Introduction
Definitive concurrent chemoradiotherapy (CCRT) is
one of the standard treatments for locally advanced non-small cell
lung cancer (NSCLC). Recently, a prospective trial of definitive
CCRT followed by durvalumab therapy showed that prognosis was
better in patients who received durvalumab than in those who
received a placebo (1,2). However, there did not appear to be a
significant increase in the local control rates. More intensive
treatments, including surgical resection, have also been attempted.
Toyooka et al (3) showed
that overall survival (OS) in the preoperative CCRT group was
significantly better than that in the preoperative chemotherapy
alone group. In a subset analysis of a phase III trial on patients
with stage III NSCLC, OS in the preoperative CCRT and lobectomy
group was found to be higher than that in the definitive CCRT group
(4). Since not all patients benefit
equally from preoperative CCRT and surgery, the attending physician
must reasonably select the treatment options in the context of
delivering personalized medicine. Moreover, research on biomarkers
that can assist in selecting a treatment regimen is required.
Historically, positron emission tomography/computed
tomography (PET/CT) has been utilized for the staging of NSCLC,
measuring response after treatment, and predicting the prognosis
after radiotherapy (5) and surgery
(6). The most well-known PET/CT
parameter for the quantification of
18F-fluorodeoxyglucose (18F-FDG) metabolism
is the maximum standardized uptake value (SUVmax), which
is the maximum voxel value in the tumor (5-7).
However, SUVmax does not indicate the overall tumor
metabolic activity and is sensitive to image noise (8). Volumetric PET parameters like total
lesion glycolysis (TLG) and metabolic tumor volume (MTV) have been
extensively evaluated and are considered more comprehensive
parameters than SUVmax for assessing NSCLC patients
(9-15).
MTV was defined as the total tumor volume above threshold, while
the TLG values were calculated by multiplying the target lesion
mean SUV by the MTV. However, only one group has examined
volumetric PET parameters as prognostic factors for preoperative
CCRT and surgery (16,17).
The aim of this study was to explore whether MTV and
TLG calculated using PET/CT are predictors of the prognosis of
NSCLC patients who have undergone preoperative CCRT and
surgery.
Materials and methods
Patients
Our hospital's review board approved this study. The
medical records of NSCLC patients who underwent preoperative CCRT
and surgery between April 2006 and March 2018 at our hospital were
retrospectively reviewed. We used the 7th edition of the TNM
Classification of Malignant Tumors for staging. Patients were
included if they had undergone pre- and post-CCRT PET/CT
examination and preoperative concurrent chemotherapy and
radiotherapy (40-60 Gy/20-30 fractions), but no other treatment
before the start of CCRT. Patients with apparent accumulation of
radiation pneumonitis during post-CCRT PET/CT were excluded. All
procedures were performed in compliance with the ethical standards
of the 1964 Declaration of Helsinki and subsequent modifications.
Before commencement of preoperative CCRT, we obtained written
informed consent for treatment. Opportunities to opt out of the
study were provided through notices displayed in the outpatient
wards and on the institution's website.
Treatment
Indications for preoperative CCRT in all cases were
discussed at the respiratory conference attended by thoracic
surgeons, respiratory physicians and radiation oncologists and
finally decided upon by board-certified thoracic surgeons. All
patients received three-dimensional conformal radiotherapy using a
linear accelerator (Primus, ONCOR or Mevatron, Canon Medical
Systems, Tochigi, Japan). Details of radiotherapy, including
targets and margins, have been previously reported (18,19).
Chemotherapy regimens consisted of cisplatin/docetaxel based on a
previous prospective study (20),
tegafur/gimeracil/oteracil (21),
and carboplatin/paclitaxel. Surgery was usually scheduled 4-6 weeks
after completion of CCRT. However, it is sometimes delayed to treat
the adverse effects of CCRT and to improve the patient's general
condition. Therefore, PET/CT as a preoperative examination may also
be delayed.
PET/CT
PET/CT scanning was performed using a Biograph 16
PET/CT scanner (Siemens Healthcare). All patients refrained from
eating and drinking for at least 6 h before the scan.
18F-FDG (3.7 MBq/kg) was administered intravenously, and
PET/CT scanning was performed 90 min later. We used the syngo.via
software (Siemens Healthcare) for the measurement of
SUVmax, MTV, and TLG. The volume of interest was
manually positioned over the primary tumor and metastatic lymph
node on the PET/CT image, and the contour of the target lesion
(primary tumor and metastatic lymph node) inside the volume of
interest was automatically delineated using the isocontour
threshold method. A SUVmax threshold of 2.5 was used to
define the MTV. In a study by Im et al (8), fixed absolute thresholds were found to
be suitable for the evaluation of the prognostic value of MTV;
another study reported that the cutoff fixed SUV value was 2.5 in
seven of the 13 studies analyzed (22). Therefore, MTV2.5 and TLG2.5 were
among the volumetric PET/CT parameters measured in this study. The
success of the target lesion segmentation was visually assessed by
a board-certificated radiologist blinded to the patients'
prognoses. SUVmax, MTV, and TLG values for the
delineated target lesion were automatically calculated. MTV was
defined as total tumor volume with 18F-FDG uptake value
above the threshold. TLG values were calculated by multiplying the
target lesion mean SUV by the MTV. In addition, we calculated the
post-CCRT percentage decrease (Δ) in each parameter value.
Statistical analysis
Early complications within 30 days of surgery were
determined by a thoracic surgeon. We used the Kaplan-Meier method
to obtain the survival curves. Variables were classified into two
categories for the statistical analysis, and the medians were
adopted as the cutoff values for the continuous PET parameters
variables. The relationships between the factors and survival rates
were then analyzed. The log-rank test was used for univariate
analysis. A factor of two-sided P<0.05 was determined to be
statistically significant. The R software (version 3.5.1, R
Foundation for Statistical Computing) was used for all
analyses.
Results
Patient characteristics
Fifty-two patients were included in this study
(Table I). The median follow-up
period after CCRT was 50.65 (range, 6.97-139.23) months. The total
preoperative radiotherapy dose was 40 Gy/20 fractions in two
patients, 46 Gy/23 fractions in 47 patients, 48 Gy/24 fractions in
one patient, and 60 Gy/30 fractions in two patients.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Value | Percentage |
|---|
| Age (years), median
(range) | 63 (35-80) | - |
| T stage, n | | |
|
1 | 7 | 11 |
|
2 | 13 | 25 |
|
3 | 18 | 35 |
|
4 | 14 | 27 |
| N stage, n | | |
|
0 | 12 | 23 |
|
1 | 5 | 10 |
|
2 | 30 | 57 |
|
3 | 5 | 10 |
| Clinical stage,
n | | |
|
II A | 1 | 2 |
|
II B | 7 | 13 |
|
III A | 30 | 58 |
|
III B | 14 | 27 |
| Histology, n | | |
|
Adenocarcinoma | 26 | 50 |
|
Squamous
cell carcinoma | 17 | 33 |
|
Non-small
cell carcinoma | 7 | 13 |
|
Undifferentiated
carcinoma | 1 | 2 |
|
Class V
(cytology only) | 1 | 2 |
| Smoking history,
n | | |
|
Never | 8 | 15 |
|
Former | 13 | 25 |
|
Current | 31 | 60 |
|
ECOG-PSa,
n | | |
|
0 | 29 | 56 |
|
1 | 20 | 38 |
|
2 | 2 | 4 |
| Lobe, n | | |
|
Upper | 42 | 81 |
|
Middle | 1 | 2 |
|
Lower | 9 | 17 |
|
Lateralitya, n | | |
|
Right | 26 | 50 |
|
Left | 26 | 50 |
| FEV1
(l)a, median
(range) | 2.38
(1.38-4.17) | - |
| %VC
(%)a, median
(range) | 99.2
(64.8-150.8) | - |
| Chemotherapy,
n | | |
|
Cisplatin +
docetaxel | 47 | 90 |
|
Carboplatin
+ paclitaxel | 2 | 4 |
|
Tegafur/gimeracil/oteracil | 3 | 6 |
| Radiation dose
(Gy), median (range) | 46 (40-60) | - |
| Surgery, n | | |
|
Wedge
resection | 1 | 2 |
|
Lobectomy | 44 | 84 |
|
Bilobectomy | 4 | 8 |
|
Pneumonectomy | 3 | 6 |
| SUVmax,
median (range) | 15.37
(4.93-32.17) | - |
| MTV2.5
(cm3), median (range) | 56.84
(2.64-305.16) | - |
| TLG2.5, median
(range) | 312.03
(9.53-2251.7) | - |
| ΔSUVmax
(%), median (range) | 7 5.72
(10.70-100.00) | - |
| ΔMTV2.5 (%), median
(range) | 97.36
(23.35-100.00) | - |
| ΔTLG2.5 (%), median
(range) | 98.56
(25.20-100.00) | - |
The median pre-CCRT pre-SUVmax, pre-MTV,
and pre-TLG values were 15.37 (range, 4.93-32.17), 56.84
cm3 (range, 2.64-305.16 cm3), and 312.03
(range, 9.53-2251.7), respectively. The median ΔSUVmax,
ΔMTV, and ΔTLG were 75.72% (range, 10.70-100.00%), 97.36% (range,
23.35-100.00%), and 98.56% (range, 25.20-100.00%),
respectively.
PET parameters and prognosis
Table II shows the
factors associated with PFS. In univariate analysis, T-stage,
forced expiratory volume in 1 sec, and ΔTLG were found to be
significant predictors of PFS (P=0.02, 0.04, and 0.03,
respectively). Pre-SUVmax, MTV, TLG, ΔSUVmax,
and ΔMTV were not predictors of PFS (P=0.4, 0.2, 0.1, 0.09, and
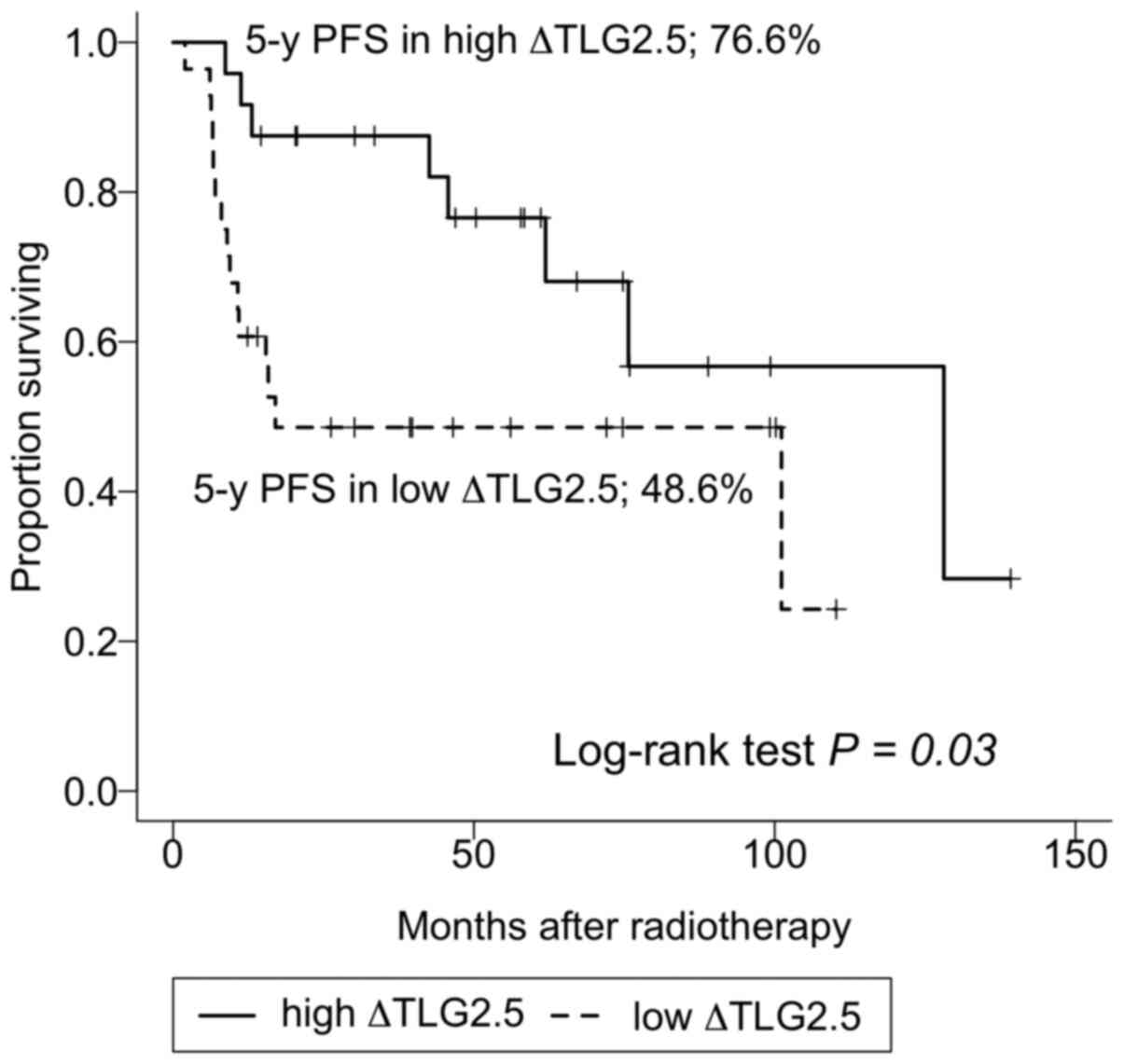
0.09, respectively). Fig. 1 shows
the PFS Kaplan-Meier curve for the high and low ΔTLG groups. In the
low ΔTLG group, the 3, 5 and 10-year PFS rates were 48.6% [95%
confidence interval (CI), 32.9-71.8%], 48.6% (95% CI, 32.9-71.8%),
and not-reached, respectively. In the high ΔTLG group, the
corresponding rates were 87.5% (95% CI, 75.2-100%), 76.6% (95% CI,
66.3-97.2%), and 56.7% (95% CI, 34.8-92.4%), respectively.
 | Table IIUnivariate analysis of factors
associated with progression-free survival. |
Table II
Univariate analysis of factors
associated with progression-free survival.
| Factor | Event/total, n | P-value |
|---|
| Age, years | | 0.20 |
|
<63 | 10/26 | |
|
≥63 | 13/26 | |
| T stage | | 0.02 |
|
1-2 | 13/20 | |
|
3-4 | 10/32 | |
| N stage | | 0.70 |
|
0-1 | 7/17 | |
|
2-3 | 16/35 | |
| Clinical stage | | 0.40 |
|
II | 2/8 | |
|
III | 21/44 | |
| Histology | | 0.10 |
|
Adenocarcinoma | 9/26 | |
|
Others | 14/26 | |
| Smoking
history | | 0.80 |
|
Never/former | 9/21 | |
|
Current | 14/31 | |
|
ECOG-PSa | | 0.70 |
|
0 | 13/22 | |
|
1-2 | 9/29 | |
| Laterality | | 0.30 |
|
Right | 10/26 | |
|
Left | 13/26 | |
| FEV1
(l)a | | 0.04 |
|
<2.4 | 15/24 | |
|
≥2.4 | 8/23 | |
| Chemotherapy | | 0.10 |
|
Cisplatin/Docetaxel | 20/47 | |
|
Others | 3/5 | |
| Radiation dose,
Gy | | 0.90 |
|
<60 | 22/50 | |
|
60 | 1/2 | |
| Surgery | | 0.10 |
|
Pneumonectomy | 2/3 | |
|
Others | 21/49 | |
|
SUVmax | | 0.40 |
|
<15 | 13/26 | |
|
≥15 | 10/26 | |
| MTV2.5,
cm3 | | 0.20 |
|
<57 | 14/25 | |
|
≥57 | 9/27 | |
| TLG2.5 | | 0.10 |
|
<310 | 12/26 | |
|
≥310 | 9/26 | |
| ΔSUVmax,
% | | 0.09 |
|
<76 | 13/26 | |
|
≥76 | 10/26 | |
| ΔMTV2.5, % | | 0.09 |
|
<97 | 13/25 | |
|
≥97 | 10/27 | |
| ΔTLG2.5, % | | 0.03 |
|
<99 | 15/28 | |
|
≥99 | 8/24 | |
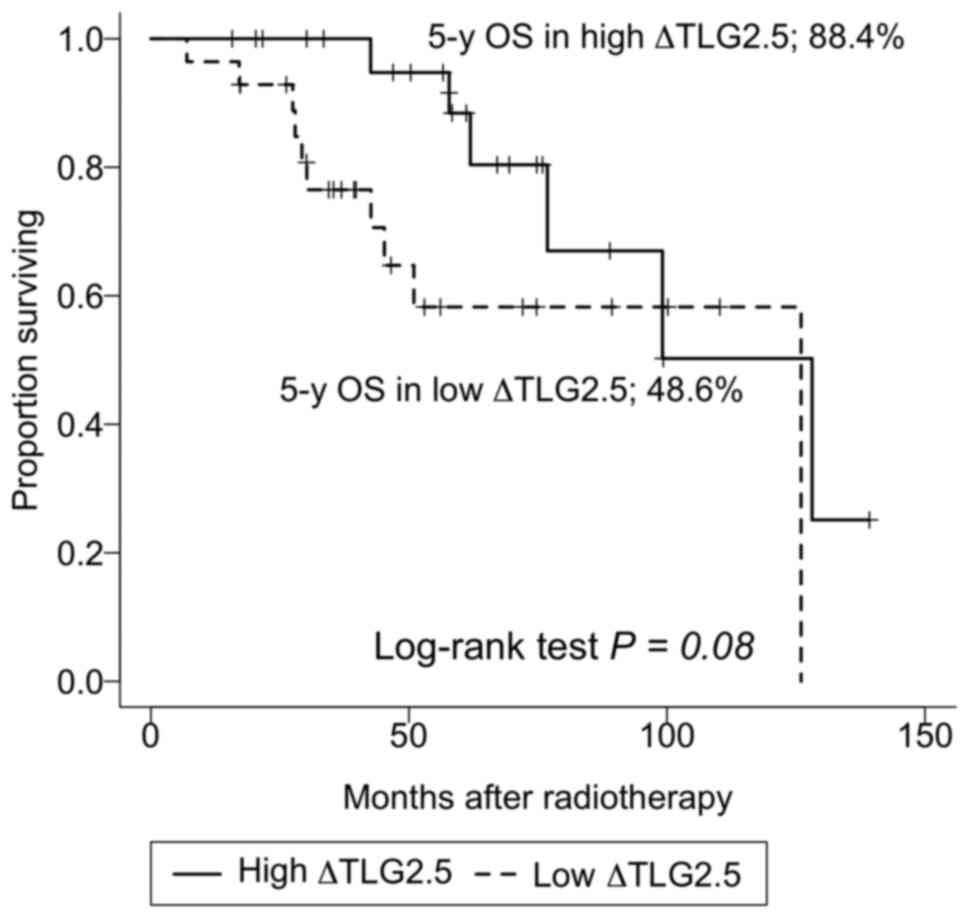
Table III shows
the factors associated with OS. In univariate analysis,
SUVmax, MTV, TLG, ΔSUVmax, and ΔMTV were not
found to be associated with OS. High ΔTLG was indicative of a
higher OS rate (P=0.08). In the low ΔTLG group, the 3, 5, and
10-year OS rates were 76.5% (95% CI, 61.6-95.0%), 58.3% (95% CI,
39.9-85.1%), and 58.3% (95% CI, 39.9-85.1%), respectively. In the
high ΔTLG group, the corresponding rates were 100% (not
applicable), 88.4% (95% CI, 74.5-100%), and 50.2% (95% CI,
24.6-100%), respectively (Fig. 2).
Pneumonectomy was found to be associated with a low OS rate
(P=0.03).
 | Table IIIUnivariate analysis of factors
associated with overall survival. |
Table III
Univariate analysis of factors
associated with overall survival.
| Factor | Event/total, n | P-value |
|---|
| Age, years | | 0.30 |
|
<63 | 8/26 | |
|
≥63 | 8/26 | |
| T stage | | 0.06 |
|
1-2 | 10/20 | |
|
3-4 | 6/32 | |
| N stage | | 0.90 |
|
0-1 | 5/17 | |
|
2-3 | 11/35 | |
| Clinical stage | | 0.70 |
|
II | 1/8 | |
|
III | 15/44 | |
| Histology | | 0.40 |
|
Adenocarcinoma | 9/26 | |
|
Others | 7/26 | |
| Smoking
history | | >0.90 |
|
Never/former | 6/21 | |
|
Current | 10/31 | |
|
ECOG-PSa | | 0.70 |
|
0 | 10/29 | |
|
1-2 | 5/22 | |
| Laterality | | 0.50 |
|
Right | 7/26 | |
|
Left | 9/26 | |
| FEV1
(l)a | | 0.20 |
|
<2.4 | 10/24 | |
|
≥2.4 | 6/23 | |
| Chemotherapy | | 0.08 |
|
Cisplatin/docetaxel | 14/47 | |
|
Others | 2/5 | |
| Radiation dose,
Gy | | 0.50 |
|
<60 | 15/50 | |
|
60 | 1/2 | |
| Surgery | | 0.03 |
|
Pneumonectomy | 2/3 | |
|
Others | 14/49 | |
|
SUVmax | | 0.90 |
|
<15 | 8/26 | |
|
≥15 | 8/26 | |
| MTV2.5,
cm3 | | 0.50 |
|
<57 | 9/25 | |
|
≥57 | 7/27 | |
| TLG2.5 | | 0.50 |
|
<310 | 9/26 | |
|
≥310 | 7/26 | |
| ΔSUVmax,
% | | 0.10 |
|
<76 | 9/26 | |
|
≥76 | 7/26 | |
| ΔMTV2.5, % | | 0.10 |
|
<97 | 11/25 | |
|
≥97 | 6/27 | |
| ΔTLG2.5, % | | 0.08 |
|
<99 | 10/28 | |
|
≥99 | 6/24 | |
Other prognostic factors and
complications
The median time from the last day of CCRT to
post-CCRT PET/CT was 3.57 weeks (range, 1.71-9.00 weeks). The
5-year survival rates for the shorter time group below the median
and longer time group above the median were 68.8% (95% CI,
50.5-93.7%) and 76.3% (95% CI, 59.7-97.6%), respectively, which
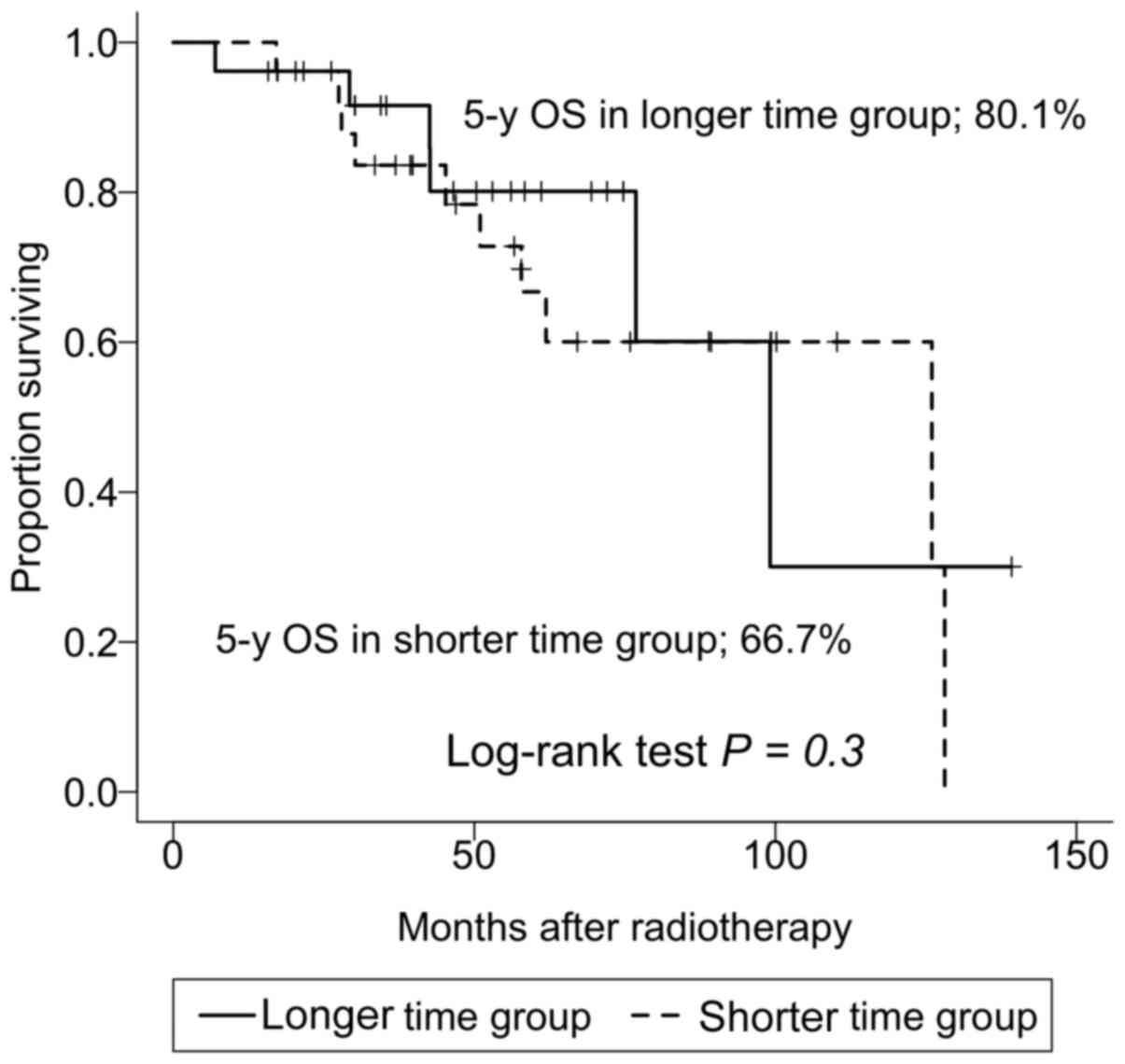
were not significantly different (P=0.5). The median time from the
last day of CCRT to surgery was 5.71 weeks (range, 3.14-12.86
weeks). The 5-year survival rates for shorter time group below the
median and longer time group above the median were 66.7% (95% CI,
48.9-91.1%) and 80.1% (95% CI, 64.2-100%), respectively, which were
not significantly different (P=0.3) (Fig. 3). There were 33 patients with early
postoperative complications within 30 days of surgery; the 5-year
survival rates for the groups with and without early complications
were 69.9% (95% CI, 54.1-90.3%) and 78.1% (95% CI, 58.3-100%),
respectively, which were not significantly different (P=0.8). The
33 patients had the following complications: Arrhythmia, 6
patients; radiation pneumonitis, 4 patients; recurrent nerve palsy,
3 patients; bacterial pneumonia, 3 patients; pleural effusion, 2
patients; chylothorax, 2 patients; pyothorax plus enteritis,
prolonged pulmonary fistula, functional pyloric ring stenosis,
chylothorax plus bacterial pneumonia plus arrhythmia, arrhythmia
plus sepsis from pneumonia, atelectasis, chylothorax plus pulmonary
artery embolus plus venous thrombosis, wound infection, recurrent
laryngeal nerve palsy plus arrhythmia plus venous thrombosis, upper
limb paresis plus recurrent laryngeal nerve paralysis, radiation
pneumonitis plus recurrent laryngeal nerve palsy, venous
thrombosis, and upper gastrointestinal bleeding, 1 patient.
Postoperative death due to complications was not observed in any of
the 33 patients here assessed.
Clinical course
Of the 52 patients included, there were 29 survivors
without recurrence, seven survivors with recurrence, 11 deaths due
to NSCLC, and five deaths due to conditions other than NSCLC. In
the low ΔTLG group, there were 13 survivors without recurrence,
five survivors with recurrence, eight deaths due to NSCLC, and two
deaths due to conditions other than NSCLC. In the high ΔTLG group,
there were 16 survivors without recurrence, two survivors with
recurrence, three deaths due to NSCLC, and three deaths due to
conditions other than NSCLC.
Discussion
In our study, ΔTLG was found to be a prognostic
factor for PFS. Previous investigators have reported that
SUVmax is a predictor of PFS and OS for NSCLC patients
receiving surgery. Liu et al (6) performed a meta-analysis that evaluated
the correlation between PET parameters and prognosis of stage I-IV
NSCLC patients who had undergone surgery. In this study, most of
the evaluated PET parameters were SUVmax values. In a
systematic review by Nair et al (7), high FDG uptake was found to be related
to poor prognosis in patients who underwent surgical treatment for
stage I NSCLC; SUVmax was measured in six of the nine
articles included in the analysis. Another meta-analysis examining
the significance of pretreatment SUVmax in NSCLC
patients who had undergone definitive radiotherapy showed that
pre-radiotherapy tumor SUVmax can predict outcomes
(5). The combined hazard ratio of
pre-radiotherapy SUVmax was 1.05 (95% CI, 1.02-1.08) for
OS and 1.26 (95% CI, 1.05-1.52) for local control. However, other
investigators have shown that volumetric PET parameters are more
predictive of patient survival than SUVmax in surgical
cases of early stage NSCLC (9,15).
Recent reports on early stage (stages I-II) NSCLC have also
documented the utility of MTV and TLG (10-11). In a study
conducted by Shrestha et al (10), MTV was found to be a predictive
factor of PFS in stage I NSCLC patients treated with carbon ion
particle therapy. A study of PET parameters and prognosis in
stereotactic radiotherapy for early stage NSCLC patients showed
that only volumetric parameters were significant predictors of PFS
for patients with large primary tumors (11). Volumetric parameters have also been
examined in locally advanced cases. Grootjans et al
(12) demonstrated that, for 27
stage III NSCLC patients who underwent definitive
chemoradiotherapy, pretreatment TLG was a significant predictor of
PFS and OS. In a prospective study, pretreatment SUVmax
was not found to be a predictor for locally advanced NSCLC treated
using definitive CCRT (13). Using
the same clinical trial dataset, Salavati et al (14) showed that the areas under the curves
for volumetric parameters were higher than those for
SUVmax, and the prognoses of the high MTV and TLG groups
were significantly worse than those of the low MTV and TLG groups.
Hyun et al (16)
demonstrated that MTV and TLG were significant predictive factors
of prognosis in a multivariate analysis of operative stage III
cases. At later stages, volumetric PET parameters are considered
more important than SUVmax as prognostic factors. Our
results also show that TLG calculated using PET/CT was a useful
predictor of prognosis and are consistent with these previous
reports.
In our study, neither SUVmax nor
ΔSUVmax was a significant predictor of PFS or OS. The
FDG metabolic imaging method evaluates tumor biology by measuring
tumor SUVs, and the major method of SUV measurement based on
SUVmax. Since this only reflects a single voxel value
indicating the highest uptake of FDG in the tumor,
SUVmax does not necessarily represent the overall burden
or metabolic activity of a tumor (8,23).
Further, SUV is influenced by various factors, including length of
the uptake period, body composition, recovery coefficient, plasma
glucose level, image noise, and partial volume effects (24,25).
As MTV and TLG consider not only single voxel information but also
highly active three-dimensional tumor volume and metabolic
activity, clinicians can use them to obtain more detailed tumor
information.
Pre-CCRT PET parameters were not found to be
prognostic factors in our study. In a study on 161 patients who
underwent preoperative CCRT, Hyun et al (17) showed that pre-CCRT MTV was a
predictor of prognosis. The chemotherapy regimen in their study
consisted of paclitaxel or docetaxel plus either cisplatin or
carboplatin, although the proportions were not specified. In our
study, cisplatin/docetaxel was used for most patients, and the
differences between our results and theirs may be due to the
differences in the size of the patient cohort, institutional
treatment strategies, and technical factors.
The correlations between the percentage decrease in
volumetric PET parameter values and prognosis of locally advanced
NSCLC treated using definitive CCRT has been investigated
previously. In the study by Huang et al (23), only ΔMTV was found to be a predictor
of OS according to a Cox regression analysis of NSCLC patients
treated with definitive CCRT. Grootjans et al (12) demonstrated that ΔTLG was
significantly associated with prognosis. To the best of our
knowledge, no studies have examined the significance of the
percentage decrease in volumetric PET parameter values after
preoperative CCRT and surgery, and further studies in this context
are warranted. In our study, high ΔTLG was indicative of a high OS
rate, but the correlation was not significant (P=0.08). There were
five living patients with recurrence in the low ΔTLG groups and two
in the high ΔTLG group. In addition to the small number of cases,
the difference in the number of living patients with recurrence is
likely to be one of the reasons why, despite the difference in PFS,
there was no difference in OS between the low and high ΔTLG groups.
The greatest advantage of utilizing this parameter as a prognostic
factor is that, in addition to measuring the size of the tumor (as
done by CT), measuring the rate of decrease in activity of the
entire tumor volume will be possible. However, limitations exist,
including i) the unavailability of a decrease rate index due to the
lack of evidence other than ours in patients undergoing
preoperative CCRT; ii) the additional costs associated with PET
imaging; and iii) the exposure to radiation, which does not concern
CT instead.
Volumetric PET parameters may accurately reflect
prognosis in NSCLC patients undergoing preoperative CCRT and
surgery. The prognosis of patients receiving definitive CCRT is
significantly improved by the addition of durvalumab after
definitive CCRT (1,2). In the future, volumetric evaluation
using PET/CT in the clinical setting may enable personalized
treatment strategies tailored to each patient's prognostic risk.
Patients who are expected to have a high likelihood of recurrence
after preoperative CCRT, with additional evidence based on
predictive biomarkers such as ΔTLG, may be recommended to receive
definitive CCRT and an immune checkpoint inhibitor. With regard to
patient follow-up after treatment, a grasp of patient clinical
information using online resources is known to improve the
prognosis of patients with lung cancer (26). Another potential significance of
volumetric PET parameter evaluation could be that rigorous
post-treatment follow-up of patients with low ΔTLG could lead to
the rapid induction of treatment after NSCLC recurrence.
Nonetheless, our study has some limitations. First,
this study was retrospective in nature and involved a single
center; therefore, there may have been some undetectable biases.
Second, the PET/CT scans were performed 90 min after injection of
FDG, while it is generally recommended that imaging be performed 60
min after injection (27). Third,
this study included only a small number of patients. Further
multi-institutional and prospective studies are necessary to
confirm the prognostic value of ΔTLG.
In conclusion, we demonstrated that ΔTLG was a
predictor of prognosis in NSCLC patients treated using preoperative
CCRT and surgery. Our results suggest that ΔTLG calculated using
PET/CT could help physicians determine treatment strategies for
locally advanced NSCLC.
Acknowledgements
The authors would like to thank Dr Shimpei Tsudaka
and Dr Hiromasa Yamamoto (Department of Thoracic Surgery, Okayama
University Hospital, Okayama, Japan) for data collection.
Funding
The present study was supported by a donation from Tsuyama Chuo
Hospital. The study sponsor was not involved in any procedure in
the present study.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KKa contributed to the design of the study,
collected the data and drafted the manuscript. TO contributed to
the design of the study and performed statistical analysis. AT and
KW contributed to the design of the study and collected the data.
The raw data have been assessed by KKa and TO. KY, MK, KKi, TH, ST
and SK contributed to the design of the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Okayama University Graduate School of Medicine,
Dentistry and Pharmaceutical Sciences and Okayama University
Hospital, Ethics Committee (Okayama, Japan) approved the present
study (approval no. 1809-018). Written informed consent was
obtained prior to treatment. The choice to opt-out was provided
through notifications displayed on the hospital's website and in
the outpatient ward before the start of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Toyooka S, Kiura K, Takemoto M, Oto T,
Takigawa N, Fujiwara T, Miyoshi S and Date H: Long-term outcome of
induction chemoradiotherapy with docetaxel and cisplatin followed
by surgery for non-small-cell lung cancer with mediastinal lymph
node metastasis. Interact Cardiovasc Thorac Surg. 14:565–569.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Albain KS, Swann RS, Rusch VW, Turrisi AT
III, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara
DR, et al: Radiotherapy plus chemotherapy with or without surgical
resection for stage III non-small-cell lung cancer: A phase III
randomised controlled trial. Lancet. 374:379–386. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Na F, Wang J, Li C, Deng L, Xue J and Lu
Y: Primary tumor standardized uptake value measured on
F18-Fluorodeoxyglucose positron emission tomography is of
prediction value for survival and local control in non-small-cell
lung cancer receiving radiotherapy: Meta-analysis. J Thorac Oncol.
9:834–842. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu J, Dong M, Sun X, Li W, Xing L and Yu
J: Prognostic value of 18F-FDG PET/CT in surgical
non-small cell lung cancer: A meta-analysis. PLoS One.
11(e0146195)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nair VS, Krupitskaya Y and Gould MK:
Positron emission tomography 18F-fluorodeoxyglucose
uptake and prognosis in patients with surgically treated, stage I
non-small cell lung cancer: A systematic review. J Thorac Oncol.
4:1473–1479. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Im HJ, Bradshaw T, Solaiyappan M and Cho
SY: Current methods to define metabolic tumor volume in positron
emission tomography: Which one is better? Nucl Med Mol Imaging.
52:5–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park SY, Cho A, Yu WS, Lee CY, Lee JG, Kim
DJ and Chung KY: Prognostic value of total lesion glycolysis by
18F-FDG PET/CT in surgically resected stage IA non-small
cell lung cancer. J Nucl Med. 56:45–49. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shrestha S, Higuchi T, Shirai K, Tokue A,
Shrestha S, Saitoh JI, Hirasawa H, Ohno T, Nakano T and Tsushima Y:
Prognostic significance of semi-quantitative FDG-PET parameters in
stage I non-small cell lung cancer treated with carbon-ion
radiotherapy. Eur J Nucl Med Mol Imaging. 47:1220–1227.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Satoh Y, Onishi H, Nambu A and Araki T:
Volume-based parameters measured by using FDG PET/CT in patients
with stage I NSCLC treated with stereotactic body radiation
therapy: Prognostic value. Radiology. 270:275–281. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grootjans W, Usmanij EA, Oyen WJ, van der
Heijden EH, Visser EP, Visvikis D, Hatt M, Bussink J and de
Geus-Oei LF: Performance of automatic image segmentation algorithms
for calculating total lesion glycolysis for early response
monitoring in non-small cell lung cancer patients during
concomitant chemoradiotherapy. Radiother Oncol. 119:473–479.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Machtay M, Duan F, Siegel BA, Snyder BS,
Gorelick JJ, Reddin JS, Munden R, Johnson DW, Wilf LH, DeNittis A,
et al: Prediction of survival by [18F]fluorodeoxyglucose
positron emission tomography in patients with locally advanced
non-small-cell lung cancer undergoing definitive chemoradiation
therapy: Results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol.
20:3823–3830. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Salavati A, Duan F, Snyder BS, Wei B,
Houshmand S, Khiewvan B, Opanowski A, Simone CB II, Siegel BA,
Machtay M and Alavi A: Optimal FDG PET/CT volumetric parameters for
risk stratification in patients with locally advanced non-small
cell lung cancer: Results from the ACRIN 6668/RTOG 0235 trial. Eur
J Nucl Med Mol Imaging. 44:1969–1983. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hyun SH, Choi JY, Kim K, Kim J, Shim YM,
Um SW, Kim H, Lee KH and Kim BT: Volume-based parameters of
(18)F-fluorodeoxyglucose positron emission tomography/computed
tomography improve outcome prediction in early-stage non-small cell
lung cancer after surgical resection. Ann Surg. 257:364–370.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hyun SH, Ahn HK, Kim H, Ahn MJ, Park K,
Ahn YC, Kim J, Shim YM and Choi JY: Volume-based assessment by
(18)F-FDG PET/CT predicts survival in patients with stage III
non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 41:50–58.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hyun SH, Ahn HK, Ahn MJ, Ahn YC, Kim J,
Shim YM and Choi JY: Volume-based assessment with
18F-FDG PET/CT improves outcome prediction for patients
with stage IIIa-N2 non-small cell lung cancer. AJR Am J Roentgenol.
205:623–628. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ogata T, Katsui K, Yoshio K, Ihara H,
Katayama N, Soh J, Kuroda M, Kiura K, Maeda Y, Toyooka S and
Kanazawa S: Dose-volume parameters predict radiation pneumonitis
after surgery with induction concurrent chemoradiotherapy for
non-small cell lung cancer. Acta Med Okayama. 72:507–513.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Katsui K, Ogata T, Watanabe K, Katayama N,
Kuroda M, Kiura K, Hiraki T, Maeda Y, Toyooka S and Kanazawa S:
Radiation pneumonitis after definitive concurrent chemoradiotherapy
with cisplatin/docetaxel for non-small cell lung cancer: Analysis
of dose-volume parameters. Cancer Med. 9:4540–4549. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Segawa Y, Kiura K, Takigawa N, Kamei H,
Harita S, Hiraki S, Watanabe Y, Sugimoto K, Shibayama T, Yonei T,
et al: Phase III trial comparing docetaxel and cisplatin
combination chemotherapy with mitomycin, vindesine, and cisplatin
combination chemotherapy with concurrent thoracic radiotherapy in
locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin
Oncol. 28:3299–3306. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takigawa N, Kiura K, Hotta K, Hosokawa S,
Nogami N, Aoe K, Gemba K, Fujiwara K, Harita S, Takemoto M, et al:
A phase I study of S-1 with concurrent thoracic radiotherapy in
elderly patients with localized advanced non-small cell lung
cancer. Lung Cancer. 71:60–64. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ,
Kim IJ, Chung JK, Kim EE and Lee DS: Prognostic value of volumetric
parameters of (18)F-FDG PET in non-small-cell lung cancer: A
meta-analysis. Eur J Nucl Med Mol Imaging. 42:241–251.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang W, Fan M, Liu B, Fu Z, Zhou T, Zhang
Z, Gong H and Li B: Value of metabolic tumor volume on repeated
18F-FDG PET/CT for early prediction of survival in
locally advanced non-small cell lung cancer treated with concurrent
chemoradiotherapy. J Nucl Med. 55:1584–1590. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Keyes JW Jr: SUV: Standard uptake or silly
useless value? J Nucl Med. 36:1836–1839. 1995.PubMed/NCBI
|
|
25
|
Boellaard R, Krak NC, Hoekstra OS and
Lammertsma AA: Effects of noise, image resolution, and ROI
definition on the accuracy of standard uptake values: A simulation
study. J Nucl Med. 45:1519–1527. 2004.PubMed/NCBI
|
|
26
|
Denis F, Lethrosne C, Pourel N, Molinier
O, Pointreau Y, Domont J, Bourgeois H, Senellart H, Trémolières P,
Lizée T, et al: Randomized trial comparing a web-mediated follow-up
with routine surveillance in lung cancer patients. J Natl Cancer
Inst. 109:2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wahl RL, Jacene H, Kasamon Y and Lodge MA:
From RECIST to PERCIST: Evolving considerations for PET response
criteria in solid tumors. J Nucl Med. 50 (Suppl 1):122S–150S.
2009.PubMed/NCBI View Article : Google Scholar
|