Introductions
Recently, the prognosis of patients with metastatic
renal cell carcinoma (RCC) has been improved by molecular targeted
therapies (1) and immune checkpoint
inhibitors (2). However, optimal
treatment strategies for metastatic non-clear cell RCC (nccRCC) has
yet to be established. In previous studies, molecular targeted
therapies, especially sunitinib, have shown clinical efficacy for
the treatment of metastatic nccRCC (3-7).
The NCCN guidelines (2020 version) indicate that clinical trial or
sunitinib is recommended at present for metastatic nccRCC. In
nccRCC papillary renal cell carcinoma (PRCC) is the leading
histology (8). PRCC is divided into
type 1 and type 2, with type 2 PRCC showing worse prognosis
compared with type 1(9). In phase 2
clinical trial evaluating the efficacy of sunitinib for metastatic
PRCC, overall survival (OS) of type1 PRCC was 17.8 months, and that
of type 2 was 12.4 months (10).
In this report, we present a patient with metastatic
type 2 PRCC whose metastatic lesions were controlled for a long
time by multidisciplinary treatments including metastasectomies,
axitinib, zoledronic acid (ZA), and radiation therapy.
Case report
A 46-year-old man with hematospermia was presented
to a urologic clinic in April, 2011. A right renal tumor was found
by ultrasound, and the patient was referred to our hospital. The
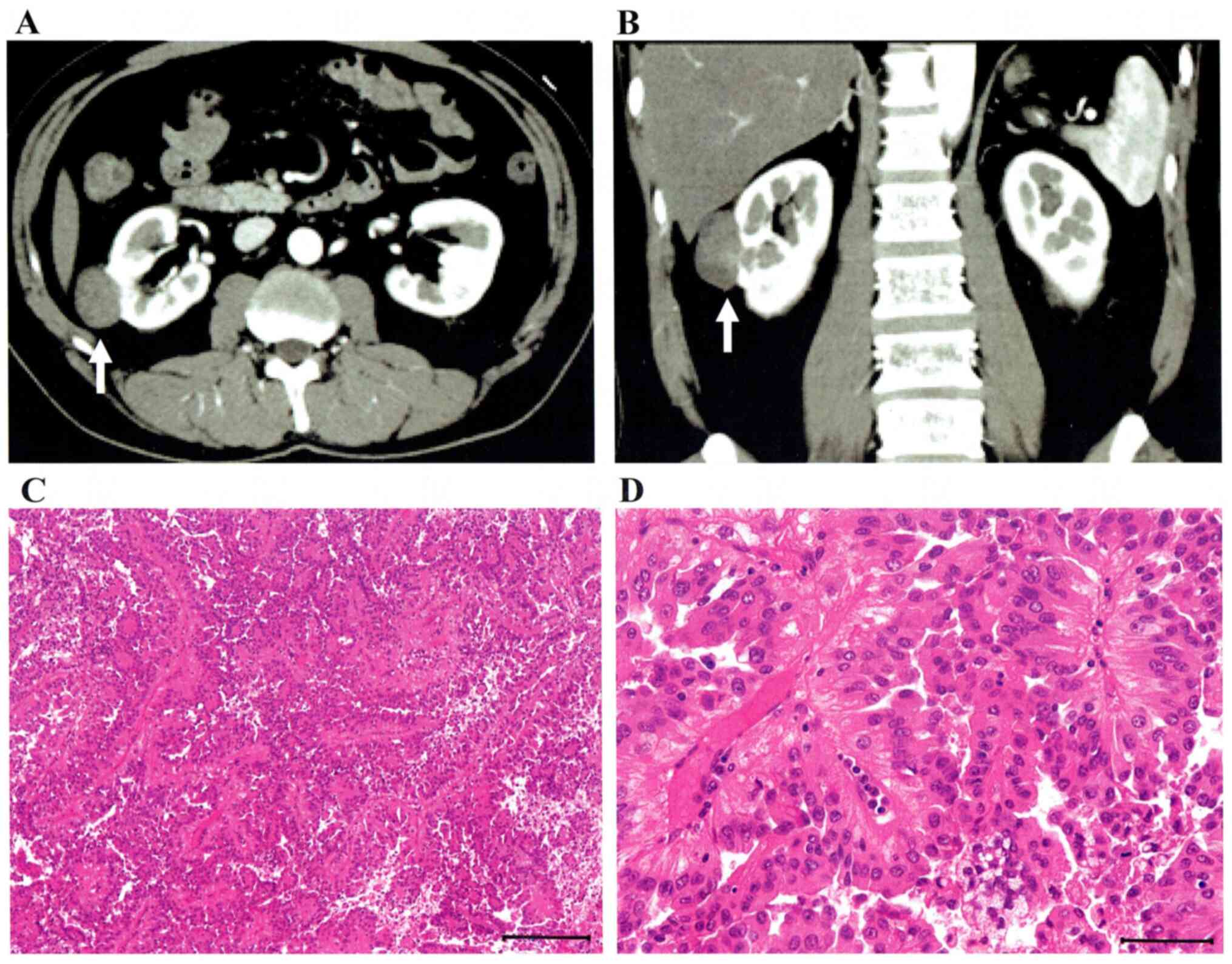
tumor (35 mm in maximal diameter) was located in the middle of the
right kidney, and RCC or fat-poor angiomyolipoma was suspected by
contrast-enhanced computed tomography (CT) (Fig. 1A and B). A right partial nephrectomy was
performed in July, 2011. Pathological diagnosis was type2 PRCC
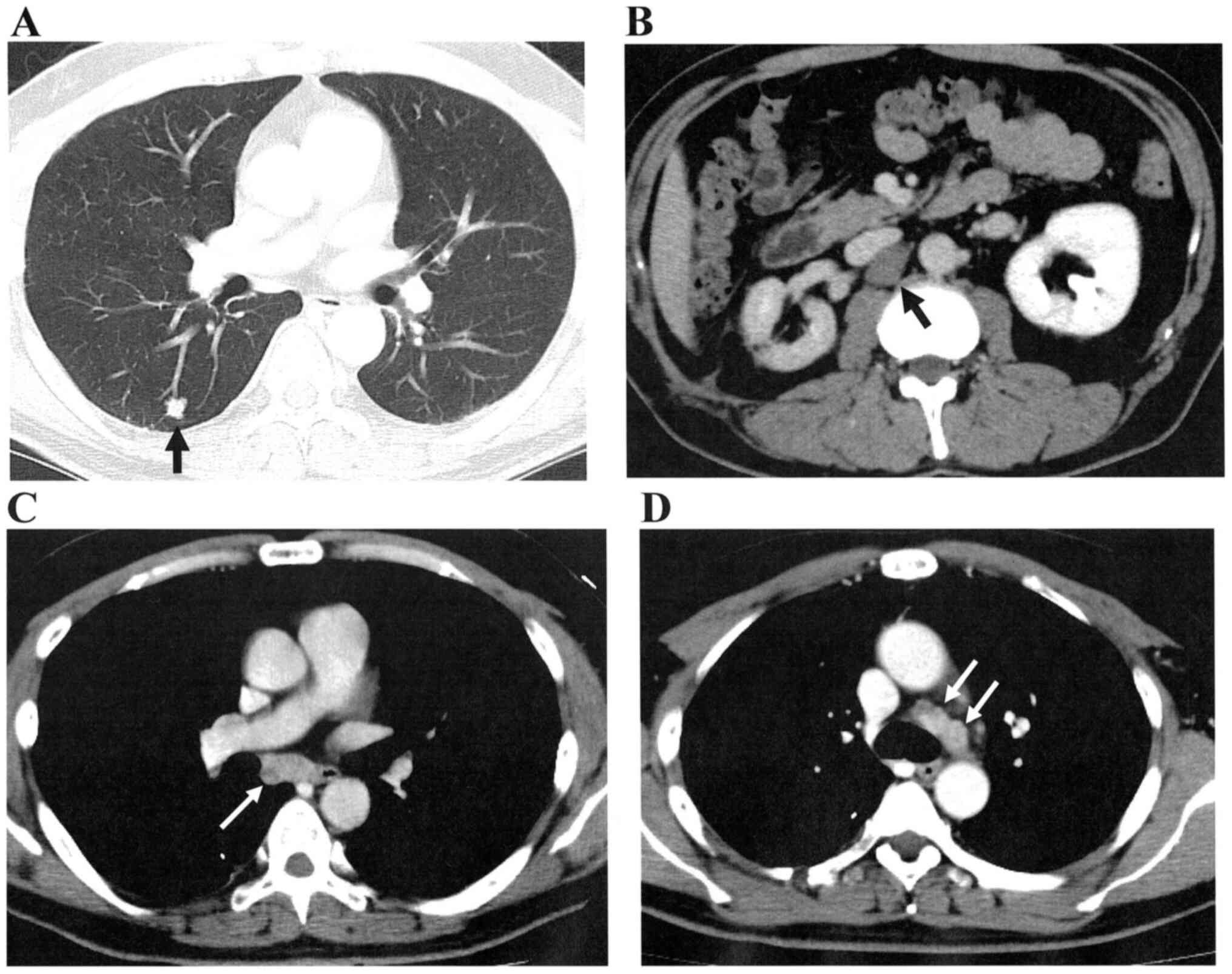
(Fig. 1C and D). A lung metastasis (S6) and paracaval
lymph node (LN) metastases appeared in March, 2012 (Fig. 2A and B). After five cycles of temsirolimus,
right radical nephrectomy and extensive LN dissection around the
inferior vena cava (IVC) and right common iliac vein were performed
in June, 2012, and the resection of the lung metastasis was
performed one month after the nephrectomy. Then, radiological
complete remission was achieved. LN swellings at the right tracheal
bifurcation was found by CT in August, 2013 (Fig. 2C). After three cycles of
temsirolimus, mediastinal LN dissection was performed in October,
2013. Mediastinal LNs (Fig. 2D)
were swollen again, and multiple bone metastases [sternum, right
fifth rib, thoracic spine (5-7th vertebrae), left ilium, and right
pubis] appeared in 2014. The clinical course of the patient,
including local treatments, medical treatments, and
treatment-related adverse events are shown in Fig. 3. Sunitinib (37.5 mg/day) and ZA (4
mg/month) were started in June, 2014. Sunitinib was stopped within
two weeks because of adverse events (AEs) including fever, malaise,
and liver dysfunction. After the patient had recovered from the
AEs, axitinib (10 mg/day) was started as a second-line treatment in
July, 2014. ZA was continued after the axitinib administration.
Because of diarrhea and hoarseness, the treatment schedule of
axitinib was changed to a periodic drug withdrawal schedule (5
days-on, 2 days-off) in October, 2014. Next, the daily dose of
axitinib was reduced to 8 mg (5 days-on, 2 days-off) to control
severe diarrhea in December, 2014. Stereotactic radiation therapy
(total 30 Gray, 10 fractions) was performed for bone metastasis at
the left ilium in November, 2015 because only the iliac metastasis
appeared to be an active lesion among all bone metastases in the
bone scintigraphy. The periodic drug withdrawal schedule of
axitinib (8 mg/day, 5 days-on, 2 days-off) was changed to the next
schedule (8 mg/day, 4 days-on, 3 days-off) due to renal dysfunction
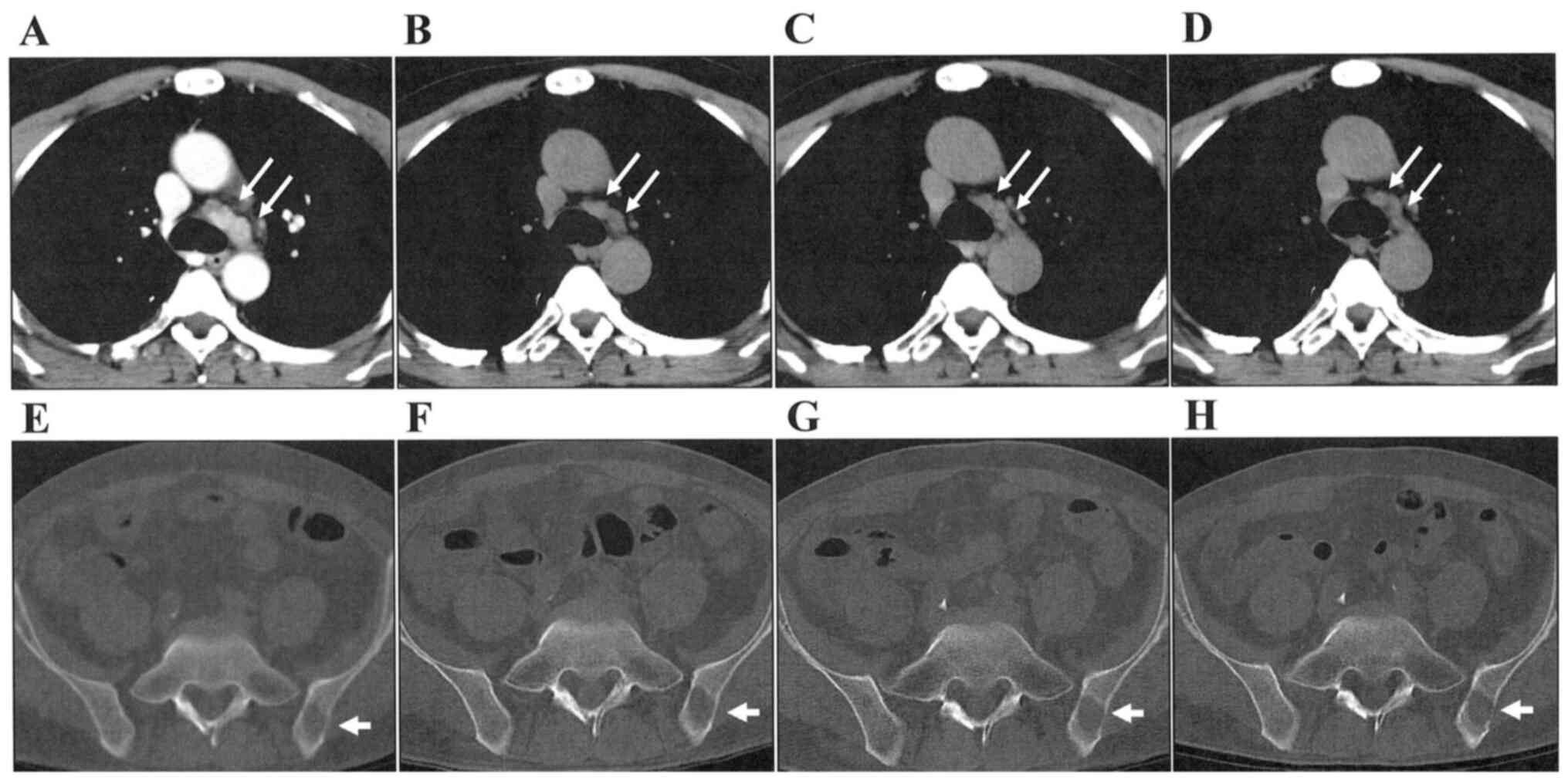
and proteinuria in April, 2017. Mediastinal LN metastases and bone
metastases was stable for 30 months after the axitinib
administration (Fig. 4). Disease
progression was confirmed in June, 2017 due to the appearance of
multiple small lung metastases. Because the metastatic lesions
progressed slowly after the disease progression, axitinib was
continued for another 19 months in accordance with the patient's
request. The schedule of axitinib was then changed to a third
schedule (8 mg/day, 3 days-on, 3 days-off) due to symptoms of
anorexia, dyspnea, and muscle pain in April, 2018. Patient was
hospitalized due to severe back pain in September, 2018, and
palliative radiotherapy was performed for a compression fracture
due to metastasis (L1 lumber vertebrae). Axitinib was stopped in
October, 2019. Temsirolimus was then administered, but interstitial
pneumonia occurred after three cycles of temsirolimus. Although
steroid pulse therapy was performed, respiratory and general
condition became worse. The patient died in November, 2019. By
multidisciplinary treatments including metastasectomy, axitinib,
ZA, and radiation therapy, the patient survived for 80 months after
the initial recurrence.
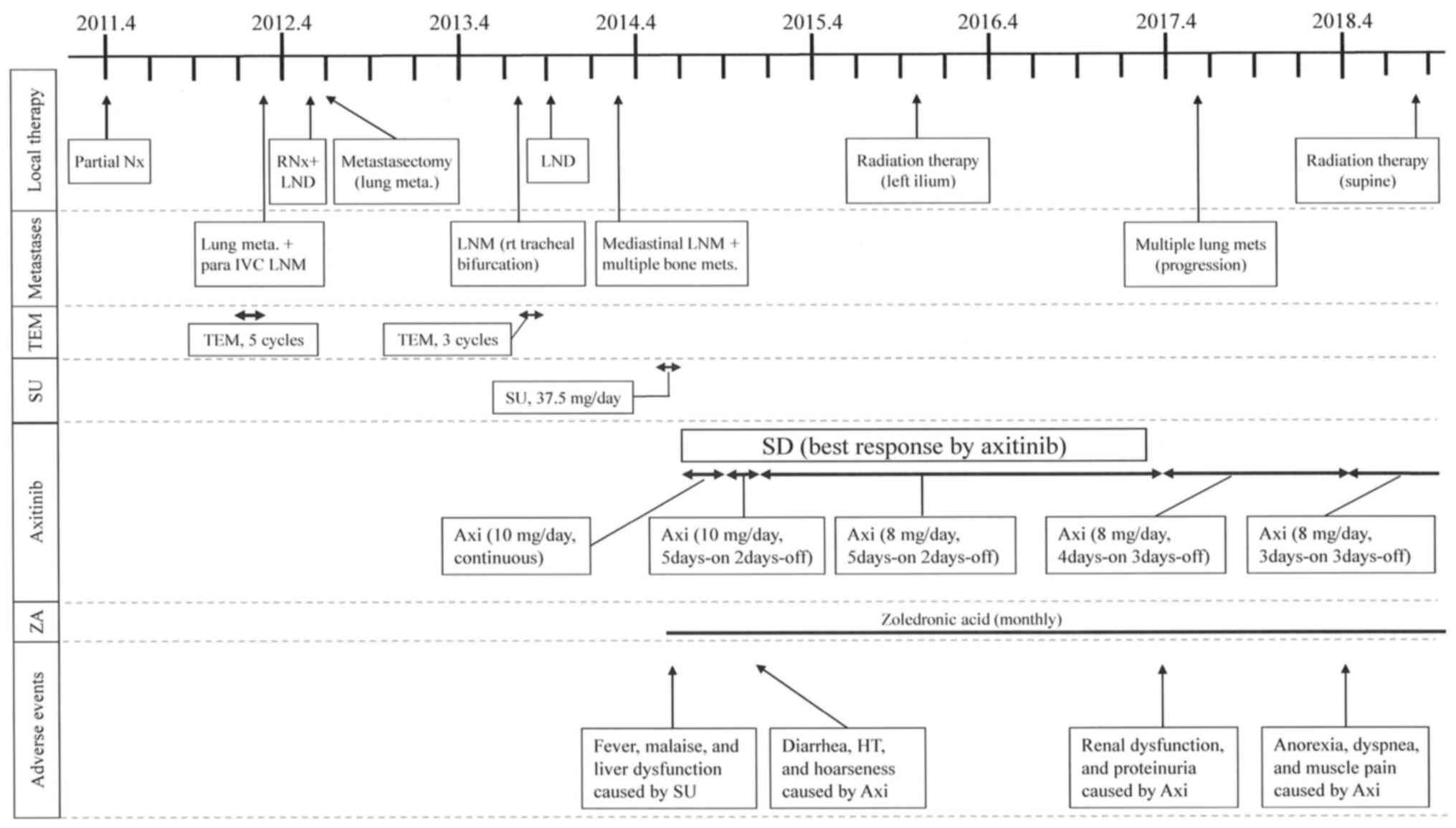 | Figure 3The time course of treatments for the
patient with metastatic type 2 papillary renal cell carcinoma. Axi,
axitinib; HT, hypertension; IVC, inferior vena cava; LND, lymph
node dissection; LNM, lymph node metastasis; RNx, radical
nephrectomy; SD, stable disease; SU, sunitinib; TEM, temsirolimus;
ZA, zoledronic acid. |
Discussion
This patient with metastatic type 2 PRCC could
survive 80 months after the initial recurrence by multidisciplinary
treatments including metastasectomies, axitinib, ZA, and radiation
therapy. Patients with metastatic type 2 PRCC generally have poor
prognosis. In a clinical trial evaluating the efficacy of sunitinib
for metastatic PRCC, the median OS in type 2 PRCC was 12.4 months
(10). Metastasectomies and
axitinib appeared to be especially effective in the present
case.
The NCCN guidelines (2020 version) indicate that
clinical trial or sunitinib is the recommended treatment for
metastatic nccRCC, and cabozantinib and everolimus are options. At
the beginning of targeted era, mammalian target of rapamycin (mTOR)
inhibitor, temsirolimus, was reportedly effective compared with
interferon-α for metastatic nccRCC (11). A comparative study between
temsirolimus and tyrosine kinase inhibitors (TKIs) in metastatic
nccRCC does not exist at present. The superiority of sunitinib
compared with everolimus in nccRCC treatment has been reported in
two clinical trials (12,13). The ASPEN trial reported that the
progression-free survival (PFS) of sunitinib was longer than that
of everolimus (12). The ESPN trial
reported that both PFS and OS of sunitinib were longer than those
of everolimus (13). Among other
TKIs, the overall response rate of pazopanib was reportedly 39% in
the treatment of metastatic nccRCC (6). There are few studies evaluating the
clinical efficacy of systemic therapies in metastatic PRCC. In
clinical trial evaluating the efficacy of sunitinib for metastatic
PRCC, the median PFS and OS were 6.6 and 17.8 months in type 1
PRCC, and 5.5 and 12.4 months in type 2, respectively (10). In a Japanese multicenter study
evaluating metastatic PRCC in which most PRCC cases (91.4%) were
type 2, the prognosis in the era of targeted therapy (OS=22.5
months) was improved compared with that in the cytokine era (OS=6.3
months). PRCC patients treated with TKIs in both first-line and
second-line treatments (OS=31.4 months) showed better prognosis
than those with mTOR inhibitors in first-line or second-line
(OS=12.9 months) (14).
In our case, the second-line use of axitinib was
effective and achieved a durable stable disease (SD). There have
been few reports in which axitinib showed efficacies for metastatic
PRCC (15,16). In our case, AEs were relieved by
dose reduction and the setting of periodic drug withdrawal
schedules of axitinib, and axitinib could be continued for a long
time. The half-life period of axitinib was reportedly short
(4.8-5.9 h) (17). Axitinib has a
characteristic that AEs can be relieved in a short period because
of its short half-life period. Then, axitinib can be started again
after the short drug withdrawal period. The risk of regrowth should
be low due to the short drug withdrawal. In our previous report of
a clear cell RCC patient with metastasis at the paranasal sinus,
AEs could be relieved effectively by using periodic drug withdrawal
schedules of axitinib and axitinib could be used for more than 30
months (18). Takayama et al
also reported a similar RCC case in which intermittent use of
axitinib was effective (19).
There is a possibility that prognosis of this
patient was improved by metastasectomies. LN metastases around the
right common iliac vein and IVC at the time of LN dissection were
pathologically confirmed. However, the relapse did not occur in the
abdominal and pelvic areas after the extensive LN dissection. This
appeared to indicate that LN metastases in the abdominal and the
pelvic areas were completely resected by the initial LN dissection.
Moreover, the three metastasectomies including the initial LN
dissection around the IVC and right iliac vein, the resection of
lung metastasis, and the mediastinal LN dissection could delay the
timing of TKI administration for two years. Those metastasectomies
might contribute to improve the patient's prognosis. In the
cytokine era, an efficacy of LN dissection on radical nephrectomy
was suggested in PRCC (20).
Metastatectomy should be performed appropriately for patients with
metastatic PRCC similar to clear cell RCC when radiological
complete remission can be achieved by metastasectomy.
Although it is difficult to evaluate the
effectiveness of ZA and local radiation therapy in this case, these
treatments might present some positive effects for disease
control.
We were able to control metastatic lesions of type 2
PRCC for a relatively long term by multidisciplinary treatment.
Axitinib was effective and periodic drug withdrawal schedules could
reduce AEs and enable to continue axitinib usage.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the present
study.
Authors' contributions
YA contributed to the study concept, design, data
collection and writing of the manuscript. YK contributed to data
collection and the revision of the manuscript. MH contributed to
data collection and the revision of the manuscript. HH contributed
to data collection and the revision of the manuscript. KM
contributed to data collection, pathological diagnosis and the
revision of the manuscript. AH contributed to the study concept,
design and the revision of the manuscript. KI contributed to the
study concept, design, data collection, writing and the revision of
the manuscript, and the supervision of the manuscript. The
authenticity of all the raw data was assessed by YA, KM, AH and KI.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was granted from the Ethics
Committee of National Defense Medical College.
Patient consent for publication
Written informed consent for the publication of any
associated data was obtained from the patient's wife.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wahlgren T, Harmenberg U, Sandström P,
Lundstam S, Kowalski J, Jakobsson M, Sandin R and Ljungberg B:
Treatment and overall survival in renal cell carcinoma: A Swedish
population-based study (2000-2008). Br J Cancer. 108:1541–1549.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tannir NM, Plimack E, Ng C, Tamboli P,
Bekele NB, Xiao L, Smith L, Lim Z, Pagliaro L, Araujo J, et al: A
phase 2 trial of sunitinib in patients with advanced non-clear cell
renal cell carcinoma. Eur Urol. 62:1013–1019. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee JL, Ahn JH, Lim HY, Park SH, Lee SH,
Kim TM, Lee DH, Cho YM, Song C, Hong JH, et al: Multicenter phase
II study of sunitinib in patients with non-clear cell renal cell
carcinoma. Ann Oncol. 23:2108–2114. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Matrana MR, Baiomy A, Campbell M, Alamri
S, Shetty A, Teegavarapu P, Kalra S, Xiao L, Atkinson B, Corn P, et
al: Outcomes of patients with metastatic non-clear-cell renal cell
carcinoma treated with pazopanib. Clin Genitourin Cancer.
15:e205–e208. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jung KS, Lee SJ, Park SH, Lee JL, Lee SH,
Lim JY, Kang JH, Lee S, Rha SY, Lee KH, et al: Pazopanib for the
treatment of non-clear cell renal cell carcinoma: A single-arm,
open-label, multicenter, phase II study. Cancer Res Treat.
50:488–494. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Park I, Lee SH and Lee JL: A multicenter
phase II trial of axitinib in patients with recurrent or metastatic
non-clear-cell renal cell carcinoma who had failed prior treatment
with temsirolimus. Clin Genitourin Cancer. 16:e997–e1002.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VE: WHO classification of tumours of the Urinary System and
Male Genital Organs. Lyon: IARCPress, 2016.
|
|
9
|
Mejean A, Hopirtean V, Bazin JP,
Larousserie F, Benoit H, Chrétien Y, Thiounn N and Dufour B:
Prognostic factors for the survival of patients with papillary
renal cell carcinoma: Meaning of histological typing and
multifocality. J Urol. 170:764–767. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ravaud A, Oudard S, De Fromont M, Chevreau
C, Gravis G, Zanetta S, Theodore C, Jimenez M, Sevin E, Laguerre B,
et al: First-line treatment with sunitinib for type 1 and type 2
locally advanced or metastatic papillary renal cell carcinoma: A
phase II study (SUPAP) by the French Genitourinary Group (GETUG)†.
Ann Oncol. 26:1123–1128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dutcher JP, de Souza P, McDermott D,
Figlin RA, Berkenblit A, Thiele A, Krygowski M, Strahs A, Feingold
J and Hudes G: Effect of temsirolimus versus interferon-α on
outcome of patients with advanced renal cell carcinoma of different
tumor histologies. Med Oncol. 26:202–209. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Armstrong AJ, Halabi S, Eisen T, Broderick
S, Stadler WM, Jones RJ, Garcia JA, Vaishampayan UN, Picus J,
Hawkins RE, et al: Everolimus versus sunitinib for patients with
metastatic non-clear cell renal cell carcinoma (ASPEN): A
multicentre, open-label, randomised phase 2 trial. Lancet Oncol.
17:378–388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tannir NM, Jonasch E, Albiges L,
Altinmakas E, Ng CS, Matin SF, Wang X, Qiao W, Dubauskas Lim Z,
Tamboli P, et al: Everolimus versus sunitinib prospective
evaluation in metastatic non-clear cell renal cell carcinoma
(ESPN): A randomized multicenter phase 2 trial. Eur Urol.
69:866–874. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ito K, Mikami S, Tatsugami K, Masumori N,
Shinohara N, Kondo T, Nakanishi S, Nagashima Y, Eto M, Kamba T, et
al: Clinical outcomes in patients with metastatic papillary
renal-cell carcinoma: A multi-institutional study in Japan. Clin
Genitourin Cancer. 16:e1201–e1214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mori J, Nakayama Y, Hiragino T and
Matsuyama H: Successful treatment of peritoneal dissemination
recurrence with axitinib in papillary renal cell carcinoma: A case
report. Hinyokika Kiyo. 64:45–48. 2018.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
16
|
Ishii G, Hatano T, Endo K, Seki K, Yamada
H, Kimura T and Egawa S: A case of papillary renal cell carcinoma
type 2 resistant to sunitinib responded to second line therapy with
axitinib. Nihon Hinyokika Gakkai Zasshi. 105:129–133.
2014.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
17
|
Mukohara T, Nakajima H, Mukai H, Nagai S,
Itoh K, Umeyama Y, Hashimot J and Minami H: Effect of axitinib
(AG-013736) on fatigue, thyroid-stimulating hormone, and
biomarkers: A phase I study in Japanese patients. Cancer Sci.
101:963–968. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Arai Y, Ito K, Tachi K, Koga A, Shinchi Y,
Masunaga A, Isono M and Asano T: Metastatic renal cell carcinoma in
paranasal sinus for which periodic drug withdrawal schedule of
axitinib was effective: A case report. Hinyokika Kiyo. 62:465–471.
2016.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
19
|
Takayama T, Nagata M, Kai F, Sugiyama T
and Ozono S: Axitinib controlled metastatic renal cell carcinoma
for 5 years. Jpn J Clin Oncol. 43:747–751. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Margulis V, Tamboli P, Matin SF, Swanson
DA and Wood CG: Analysis of clinicopathologic predictors of
oncologic outcome provides insight into the natural history of
surgically managed papillary renal cell carcinoma. Cancer.
112:1480–1488. 2008.PubMed/NCBI View Article : Google Scholar
|