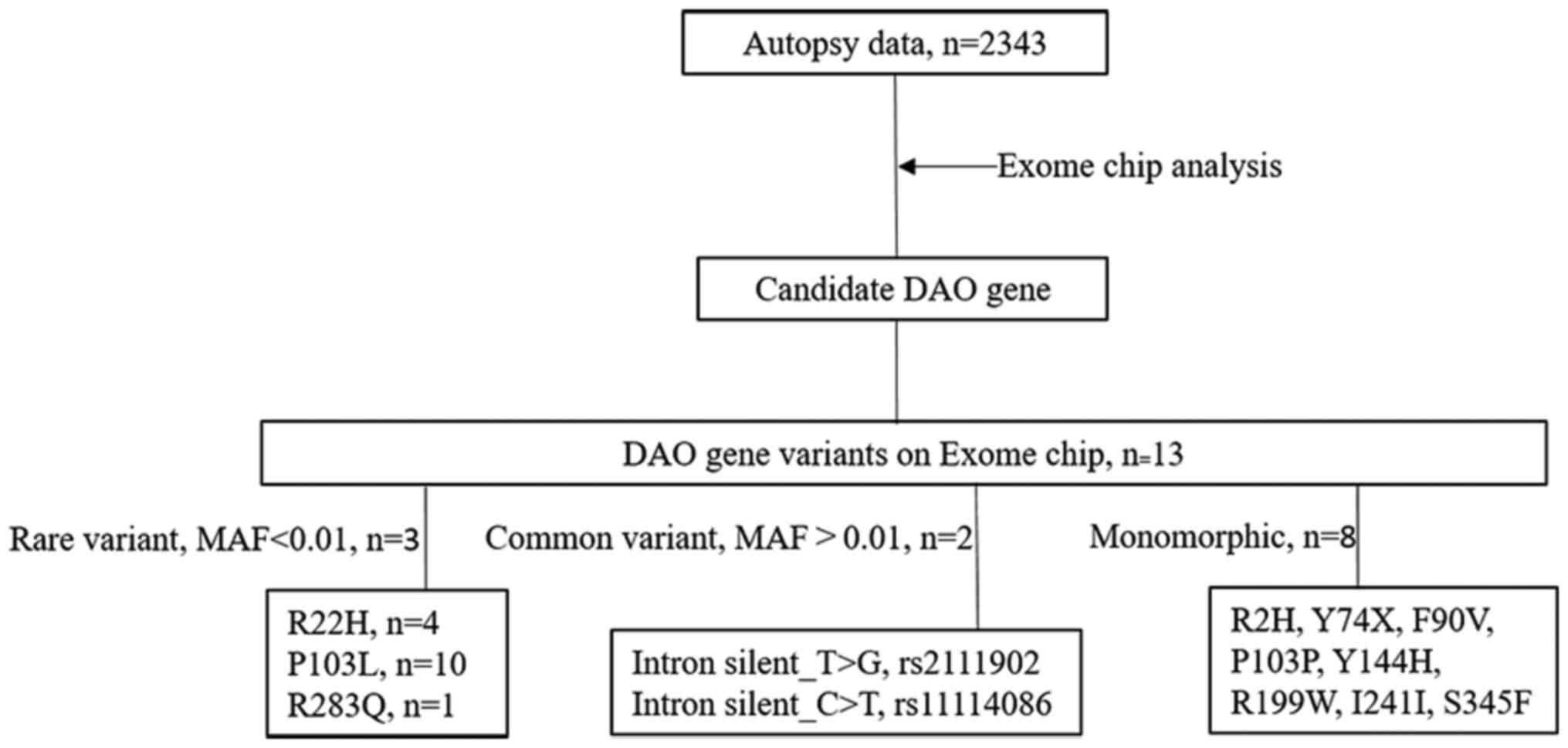
On the exome chip, there were altogether 13 variants
of the DAO gene. Among them, 8 variants were monomorphic, 2 common
variants in the intron, and 3 rare (MAF<0.01) variants (Fig. 1).
Cancer bearing state of the heterozygotes of these
three non-synonymous variants, p.R22H (rs200257378), p.P103L
(rs200127576), and p.R283Q (rs143550642) were further analyzed
(Table I). The number of p.R22H,
p.P103L, and p.R283Q heterozygote carriers was four, ten and one,
respectively. Thus, the minor allele frequency of these variants
was 0.0009, 0.0021 and 0.0002, respectively. According to The Human
Gene Mutation Database, the non-synonymous variants p.R22H, p.R283Q
and p.P103L has been reported to be related with Frontotemporal
dementia (13,14), Alzheimer's Disease (15) and amyotrophic lateral sclerosis
(ALS), separately (16).
 | Table ICancer bearing state of the
heterozygotes. |
Table I
Cancer bearing state of the
heterozygotes.
| SNV |
Predictiona | Heterozygotes | MAF | Related
diseaseb |
|---|
| p.R22H | Benign | 4 | 0.0009 | Frontotemporal
dementia |
| p.P103L | Probably
damaging | 10 | 0.0021 | Amyotrophic lateral
sclerosis |
| p.R283Q | Probably
damaging | 1 | 0.0002 | Alzheimer's
disease |
Characteristics of heterozygotes with
DAO rare variants
Table II shows the
demographic of the subjects carrying DAO rare variants. Among ten
carriers of p.P103L, four were men and six were women. All of the
men had gastric cancer. One man had an additional lung cancer.
Among six women, one had colon cancer, one had small intestine
cancer, one had multiple cancers of pancreas, breast and thyroid.
Other three women had no cancer. All men had smoking and drinking
habit. One woman had missing data, the others had no drinking habit
and no smoking except for one smoker.
 | Table IIDemographic and characteristics of
heterozygotes with SNVs. |
Table II
Demographic and characteristics of
heterozygotes with SNVs.
| Variant | Sample | Sex | Age | Cancer | Drinking | Smoking |
|---|
| p.P103L | p.P103L 1 | M | 84 | Gastric | + | + |
| | p.P103L 2 | M | 71 | Gastric | + | + |
| | p.P103L 3 | M | 70 | Gastric | + | + |
| | p.P103L 4 | M | 83 | Gastric, lung | + | + |
| | p.P103L 5 | F | 79 | Colon | - | - |
| | p.P103L 6 | F | 86 | Small Intestine | - | - |
| | p.P103L 7 | F | 96 | Pancreas, breast,
thyroid | - | - |
| | p.P103L 8 | F | 93 | None | - | + |
| | p.P103L 9 | F | 93 | None | - | - |
| | p.P103L 10 | F | 82 | None | Missing | Missing |
| p.R22H | p.R22H 1 | M | 69 | Malignant
lymphoma | - | - |
| | p.R22H 2 | M | 88 | None | Missing | Missing |
| | p.R22H 3 | M | 91 | None | Missing | + |
| | p.R22H 4 | M | 61 | None | Missing | - |
| p.R283Q | p.R283Q | F | 71 | Lung | - | + |
Four p.R22H carriers were all men, among them one
man had malignant lymphoma and the others had no cancer. One
p.R283Q carrier was a woman who had lung cancer with habit of
smoking but not drinking.
Association of DAO p.P103L with
cancer
Among 2,343 cases, 1,446 (61.72%) were affected by
different type of cancer. There are 262 (11.18%) gastric cancer,
222 (9.48%) colorectal cancer and 12 (0.51%) small intestine
cancer. Association of gastric cancer and p.P103L variant showed
positive result with P=0.018 by Fisher's exact test, odds ratio,
5.36 and 95% CI, 1.50-19.13 (Table
III). Gastrointestinal cancer and p.P103L were also positively
associated with P=0. 008, odds ratio, 5.64, and 95% CI, 1.59-20.07
(Table III).
 | Table IIIp.P103L and cancer. |
Table III
p.P103L and cancer.
| Cancer | p.P103L (+) | p.P103L (-) | P-value | OR | 95% CI |
|---|
| GC (+) | 4 | 258 | 0.018 | 5.36 | 1.50-19.13 |
| GC (-) | 6 | 2075 | | | |
| GIC (+) | 6 | 490 | 0.008 | 5.64 | 1.59-20.07 |
| GIC (-) | 4 | 1843 | | | |
Discussion
We surveyed rare non-synonymous SNVs of the DAO gene
in consecutive autopsy cases of the JG-SNP data base and found
potential association between DAO p.P103L and gastric cancer in
men. Due to the small sample size and the low allele frequency of
the variant, this result should not be taken as a true association,
but rather as a hypothesis generation, which needs to be confirmed
in a larger size cohort study.
DAO is a flavin enzyme, which catalyzes oxidative
deamination of natural D-amino acids. DAO gene is present in a
single copy in human chromosome 12q23-24 region. It is ubiquitously
expressed in tissues with high expression in liver, kidney and
brain. Human DAO have various roles in modulating physical
activity, including control of D-serine levels in the brain
(17), production of H2S
from D-cysteine in brain, kidney and GI system (6,8), and
protection from microbes in the mucosa by generating
H2O2 (9).
Thus DAO appear to have important roles in the protection of GI
tract by generating H2S in the stomach and
H2O2 in the intestine. This may in the same
line that we found a sign of association between p.P103L and GI
cancer (Table III).
Recent studies have shown detailed analysis of DAO
non-synonymous variants and their structure function relationship
(18). The p.P103L in our study has
not been specified in the analysis, thus the functional consequence
of this amino acid exchange is not fully understood. According to
Polyphen2, a software to predict the impact of an amino acid
substitution on the structure and function of a human protein, the
p.R22H, p.P103L and p.R283Q are benign, probably damaging and
probably damaging, respectively (Table
I). Indeed, p. P103L has been reported as a phenotypic
modulator of ALS (16).
Nevertheless, none of the p.P103L carrier in our autopsy samples
was diagnosed as ALS.
The striking difference in sex was seen for the
effect of p.P103L on gastric cancer. While all of the heterozygote
men were gastric cancer, none women heterozygote had gastric cancer
but each one had colon cancer, intestine cancer and multiple
cancers, respectively. According to the age-standardized incidence
rate (ASIR) gastric cancer in men were generally twice than women
(19). Although the reason for this
gender difference is not clear, the fact that all four men were
drinker and smoker might have accounted since these factors induce
mucosal damage. While there were only three non-synonymous variants
on the Exome Bead Chip, whole exome sequencing of familial ALS
identified nine non-synonymous rare variants of p.P103L, p.E121K,
p.R199Q, p.R199W, p.Q201R, p.T269I, p.A323V, p.G331E and p.S345F,
according to the Human Genome Mutation Database (HGMD). It would be
interesting to study whether other hypomorphic DAO variants also
associate with gastric cancer, since co-occurrence of ALS and
gastric cancer in Japanese are reported (20).
In conclusion, we found a cluster of DAO rare
variant p.P103L carriers in gastric cancer men. DAO variants
warrants further study with regard to GI diseases in larger size
cohort.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Ministry of
Education, Culture, Sports, Science and Technology/Japan Society
for the Promotion of Science (grant no. 17 K09081).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM designed the present study. TA performed
pathological analysis. MT acquired and analyzed clinical data. YZ
performed genotyping and statistical analyses. MM, TA and MT
confirm the authenticity of all the raw data. YZ and MM wrote the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committees of Tokyo Geriatric Hospital and Tokyo Medical and Dental
University and authorized by TMDU Research Ethics Committee
(approval no. 2016-011-02). Written informed consent was obtained
from family member of all participants involved in the present
study before autopsy.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lott PC and Carvajal-Carmona LG: Resolving
gastric cancer aetiology: An update in genetic predisposition.
Lancet Gastroenterol Hepatol. 3:874–883. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yusefi AR, Bagheri Lankarani K, Bastani P,
Radinmanesh M and Kavosi Z: Risk factors for gastric cancer: A
systematic review. Asian Pac J Cancer Prev. 19:591–603.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang KL, Mashl RJ, Wu Y, Ritter DI, Wang
J, Oh C, Paczkowska M, Reynolds S, Wyczalkowski MA, Oak N, et al:
Pathogenic germline variants in 10,389 adult cancers. Cell.
173:355–370.e14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kimura H: Signalling by hydrogen sulfide
and polysulfides via protein S-sulfuration. Br J Pharmacol.
177:720–733. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shen F, Zhao CS, Shen MF, Wang Z and Chen
G: The role of hydrogen sulfide in gastric mucosal damage. Med Gas
Res. 9:88–92. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shibuya N, Koike S, Tanaka M,
Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N and
Kimura H: A novel pathway for the production of hydrogen sulfide
from D-cysteine in mammalian cells. Nat Commun.
4(1366)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang S, Huang D, An N, Chen D and Zhao D:
A novel pathway for the production of H2 S by DAO in rat jejunum.
Neurogastroenterol Motil. 28:687–692. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sasabe J, Miyoshi Y, Rakoff-Nahoum S,
Zhang T, Mita M, Davis BM, Hamase K and Waldor MK: Interplay
between microbial d-amino acids and host d-amino acid oxidase
modifies murine mucosal defence and gut microbiota. Nat Microbiol.
1(16125)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Souza LK, Araújo TS, Sousa NA, Sousa FB,
Nogueira KM, Nicolau LA and Medeiros JV: Evidence that d-cysteine
protects mice from gastric damage via hydrogen sulfide produced by
d-amino acid oxidase. Nitric Oxide. 64:1–6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sawabe M, Arai T, Kasahara I, Esaki Y,
Nakahara K, Hosoi T, Orimo H, Takubo K, Murayama S and Tanaka N:
Developments of geriatric autopsy database and Internet-based
database of Japanese single nucleotide polymorphisms for geriatric
research (JG-SNP). Mech Ageing Dev. 125:547–552. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oda K, Tanaka N, Arai T, Araki J, Song Y,
Zhang L, Kuchiba A, Hosoi T, Shirasawa T, Muramatsu M and Sawabe M:
Polymorphisms in pro- and anti-inflammatory cytokine genes and
susceptibility to atherosclerosis: A pathological study of 1503
consecutive autopsy cases. Hum Mol Genet. 16:592–599.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim EJ, Kim YE, Jang JH, Cho EH, Na DL,
Seo SW, Jung NY, Jeong JH, Kwon JC, Park KH, et al: Analysis of
frontotemporal dementia, amyotrophic lateral sclerosis, and other
dementia-related genes in 107 Korean patients with frontotemporal
dementia. Neurobiol Aging. 72:186.e1–186.e7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang H, Cai W, Chen S, Liang J, Wang Z,
Ren Y, Liu W, Zhang X, Sun Z and Huang X: Screening for possible
oligogenic pathogenesis in Chinese sporadic ALS patients. Amyotroph
Lateral Scler Frontotemporal Degener. 19:419–425. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barber IS, Braae A, Clement N, Patel T,
Guetta-Baranes T, Brookes K, Medway C, Chappell S, Guerreiro R,
Bras J, et al: Mutation analysis of sporadic early-onset
Alzheimer's disease using the NeuroX array. Neurobiol Aging.
49:215.e1–215.e8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pang SY, Hsu JS, Teo KC, Li Y, Kung MHW,
Cheah KSE, Chan D, Cheung KMC, Li M, Sham PC and Ho SL: Burden of
rare variants in ALS genes influences survival in familial and
sporadic ALS. Neurobiol Aging. 58:238.e9–238.e15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pollegioni L, Sacchi S and Murtas G: Human
D-Amino acid oxidase: Structure, function, and regulation. Front
Mol Biosci. 5(107)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sacchi S, Cappelletti P and Murtas G:
Biochemical properties of human D-amino acid oxidase variants and
their potential significance in pathologies. Front Mol Biosci.
5(55)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ang TL and Fock KM: Clinical epidemiology
of gastric cancer. Singapore Med J. 55:621–628. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nozaki I, Kato-Motozaki Y, Ikeda T, Tagami
A, Takahashi K, Ishida C and Komai K: Clinical features in
association with neurodegenerative diseases and malignancies. Eur
Neurol. 71:99–105. 2014.PubMed/NCBI View Article : Google Scholar
|