Introduction
Cancer is not the leading cause of death worldwide,
but cancer associated mortality has increased in recent years
(1,2). With stratification by income, it has
been indicated that cancer mortality rates are steadily increasing
in high-income countries compared with low-income countries
(1,2). According to data from the
International Agency for Research on Cancer (IARC) World Cancer
Statistics GLOBOCAN, breast cancer is the most frequent cancer in
women, accounted for 24% of newly diagnosed cancers in 2018 and 15%
of cancer deaths, and these rates are expected to increase in the
future (1,2). In addition, it is considered that the
worldwide population will be aging in the future (3). Moreover, in 2019, a total of 463
million people were estimated to be living with diabetes (4), representing 9.3% of the global adult
population (20-79 years), with a prevalence of 9.0% in women and
9.6% in men. The number of people living with diabetes is projected
to increase by 25% to 578 million by 2030 and by 51% to 700 million
by 2045 globally (4). The morbidity
and mortality associated with aging, diabetes, and breast cancer
are also very relevant concerns for the Japanese population.
Therefore, novel therapeutic drugs for breast cancer and diabetes
are continuously being developed; However, with increasing numbers
of patients with comorbidities, the interactions, side effects, and
adverse events of these therapeutic drugs are becoming increasingly
more complicated. Under these circumstances, it is important to
provide safe and secure medical care to elderly patients in
particular, and it is expected that the need for a team approach to
medical care consisting of many specialists, including doctors and
pharmacists, will become even more important in the future. The
current reports describes a case of severe hypoglycaemia in a
patients with breast cancer that persisted for >24 h after the
administration of abemaciclib, an antitumor agent and dual
inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6). In November
2018, Japan approved the use of abemaciclib for the treatment of
hormone receptor-positive and HER2-negative advanced and metastatic
breast cancer (5-7).
Currently, to the best of our knowledge, there have been no
detailed reports regarding cases of severe hypoglycaemia associated
with the use of abemaciclib to date.
Case report
In March 2013, an 80-year-old woman who had
developed multiple bone, liver, and ovarian metastases from right
breast cancer (ER+: 90%, PgR8+: 90%,
HER2-) was administered letrozole orally and denosumab
subcutaneously. In June 2015, letrozole treatment was replaced with
fulvestrant (Ful) owing to progressive disease (PD). In February
2018, haemorrhagic advanced breast cancer [Br+AX (level 1), T4N1M1,
pT4bN1MX, stage IV] mastectomy was performed. Administration of Ful
was continued thereafter. In July 2018, despite treatment with Ful,
the patient experienced PD; therefore, bevacizumab plus paclitaxel
therapy was initiated. In March 2019, due to PD as detected by
computed tomography, abemaciclib plus Ful therapy was initiated.
The pateent's glycated haemoglobin (HbA1c) level was 5.9% 3 weeks
before the initiation of abemaciclib treatment, which commenced 4
days before hospitalisation (-day 4) at an oral dose of 150 mg,
twice daily. However, adverse events such as poor physical
condition, abdominal pain, and diarrhoea occurred on the second day
post abemaciclib treatment (-day 3). Therefore, the dose of
abemaciclib was reduced to 100 mg, twice a day, from -day 1 to
hospitalisation. The patient reported tremors and insomnia that
same night. Furthermore, on the following day (day 1: Day of
hospitalisation), she reported subjective symptoms of diplopia, but
she was able to eat a full meal three times that day. At ~21:45 h,
the patient's family noticed that the patient was unfocused, with
impaired speech; therefore, they contacted the emergency department
of the hospital 5 min later. The patient was rushed to the hospital
by her family at 22:40 h. As she was in the supine position in the
back seat of the car, the staff transferred her to a stretcher with
full assistance. The patient responded slightly to our call, but
her level of consciousness declined, and she was unable to speak
and focus. Her breathing was normal and there were no abnormal
laboratory findings in her chest and abdomen during emergency room
(ER) observation. The laboratory findings in the emergency
outpatient clinic revealed extremely low blood glucose level of 24
mg/dl (Fig. 1A), indicating
hypoglycaemia (Tables I and
II). Moreover, renal dysfunction
was indicated as her serum creatinine level was 1.40 mg/dl.
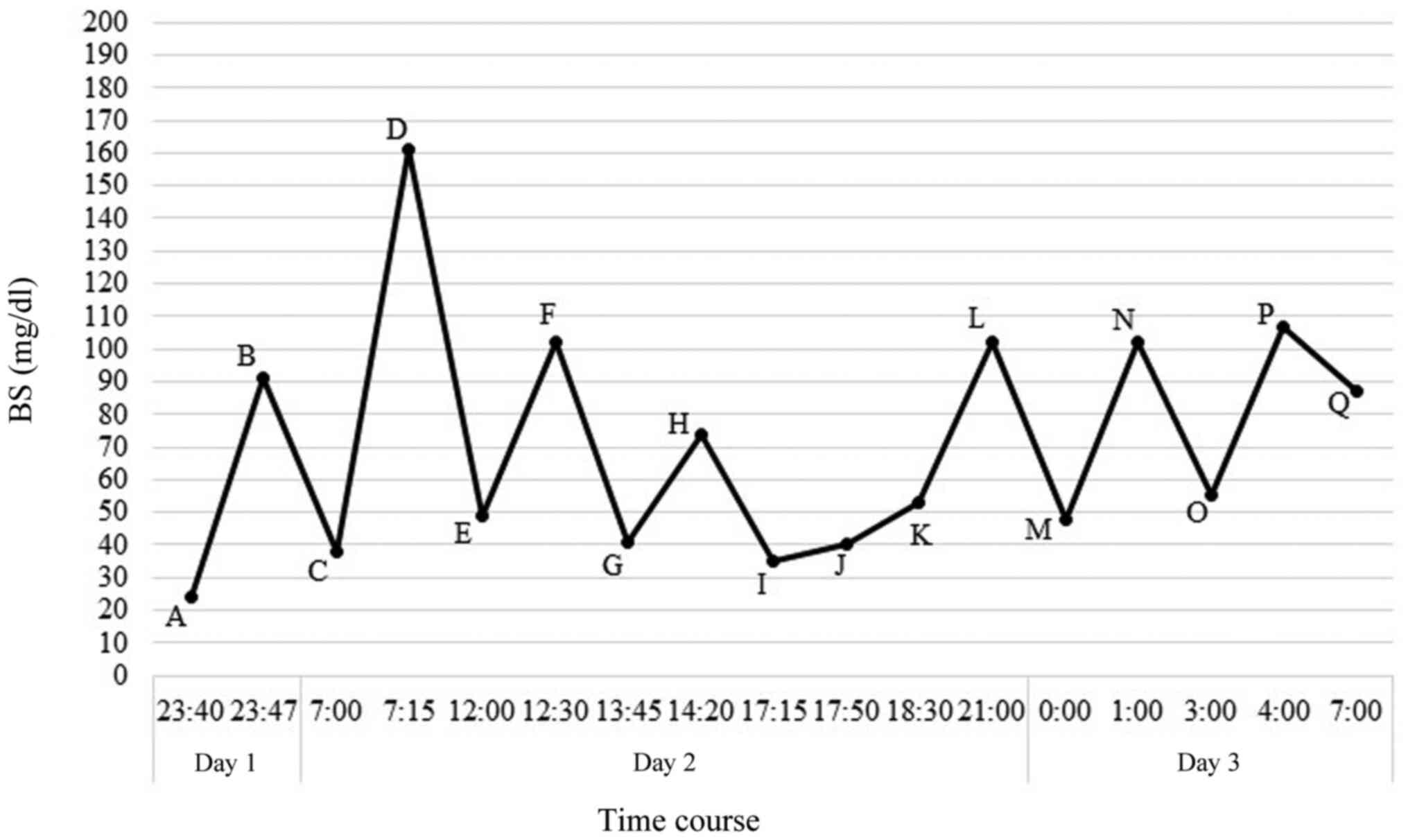 | Figure 1Patient's time course of blood glucose
level and treatment after admission (A) day 1, 23:40 h, measurement
of blood glucose and i.v. administration of 20 ml of 40% glucose
solution; (B) day 1, 23:47 h, measurement of blood glucose, and the
patient consumed a midnight snack; (C) day 2, 7:00 h, measurement
of blood glucose and i.v. administration of 40 ml of 40% glucose
solution; (D) day 2, 7:15 h, measurement of blood glucose, and the
patient consumed breakfast; (E) day 2, 12:00 h, measurement of
blood glucose and i.v. administration of 20 ml of 40% glucose; (F)
day 2, 12:30 h, measurement of blood glucose, and the patient
consumed lunch; (G) day 2, 13:45 h, measurement of blood glucose
and oral administration of 10 g glucose; (H) day 2, 14:20 h,
measurement of blood glucose; (I) day 2, 17:15 h, measurement of
blood glucose and i.v. administration of 20 ml of 40% glucose
solution; (J) day 2, 17:50 h, measurement of blood glucose and i.v.
administration of 20 ml of 40% glucose; the patient consumed
dinner; (K) day 2, 18:30 h, measurement of blood glucose and oral
administration of 10 g glucose; (L) day 2, 21:00 h, measurement of
blood glucose; (M) day 3, 0:00 h, measurement of blood glucose and
oral administration of 10 g glucose; (N) day 3, 1:00 h, measurement
of blood glucose; (O) day 3, 3:00 h, measurement of blood glucose
and oral administration of 10 g glucose; (P) day 3, 4:00 h,
measurement of blood glucose; (Q) day 3, 7:00 h, measurement of
blood glucose. BS, blood sugar. |
 | Table IObservations at admission. |
Table I
Observations at admission.
| Clinical
characteristics | Value |
|---|
| Body height | 154.5 cm |
| Body-weight
(BMI) | 57.2 kg (23.96
kg/m2) |
| Body temperature | 34.5˚C |
| Blood pressure | 118/52 mmHg |
| Pulse | 67/min |
| Oxygen
saturation | 94% |
| Consciousness level
(GCS) | 13 = E4+V4+M5 |
 | Table IIPhysiological data at the time of
hospitalization. |
Table II
Physiological data at the time of
hospitalization.
| Blood and biochemical
tests | Value |
|---|
| AST | 29 IU/l |
| ALT | 21 IU/l |
| BUN | 20.4 mg/dl |
| Cr | 1.4 mg/dl |
| CK | 108 IU/l |
| Na | 141 mEq/l |
| Cl | 108 mEq/l |
| Ca | 8.6 mEq/l |
| BS | 24 mg/dl |
| WBC |
66.1x103/µl |
| RBC |
342x104/µl |
| Hb | 108 g/dl |
| Ht | 31.40% |
| PLT |
12.7x103/µl |
| Neut |
55.2x102/µl |
| Lymph |
7.9x102/µl |
Emergency outpatient clinical course: In the ER, the
patient was not able to focus her eyes and she was unable to answer
our call. As the blood tests revealed hypoglycaemia, we
administered 40 ml of 40% glucose solution intravenously. The
patient's level of consciousness returned to normal immediately
after the injection. She could make eye contact and converse with
us. After 60 min, at 23:40 h, her blood glucose level increased to
91 mg/dl (Fig. 1B), and she was
able to urinate independently in the toilet and move to a
wheelchair stably. The patient did not remember the time she
arrived at the hospital because of hypoglycaemia. In the ER, we
asked her family about her medical history and medications,
including whether she had diabetes. The patient had no history of
receiving antidiabetic treatment at other hospitals and had no
experience of hypoglycaemia while receiving antidiabetic drugs for
more than a year (Table III).
Additionally, she had eaten all her meals on the day of
hospitalisation (day 1). She had self-managed abemaciclib
medication (Verzenio), which was recorded in the ‘Verzenio Diary’.
We were able to confirm that there was no overdose of glimepiride
(Gli) tablets from the patient's remaining medication.
 | Table IIIPrescription drugs: Daily dose. |
Table III
Prescription drugs: Daily dose.
| Oral
medication | Dose |
|---|
| Abemaciclib (100
mg) | Twice (after
breakfast and dinner) |
| Glimepiride (1
mg) | Twice (after
breakfast and dinner) |
| Loxoprofen sodium
hydrate (60 mg) | Twice (after
breakfast and dinner) |
| Rebamipide (100
mg) | Twice (after
breakfast and dinner) |
| Doxazosin mesylate
(2 mg) | Twice (after
breakfast and dinner) |
| Valsartan (80
mg) | Once after
breakfast |
| Amlodipine besilate
(5 mg) | Once after
breakfast |
| Pravastatin sodium
(10 mg) | Once after
dinner |
| Loperamide
hydrochloride (1 mg) | Up to 3 times a day
in case of diarrhoea |
| Brotizolam (0.25
mg) | Before sleeping in
case of insomnia |
| Indomethacin
patch | Topical |
The patient was admitted to the surgical department
on the same day for hypoglycaemia treatment and follow-up. All
outpatient prescription drugs were discontinued at the time of
hospitalisation. The patient's clinical course after admission is
shown in Fig. 1. She was conscious
at 00:10 h on the second day of admission. As she complained of
hunger, she was provided a banana and tea by her family. We did not
detect symptoms such as diplopia, numbness, and cold sweats. The
patient seemed to have independently used the toilet during the
night without the aid of a nurse, as we detected a large quantity
of urine in a portable toilet. The patient was asleep during the
nurse's patrol. At 7:00 h, during the nurse's patrol, the patient
was changing clothes on the bed, but she did not respond to the
nurse's calling; moreover, she could not focus her eyes. Her blood
glucose level was 38 mg/dl (Fig.
1C); thus, we immediately injected 40 ml of 40% glucose
solution intravenously. Shortly after, her blood glucose level
increased to 161 mg/dl (Fig. 1D),
and her consciousness level returned to normal. She could maintain
eye contact and we could converse with her. However, the patient
did not remember any of her hypoglycaemic events. Immediately after
the intravenous injection of glucose solution and oral glucose
intake, her blood glucose levels increased and her consciousness
improved, but the blood glucose levels later dropped back to 30-50
mg/dl. This hypoglycaemic event repeated until the third day
post-admission. On all 3 days in the surgical department, the
patient ate all of her meals (breakfast, lunch, and dinner). At
7:00 h on the third day of admission, her blood glucose level was
87 mg/dl, indicating no hypoglycaemia (Fig. 1Q). The patient's blood glucose level
was maintained over 80 mg/dl, and there was no relapse of
hypoglycaemia. Her immunoreactive insulin level was normal at 5.56
µIU/ml. In summary, the total administered glucose content from
admission to recovery of severe hypoglycaemia was 48 g administered
intravenously and 40 g administered orally, plus a regular meal of
1,600 kcal/day and a banana. Finally, the time required to recover
from severe hypoglycaemia was ~46 h. Summary of the treatment
(Table IV).
 | Table IVSummary of the treatment. |
Table IV
Summary of the treatment.
| A, Day 1 |
|---|
| Point | Time | Blood glucose
level | Treatment |
|---|
| A | 23:40 h | 24 | 20 ml of 40%
glucose solution i.v. injection |
| B | 23:47 h | 91 | Patient ate a
midnight snack |
| B, Day 2 |
| Point | Time | Blood glucose
level | Treatment |
| C | 7:00 h | 38 | 40 ml of 40%
glucose solution i.v. injection |
| D | 7:15 h | 161 | Patient ate
breakfast |
| E | 12:00 h | 49 | 20 ml of 40%
glucose solution i.v. injection |
| F | 12:30 h | 102 | Patient ate
lunch |
| G | 13:45 h | 41 | Oral administration
of 10 g |
| H | 14:20 h | 74 | Oral administration
of 10 g |
| I | 17:15 h | 35 | 20 ml of 40%
glucose solution i.v. injection |
| J | 17:50 h | 40 | 20 ml of 40%
glucose solution i.v. injection |
| K | 18:30 h | 53 | Oral administration
of 10 g glucose |
| L | 21:00 h | 102 | Medical
follow-up |
| C, Day 3 |
| Point | Time | Blood glucose
level | Treatment |
| M | 0:00 h | 48 | Oral administration
of 10 g glucose |
| N | 1:00 h | 102 | Medical
follow-up |
| O | 3:00 h | 55 | Oral administration
of 10 g glucose |
| P | 4:00 h | 107 | Medical
follow-up |
| Q | 7:00 h | 87 | Medical
follow-up |
Discussion
We present a case report of severe hypoglycaemia
under abemaciclib administration. When the patient arrived at our
hospital, she had taken prescription medicines, including
abemaciclib, after a full portion of dinner. We confirmed with the
family regarding the absence of any overlapping medications. In
this case (from -day 4 to 0), no additional new medicines were
administered other than abemaciclib. The patient had been using Gli
and loxoprofen sodium hydrate (Lox) since a long time, and no
associated hypoglycaemic events had occurred previously. Therefore,
the possibility of drug (Gli and Lox)-interaction-induced
hypoglycaemia was low. Gli has a high protein-binding rate
according to dosage studies in patients with type 2 diabetes
(8-11).
Allylpropionic acid-based Lox also has a high protein-binding rate
(12-15).
Therefore, when Gli is used together with Lox, the binding of Gli
to blood protein is suppressed, and the free form of Gli increases
(8-11).
Therefore, the combined usage of Lox with Gli may enhance the
hypoglycaemic effect (16).
Abemaciclib, a pyrido[2,3-d]pyrimidin-7-one inhibitor, is a
selective inhibitor of CDK4 and CDK6 (17-19)
that phosphorylates Rb and activates transcription factor E2F1/2.
Thus, abemaciclib pushes cells into the S phase and triggers DNA
synthesis (20,21). The time to reach abemaciclib
Cmax is ~5 h (Tmax, 4-6 h) (22,23),
and the half-life of 150 mg of abemaciclib is 17.5 h (nearly lower
limit: 17.4 to 38.1 h) (22,23).
Therefore, the total time to reach half the maximum blood
concentration after abemaciclib administration is ~24 h
[Tmax + t1/2 =
5+17.5=22.5 h (22,23)]. In other words, it takes 24 h for
abemaciclib blood concentration to drop by half (1/2). As
abemaciclib is administered twice daily, a considerable amount of
abemaciclib may persist in the blood when the second dose (~12 h
later) is administered. In patients with severe liver dysfunction,
the blood concentration of this drug increases (24). With repeated dosing of abemaciclib,
the blood concentration of abemaciclib in patients with metastatic
liver tumours may be higher than anticipated, even with normal
liver function (11). Abemaciclib
has been shown to have a high human plasma protein-binding rate in
in vitro studies (5-7,22).
Gli is primarily metabolised by the liver metabolic enzyme CYP2C9
and excreted via the kidney (urine) and liver (bile) (8-11,25),
while abemaciclib is metabolised by CYP3A and excreted via the
liver (24). Therefore, the
possibility that they influence each other's metabolism is low.
Increase in blood creatinine level has been described as an adverse
event of abemaciclib (5-7,24).
The patient's creatinine level was 1.05 mg/dl at the start of
administration (-day 4). On the fifth day of abemaciclib
administration (day 1: Day of hospitalisation), the creatinine
level increased to 1.40 mg/dl; however, dehydration due to loose
stools, diarrhoea, and other symptoms was not observed on
admission.
Abemaciclib has been shown to slow metabolism in the
blood of patients with impaired liver function (5-7,24).
In metastatic liver cancer, CYP2C9 metabolism in the liver
decreases; therefore, the blood concentration of Gli increases
(11,25). However, our patient was administered
the same dose of Gli for over 1 year and had never experienced
hypoglycaemia. In addition, CYP3A4 metabolism in the liver
decreases in metastatic liver cancer (26,27).
Consequently, the blood concentration of abemaciclib increases,
which increases the creatinine level (5-7,24).
Furthermore, an increase in creatinine level suggests a decrease in
renal function, which is thought to increase the blood
concentration of Gli (25,28-31).
Although it is unclear at present whether this case is an isolated
incident of the combined biochemical and genetic profile of the
patient, severe hypoglycaemia may well occurs in elderly breast
cancer patients with diabetes and a history of liver metastases
when abemaciclib is combined used with Gli (high protein-binding
affinity) and allylpropionic acid-based Lox (high protein-binding
affinity). Therefore, adverse events of the drug for these patients
are likely to be worth investigating in a larger population size
and those awaits further elucidation. The increase in creatinine
levels following abemaciclib administration does not necessarily
indicate glomerular injury. However, it is difficult to argue that
the increase in creatinine levels is not related to the decrease in
renal function (5,23,32).
Although it can not be ruled out that, the increased creatinine
levels after abemaciclib treatment in patients without liver
metastases, it may be lead to hyperglycemia caused by decreased
water reabsorption, Low levels of Ht and BUN/Cre in the labo data
indicated that the patient was not dehydrated at the time of
transport. Although since this patient has liver metastasis, it is
considered that the blood concentration of abemaciclib is increased
due to the metabolic delay of abemaciclib and the blood creatinine
level is increased. Increased creatinine levels suggested a
decrease in renal function, which may have caused an increase in
the blood concentration of Gli and the strong effect of Gli may
have caused the patient's hypoglycaemia. Moreover, glucagon blood
sugar increasing action is mainly due to the decomposition of
hepatic glycogen, it is said that the effect of raising blood sugar
can hardly be expected for liver metastasis patients (33). And in severe hypoglycemia with
unconsciousness, it may be difficult to take glucose tablets or
glucose powder. Based on the above, we must attend to the presence
or absence of liver metastases, use of drugs that depend on renal
excretion, blood glucose level should be carefully monitored, when
we are using abemaciclib with diabetes patients. Then, if renal
function is poor, it is necessary to immediately stop SU drugs such
as Gli and switch to insulin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH, TK and KK were involved in the conception and
design of the case study; TH, MY, MH, KI and SS were involved in
data acquisition; TH, MY, SH, KI, SY and SS analysed and
interpreted the data. TH and KK were responsible for confirming the
authenticity of the raw data. The manuscript was written by TH and
was critically reviewed by TH, TK, MY, SH, MH, KI, SY, SS and KK.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jamison DT, Summers LH, Alleyne G, Arrow
KJ, Berkley S, Binagwaho A, Bustreo B, Evans D, Feachem RGA, Frenk
J, et al: Global health 2035: A world converging within a
generation. Lancet. 382:1898–1955. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raftery AE, Li N, Ševčíková H, Gerland P
and Heilig GK: Bayesian probabilistic population projections for
all countries. Proc Natl Acad Sci USA. 109:13915–13921.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International Diabetes Federation Diabetes Atlas, 9(th)
edition. Diabetes Res Clin Pract. 157(107843)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dickler MN, Tolaney SM, Rugo HS, Cortés J,
Diéras V, Patt D, Wildiers H, Hudis CA, O'Shaughnessy J, Zamora E,
et al: MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6
inhibitor, as a single agent, in patients with refractory
HR+/HER2- metastatic breast cancer. Clin
Cancer Res. 23:5218–5224. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib, in combination with Fulvestrant in women
with HR+/HER2- advanced breast cancer who had
progressed while receiving endocrine therapy. J Clin Oncol.
35:2875–2884. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib, as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Draeger E: Clinical profile of
glimepiride. Diabetes Res Clin Pract. 28:139–146. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Draeger KE, Wernicke-Panten K, Lomp HJ,
Schüler E and Rosskamp R: Long-term treatment of type 2 diabetic
patients with the new oral antidiabetic agent glimepiride (Amaryl):
A double-blind comparison with glibenclamide. Horm Metab Res.
28:419–425. 1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Badian M, Korn A, Lehr KH, Malerczyk V and
Waldhäusl W: Absolute bioavailability of Glimepiride (Amaryl) after
oral administration. Drug Metabol Drug Interact. 11:331–339.
1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Niemi M, Cascorbi I, Timm R, Kroemer HK,
Neuvonen PJ and Kivistö KT: Glyburide and glimepiride
pharmacokinetics in subjects with different CYP2C9 genotypes. Clin
Pharmacol Ther. 72:326–332. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Otagiri M: Study on binding of drug to
serum protein. Yakugaku Zasshi. 129:413–425. 2009.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
13
|
Meyer MC and Guttman DE: The binding of
drugs by plasma proteins. J Pharm Sci. 57:895–918. 1968.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jusko WJ and Gretch M: Plasma and tissue
protein binding of drugs in pharmacokinetics. Drug Metab Rev.
5:43–140. 1976.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vallner JJ: Binding of drugs by albumin
and plasma protein. J Pharm Sci. 66:447–465. 1977.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Langtry HD and Balfour JA: Glimepiride. A
review of its use in the management of type 2 diabetes mellitus.
Drugs. 55:563–584. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang D, Sun Y, Li W, Ye F, Zhang Y, Guo Y,
Zhang DY and Suo J: Antiproliferative effects of the CDK6 inhibitor
PD0332991 and its effect on signaling networks in gastric cancer
cells. Int J Mol Med. 41:2473–2484. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Toogood PL, Harvey PJ, Repine JT, Sheehan
DJ, VanderWel SN, Zhou H, Keller PR, McNamara DJ, Sherry D, Zhu T,
et al: Discovery of a potent and selective inhibitor of
cyclin-dependent kinase 4/6. J Med Chem. 48:2388–2406.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu H, Yu S, Liu Q, Yuan X, Mani S, Pestell
RG and Wu K: Recent advances of highly selective CDK4/6 inhibitors
in breast cancer. J Hematol Oncol. 10(97)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li B, He H, Tao BB, Zhao ZY, Hu GH, Luo C,
Chen JX, Ding XH, Sheng P, Dong Y, et al: Knockdown of CDK6
enhances glioma sensitivity to chemotherapy. Oncol Rep. 28:909–914.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Fujiwara Y, Tamura K, Kondo S, Tanabe Y,
Iwasa S, Shimomura A, Kitano S, Ogasawara K, Turner PK, Mori J, et
al: Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a
single agent for Japanese patients with advanced cancer. Cancer
Chemother Pharmacol. 78:281–288. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Patnaik A, Rosen LS, Tolaney SM, Tolcher
AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW,
Hilton JF, et al: Efficacy and safety of abemaciclib, an inhibitor
of CDK4 and CDK6, for patients with breast cancer, non-small cell
lung cancer, and other solid tumors. Cancer Discov. 6:740–753.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tate SC, Sykes AK, Kulanthaivel P, Chan
EM, Turner PK and Cronier DM: A population pharmacokinetic and
pharmacodynamic analysis of abemaciclib, in a phase I clinical
trial in cancer patients. Clin Pharmacokinet. 57:335–344.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rosenkranz B: Pharmacokinetic basis for
the safety of glimepiride in risk groups of NIDDM patients. Horm
Metab Res. 28:434–439. 1996.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kacevska M, Robertson GR, Clarke SJ and
Liddle C: Inflammation and CYP3A4-mediated drug metabolism in
advanced cancer: Impact and implications for chemotherapeutic drug
dosing. Expert Opin Drug Metab Toxicol. 4:137–149. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fahy BN, Guo T and Ghose R: Impact of
hepatic malignancy on CYP3A4 gene expression. J Surg Res.
178:768–772. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rosenkranz B, Profozic V, Metelko Z,
Mrzljak V, Lange C and Malerczyk V: Pharmacokinetics and safety of
glimepiride at clinically effective doses in diabetic patients with
renal impairment. Diabetologia. 39:1617–1624. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hou L, Zhao T, Liu Y and Zhang Y: Efficacy
and safety of sitagliptin compared with sulfonylurea therapy in
patients with type 2 diabetes showing inadequately controlled
glycosylated hemoglobin with metformin monotherapy: A
meta-analysis. Exp Ther Med. 9:1528–1536. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
DeFronzo RA: Pharmacologic therapy for
type 2 diabetes mellitus. Ann Intern Med. 131:281–303.
1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Inzucchi SE: Oral antihyperglycemic
therapy for type 2 diabetes: Scientific review. JAMA. 287:360–372.
2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Levey AS, Perrone RD and Madias NE: Serum
creatinine and renal function. Annu Rev Med. 39:465–490.
1988.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pun KK, Young RTT, Wang C, Tam CF and Ho
PWM: The use of glucagon challenge tests in the diagnostic
evaluation of hypoglycemia due to hepatoma and insulinoma. J Clin
Endocrinol Metab. 67:546–550. 1988.PubMed/NCBI View Article : Google Scholar
|















