Introduction
It is well known that a high dose of radiation is
deleterious to cells or tissue (1-4).
However, the health risks of exposure to low-dose radiation remain
unclear. Several researchers have studied the biological response
of normal cells or tissue to low doses of radiation using various
biological endpoints such as neoplastic transformation, chromosome
or DNA damage and immune function (5-17).
In addition, several studies investigated the biological response
of cancer cells or tissue to low doses of radiation using
biological endpoints such as cell cycle and cell death (18-20).
These studies showed differences in the biological response of
normal and cancer cells or tissues to low dose radiation.
Nonetheless, the limitation of the previous data on the response of
normal and cancer cell or tissue to low-doses radiation is
radiation doses in the range of centi Gray (cGy). Hence, the
present study investigated the different responses of normal cells
(blood cells) and cancer cells to low dose gamma-rays in the milli
Gray (mGy) range.
These current studies focused on the four endpoints
of biological responses that are recognized to be associated with
oxidative stress induced by radiation. These biological responses
are reactive oxygen species (ROS) levels, mitochondrial activity
(which represents mitochondrial function), hemolysis (which
represents plasma membrane integrity in red blood cells (RBCs)] and
complete blood count (CBC). The focus was on ROS levels since it
radiation (both low- and high-dose radiation) generates free
radicals including ROS, resulting in oxidative damage in cells or
tissues (21,22). Oxidative stress is a disturbance in
the balance between the yields of free radicals including ROS and
antioxidant defenses (23).
Typically, oxidative damage in cell or tissue induces mitochondria
dysfunction or lipid peroxidation in plasma membranes (21,24-27).
Mitochondrial dysfunction and plasma membrane damage is known to be
involved in mitochondrial activity and red blood cell hemolysis,
respectively. Moreover, the abnormal deformability of RBCs was
observed in conditions linked to oxidative stress (28). In addition, radiation induced
deleterious effects in blood cells in irradiated whole blood when
compared with non-irradiated whole blood (29).
Materials and methods
Blood samples
Blood samples (n=10) were collected from remaining
normal blood test group (males; age range, 40-50 years) at the
Associated Medical Sciences Clinical Service Center, Faculty of
Associated Medical Sciences, Chiang Mai University, Thailand.
Irradiation
Blood samples were given a dose of 0.03, 0.05 and
0.1 mGy gamma-rays (at a dose rate of 0.001 Gy/min) using a
137Cs radioactive standard source (located at the
Department of Radiologic Technology, Faculty of Associated Medical
Sciences, Chiang Mai University, Thailand). Samples exposed to 0 Gy
served as controls. The equations were used to calculate radiation
dose as following; i) At =
A0e-λt; ii) D =
At x Γ/d2.
When, A0 and At are the
activity of radioactive present at t=0 and time=t; λ, is decay
constant; D, is radiation dose; d, is distance from radioactive; Γ,
is specific gamma-ray constant.
Cancer cells and culture
Doxorubicin-sensitive erythroleukemia K562 cells
(K562) and doxorubicin-resistant erythroleukemia K562 cells
(K562/Dox, overexpressing P-glycoprotein) were provided by Dr
Udomtanakunchai C. The cells were grown in RPMI-1640 medium
supplemented with 10% fetal calf serum and 1%
penicillin/streptomycin at 37˚C, 95% humidity and 5%
CO2. The cells were seeded at a density of
1x105 cells/ml then exponentially grown to
8-10x105 cells/ml in 3 days. To obtain cells in the
exponential growth phase for the experiments, cells were initiated
at a density of 5x105 cells/ml. Cells were used for
experiments 24 h later after reaching a density of
8-10x105 cells/ml.
Measurement of intracellular ROS
levels
Cells (5x105 cells/ml) were incubated
with 10 µM 2',7'-dichlorofluorescein (DCF) diacetate for 30 min in
the dark. Subsequently, intracellular ROS levels were measured
using fluorescence intensity at an emission wavelength of 523 nm
(excitation wavelength, 502 nm) using a fluorescence spectrometer
(PerkinElmer, Inc.).
Mitochondrial activity
Living cells are able to reduce the nonfluorescent
dye resazurin into the fluorescent dye resorufin via mitochondrial
reductase. Hence, resazurin sodium salt (Sigma-Aldrich; Merck KGaA)
was used to determine mitochondrial activity. Cells
(5x105) were incubated with 100 µl resazurin solution
(0.1 mg/ml) in 1 ml PBS at 37˚C and were humidified with 5%
CO2 for 2 h. Subsequently, resazurin fluorescence
intensity at a wavelength of 590 nm (excitation wavelength, 570 nm)
which is an indicator of mitochondrial activity in living cells was
measured on a spectrofluorometer using a well-plate reader.
Hemolysis in normal RBCs
The hemolysis assay was performed based on
previously published studies (30,31).
Briefly, 25 µl of blood sample was incubated in 725 µl PBS and in
725 µl distilled H2O for 30 min at 37˚C. Next, blood
samples were centrifuged at 7,000 rpm for 1 min. The absorbance at
wavelength 415 nm was recorded using a spectrophotometer (Agilent
8453 UV-vis spectrophotometer; Agilent Technologies, Inc.). The
percentage of hemolysis was then calculated.
Determination of CBC parameters in
whole blood
CBC parameters were measured at the AMS Clinical
Service Center, Faculty of Associated Medical Sciences, Chiang Mai
University, Thailand. CBC parameters considered for the current
included red blood cell count, hematocrit (HCT), mean corpuscular
volume (MCV), red cell distribution width standard deviation
(RDW-SD), white blood cell (WBC) count, neutrophil (NEUT) count,
lymphocyte (LYMPH) count, monocyte (MONO) count, eosinophil (EO)
count, basophil (BASO) count, platelets (PLT) count, platelet
distribution width (PDW), pateletcrit (PCT) and mean platelet
volume (MPV).
Statistical analysis
The data were expressed as the mean ± SEM. An
analysis of variance (ANOVA) method appropriate for a one-factor
experiment (radiation dose) was used to assess the significance of
radiation dose. Further, the post hoc test (Tukey test) was used to
evaluate statistical differences in the mean values between each
group. Student's t-test was used independently to evaluate
statistical differences in the mean values between each test group
and the corresponding control group. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of low-doses gamma-rays on
blood cells
Effect on ROS in normal RBCs
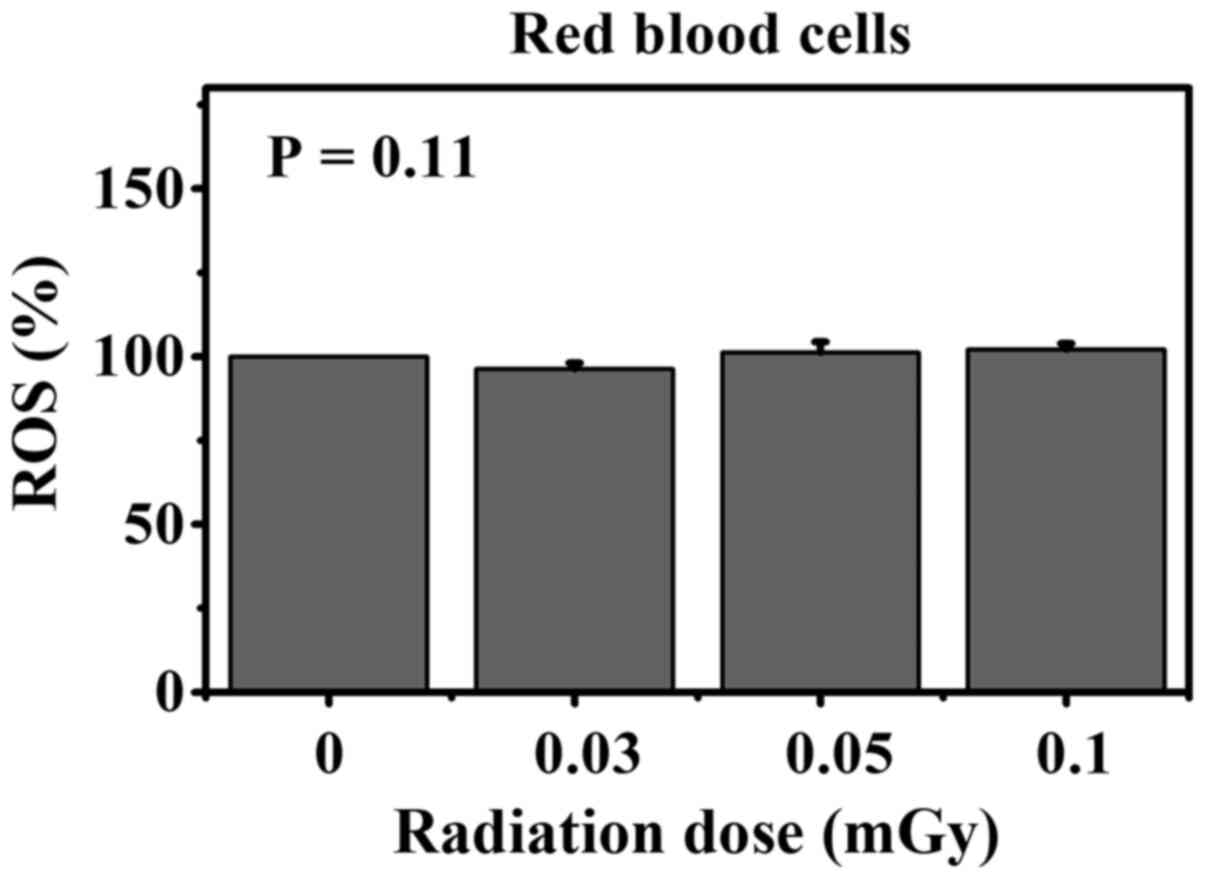
Fig. 1 shows the
percentage of ROS in RBCs following in vitro exposure to
various low doses of gamma-rays and in the corresponding
non-irradiated control groups. The data showed no change in the
percentage of ROS in irradiated RBCs relative to the corresponding
non-irradiated RBCs (ANOVA test; P-value =0.11).
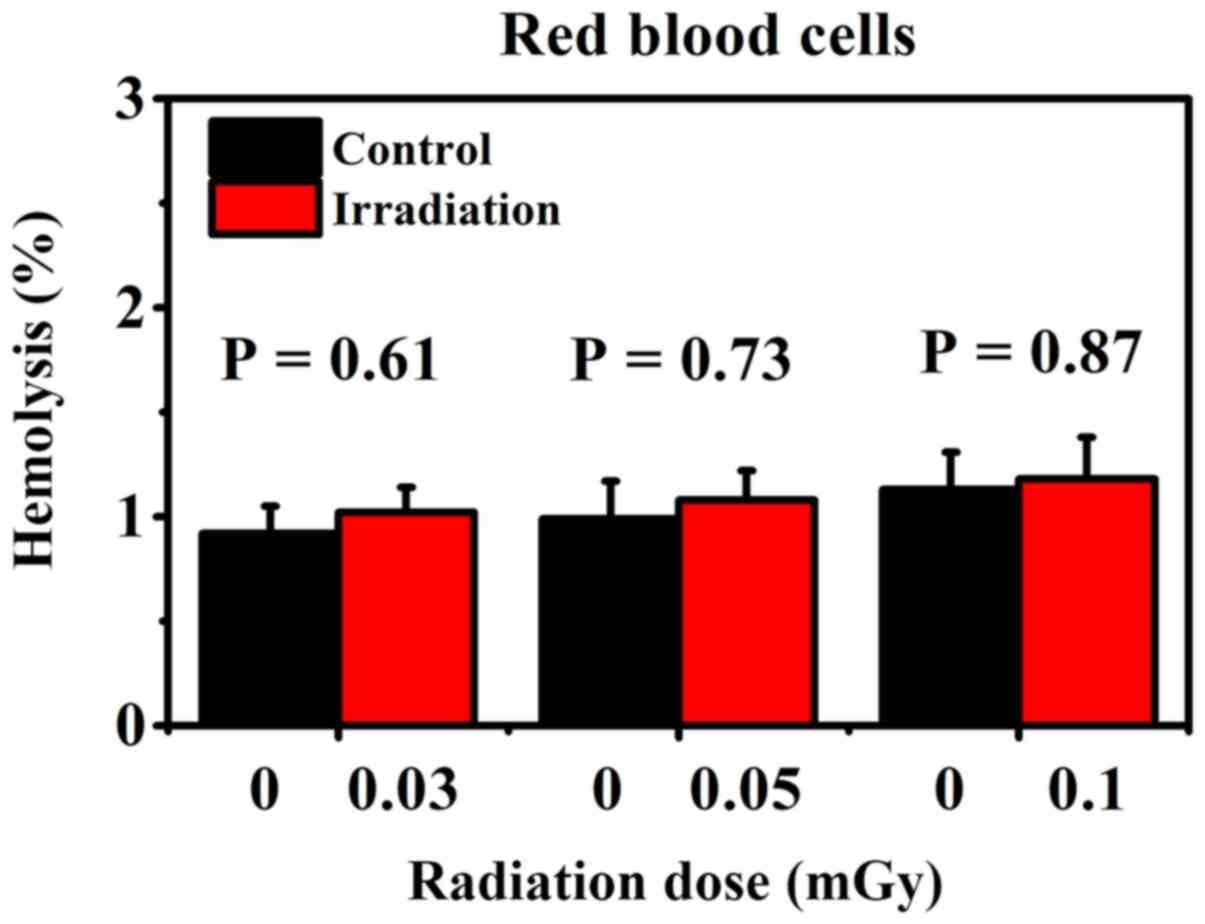
Effect on the percentage of hemolysis in normal
RBCs. Fig. 2 shows the
percentage of hemolysis in RBCs following in vitro exposure
to various low doses of gamma-rays and in the corresponding
non-irradiated control groups. The results showed that the
percentage of hemolysis did not change in irradiated RBCs compared
with corresponding non-irradiated RBCs (Student's t-test; P-value
range, 0.61-0.87).
Effect on CBC parameters in whole blood.
Table I shows the CBC parameters in
whole blood following in vitro exposure to various low doses
of gamma-rays. Similar to the percentage of ROS and hemolysis, this
data indicated no alteration in the complete blood count in
irradiated whole blood compared with the corresponding
non-irradiated whole blood.
 | Table IComplete blood count parameters in
whole blood following in vitro exposure to various low doses
of gamma-rays. |
Table I
Complete blood count parameters in
whole blood following in vitro exposure to various low doses
of gamma-rays.
| | Radiation dose |
|---|
| | 0 mGy | 0.03 mGy | 0.05 mGy | 0.1 mGy |
|---|
| Parameters | Mean ± SE | Mean ± SE | P-value | Mean ± SE | P-value | Mean ± SE | P-value |
|---|
| RBC
(106/µl) | 2.86±0.36 | 2.40±0.05 | 0.29 | 2.30±0.14 | 0.23 | 2.20±0.14 | 0.17 |
| HCT (%) | 24.58±3.22 | 20.90±0.55 | 0.34 | 20.02±1.16 | 0.26 | 19.14±1.28 | 0.19 |
| MCV (fl) | 85.93±2.88 | 87.20±2.53 | 0.75 | 87.20±2.58 | 0.75 | 86.98±2.51 | 0.79 |
| RDW-SD (fl) | 40.05±1.32 | 41.76±2.08 | 0.51 | 41.68±2.02 | 0.52 | 41.58±1.87 | 0.53 |
| PLT
(103/µl) | 77.75±11.24 | 105.00±21.10 | 0.30 | 106.40±20.60 | 0.27 | 107.00±21.34 | 0.27 |
| PDW (fl) | 11.88±0.83 | 11.52±0.99 | 0.79 | 11.34±0.71 | 0.64 | 11.08±0.81 | 0.51 |
| MPV (fl) | 10.65±0.29 | 10.04±0.44 | 0.29 | 10.10±0.39 | 0.30 | 10.08±0.36 | 0.26 |
| PCT (%) | 0.08±0.01 | 0.10±0.02 | 0.39 | 0.11±0.02 | 0.32 | 0.10±0.02 | 0.39 |
| WBC
(103/µl) | 4.03±0.32 | 4.12±0.24 | 0.83 | 4.15±0.37 | 0.82 | 4.05±0.37 | 0.98 |
| NEUT (%) | 62.63±3.95 | 59.42±3.63 | 0.57 | 59.06±3.79 | 0.54 | 58.14±3.45 | 0.42 |
| LYMPH (%) | 28.75±3.82 | 30.32±3.36 | 0.77 | 30.86±3.38 | 0.69 | 31.60±3.31 | 0.59 |
| MONO (%) | 5.73±0.60 | 6.84±0.16 | 0.16 | 6.48±0.25 | 0.31 | 6.86±0.24 | 0.16 |
| EO (%) | 2.73±0.36 | 3.28±0.97 | 0.61 | 3.46±1.10 | 0.55 | 3.36±1.02 | 0.58 |
| BASO (%) | 0.18±0.06 | 0.14±0.06 | 0.70 | 0.14±0.06 | 0.70 | 0.04±0.04 | 0.13 |
Effect of low-doses gamma-rays on K562
and K562/Dox cancer cells
Effect on ROS in cancer cells
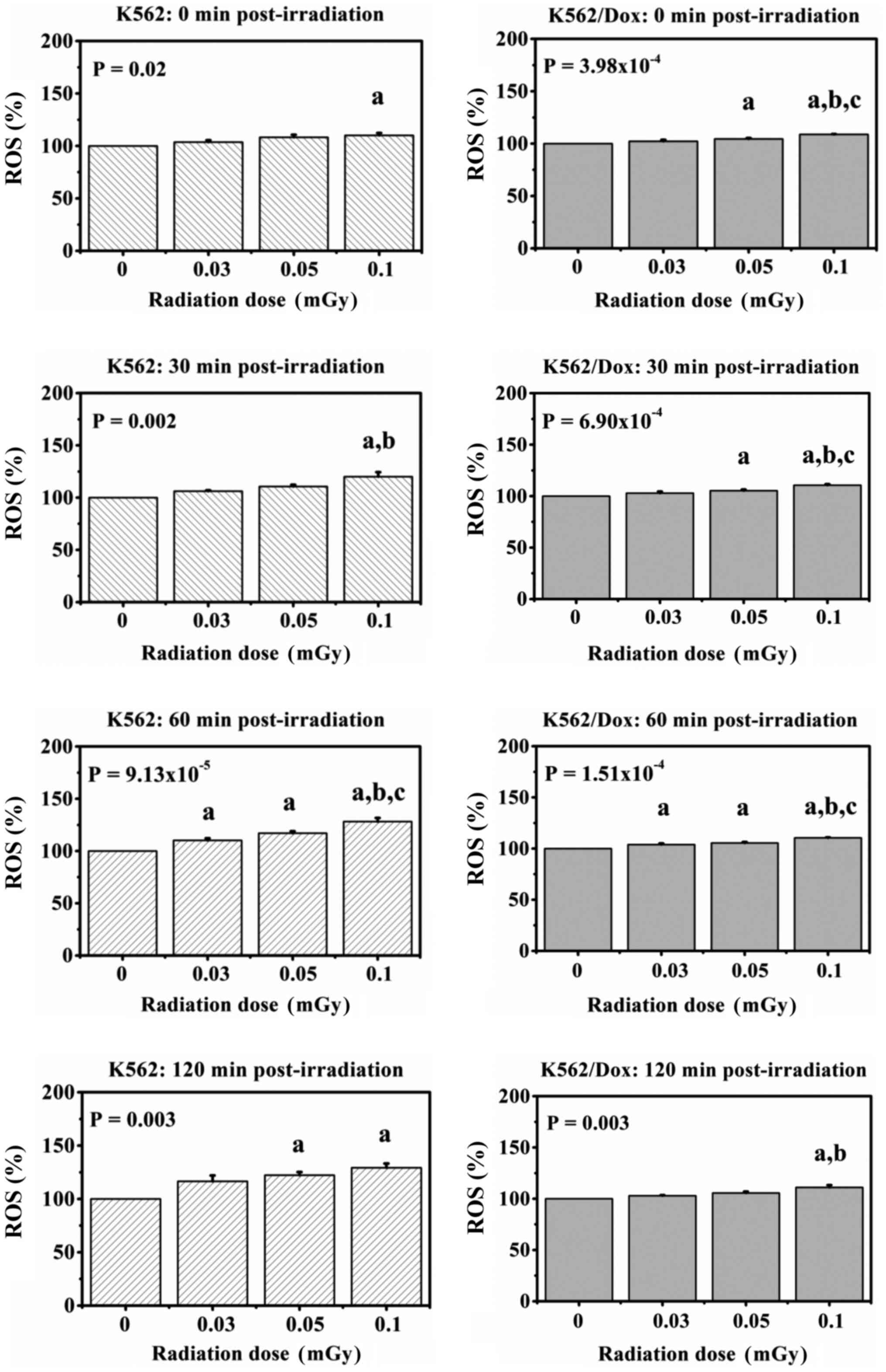
Fig. 3 shows the
percentage of ROS in K562 and K562/Dox cancer cells collected at 0,
30, 60 and 120 min after exposure to various low doses of
gamma-rays. The data showed statistically significant
dose-dependent increases in the percentage of ROS in the K562 and
K562/Dox cancer cells from 0 min up to 120 min
post-irradiation.
In K562 cancer cells, the increases were 1.04-,
1.08- and 1.10-fold higher compared with the control at 0 min
post-irradiation; 1.06-, 1.11- and 1.20-fold higher compared with
the control at 30 min post-irradiation and 1.10-, 1.17- and
1.28-fold higher compared with the control at 60 min
post-irradiation. Likewise, the increase in ROS levels in exposed
cells at 120 min post-irradiation were 1.17-, 1.22- and 1.29-fold
higher compared with the control.
In K562/Dox cancer cells, at 0 min post-irradiation,
the increases were 1.02-, 1.05- and 1.09-fold higher compared with
the control; at 30 min post-irradiation, the increases were 1.03-,
1.05- and 1.11-fold higher compared with the control and at 60 min
post-irradiation, the increases were 1.04-, 1.05- and 1.11-fold
higher compared with the control. Likewise, the increase in ROS
levels in exposed cells at 120 min post-irradiation were 1.03-,
1.06- and 1.11-fold higher compared with the control.
Effect on mitochondrial activity in
cancer cells
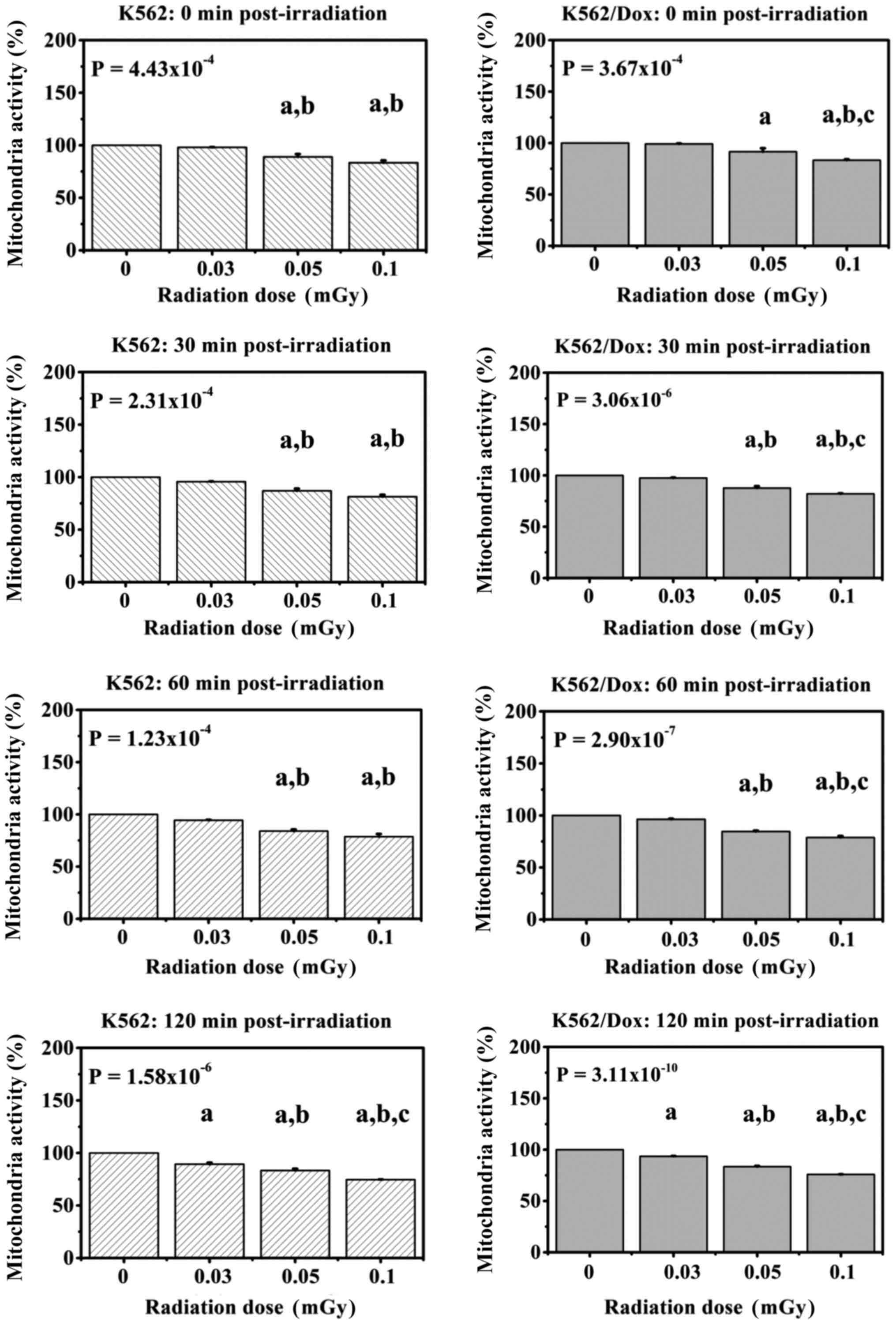
Fig. 4 shows the
mitochondrial activity in K562 and K562/Dox cancer cells, collected
at 0, 30, 60, and 120 min following exposure to various low doses
of gamma-rays. The results showed statistically significant
dose-dependent decreases in the mitochondrial activity of K562 and
K562/Dox cancer cells from 0 min up to 120 min
post-irradiation.
In K562 cancer cells, at 0 min post-irradiation, the
decreases were 0.98-, 0.89- and 0.83-fold lower compared with the
control; at 30 min post-irradiation, the decreases were 0.96-,
0.87- and 0.81-fold lower compared with the control, and at 60 min
post-irradiation, the decreases were 0.94-, 0.84- and 0.79-fold
lower compared with the control. Likewise, the decreases in the
mitochondrial activity of exposed cells at 120 min post-irradiation
were 0.89-, 0.83- and 0.75-fold lower compared with the
control.
In K562/Dox cancer cells, the fold decrease in the
mitochondrial activity were dose-dependent at all four timepoints
relative to the corresponding controls: 0.99, 0.92 and 0.83 at 0
min post-irradiation; 0.98, 0.88 and 0.82 at 30 min
post-irradiation; 0.96, 0.85 and 0.79 at 60 min post-irradiation
and 0.94, 0.83 and 0.76 at 120 min post-irradiation.
Discussion
The dose ranges of the gamma-rays damage to normal
RBCs in vitro were reported in IAEA-TECDOC-934 document.
This document reported that examining the nature of the membrane
injury in gamma irradiated RBCs in the dose range 2 to 200 Gy, It
was concluded that the sulphydryl group was the major target in
radiation-induced alteration of sodium and potassium ion
permeability. In addition, an in vitro study on the effect
of X-rays on movement of sodium in human RBCs, showed a loss of
sodium/potassium ion balance in RBCs, following radiation doses in
the range of 8.9 to 89 Gy. This phenomenon was due in part to
discontinuation of membrane integrity (32). However, those radiation dose ranges
are rather highly and most that dose find in radiation accident or
radiotherapy. Whereas radiation dose in low-dose range that find in
diagnostic radiology or nuclear medicine examination is still
challenges. Our previous studies investigated biological responses
to radiation after blood tissue was exposed to low dose X-rays in
an in vitro system. The results showed that hemolysis and
osmotic fragility in irradiated human RBCs did not significantly
differ from non-irradiated RBCs. The results also showed that
low-dose X-rays did not induce a change in mitochondrial membrane
potential, number of apoptotic cells and perturbation of the cell
cycle in irradiated human lymphocytes compared with non-irradiated
lymphocytes. The authors suggested that there were no deleterious
effects of low-dose X-rays when blood tissues were exposed in an
in vitro system (30,31,33).
The present data demonstrated no changes in ROS
levels and percentage of hemolysis of RBCs in irradiated whole
blood when compared to the non-irradiated control groups. In
addition, the CBC values in whole blood following in vitro
exposure to low-dose gamma-ray groups have not differed compared
with the non-irradiated control groups. The current findings
suggested that low-dose gamma-ray do not induce any harmful effects
to human blood cells. It should be noted that the current results
are in agreement with our previous studies (30,31,33)
and El-Shanshoury et al (34). These authors showed that
statistically significant alteration in white blood cell, red blood
cell and platelet count did not occur in rats after exposure to
low-dose gamma radiation when compared with non-irradiated groups
(34). Conversely, studies have
demonstrated radiation-induced red blood cell damage such as
increment of hemolysis and lipid peroxidation in RBCs. However,
those studies on irradiated RBCs involved high dose gamma radiation
(27,35-37).
It could be suggested that, depending on radiation dose, there are
different responses in normal cells (red blood cells) between low-
and high-dose radiation.
By contrast, normal cells (red blood cells) with low
dose gamma irradiation caused significant increase in ROS levels in
both irradiated K562 and K562/Dox cancer cells at all harvest time
points, whereas the mitochondrial activity was decreased in both
irradiated K562 and K562/Dox cancer cells at all harvest time
points relative to non-irradiated cells. ROS and cell type [normal
cells (RBCs) vs. cancer cells (K562 and K562/Dox)] were also
compared. In the present study, K562 and K562/Dox exhibited
sensitivity to low dose gamma radiation more than RBCs. Cancer
cells show a wide range of sensitivity to radiation with different
radiosensitivities. Low-dose hypersensitivity is found in various
cancer cell lines upon receiving radiation (38-41).
In addition, Dai et al investigated low dose
hyper-radiosensitivity in the cancer cell line A549 irradiated with
60Co gamma-rays at doses of 0-2 Gy. The results showed
that A549 cells exhibited low dose hyper-radiosensitivity. The type
of death observed in cells was mainly apoptosis (18). Enns et al studied the
response of three cancer cell lines, A549, T98G and MCF7, exposed
to 0-200 cGy radiation doses from 137Cs source
gamma-rays. The authors found that hypersensitivity occurred in the
A549 and T98G cancer cells, but not in MCF7 cancer cells at
radiation doses <50 cGy. The authors also suggested that
hyper-radiosensitivity was involved in p53-dependent apoptosis
(19). Short et al (20) investigated low dose
hyper-radiosensitivity in the cancer cell lines T98G and U373
irradiated with X-rays. The results showed that
hyper-radiosensitivity was observed in both T98G and U373 cancer
cells. The authors also demonstrated that low-dose
hyper-radiosensitivity depended on the cell cycle phase (20). Therefore, the present results agree
with the hypothesis that cancer cell lines exhibit low-dose
hypersensitivity to radiation.
ROS have been shown to play important roles in cell
proliferation and cell death (42,43).
Typically, ROS are produced in cells upon cells that are exposed to
radiation in which ROS is mediated from the indirect effects of low
linear energy transfer radiation as gamma-rays (44,45). A
study has demonstrated that radiation potently induced cancer cell
death via generation of ROS and oxidative response in cell
organelles such as the mitochondria (46). In addition, Walsh et al
performed mitochondrial staining with tetramethyl rhodamine ethyl
ester in live MCF-7 and A549 cancer cells after exposure to 55 MeV
carbon ions or 3 MeV proton radiation. The results showed that
tetramethyl rhodamine ethyl ester levels were decreased in the
mitochondria. The authors suggested that there was an induction of
mitochondrial membrane depolarization after cancer cells received
either protons or carbon ions (47). Leach et al had shown
increased DCF fluorescence in A431 cancer cells. It was found that
radiation stimulated ROS production in cells after exposure to 3 Gy
of 90Sr radiation source. The authors also showed transient
depolarizing effects of radiation on the mitochondrial membrane
potential in A431 cancer cells (48). However, ROS is not only generated in
cells by high dose radiation, but also by low-dose radiation,
resulting in a number of deleterious effects on cells (21). Hence, the present study hypothesized
that low-dose gamma-rays might induce increments of ROS in K562 and
K562/Dox cancer cells, resulting in occurrence of oxidative stress
that plays a role in decreasing mitochondria activity.
The current study showed the biological responses in
K562 and K562/Dox cancer cells to low-dose gamma-rays but did not
show that in RBCs. These findings suggested that erythroleukemia
was more sensitive to low-dose gamma-rays compared with normal
RBCs. In addition, the results of the current study suggested the
possibility of using low-dose gamma radiation to treat
erythroleukemia.
In conclusion, the current study showed the
difference in biological responses in normal cells (RBCs) and
cancer cells (K562 and K562/Dox) to low-dose gamma-rays when cells
were exposed under in vitro conditions.
Acknowledgements
Not applicable.
Funding
This research was partially supported by Chiang Mai University
(grant no. R000023314).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BS, PH, NK and NS performed the experiments. ST, SK
and CU interpreted the data and revised the manuscript. MT
conceived and designed the current study, performed the
experiments, analyzed and interpreted the data, and drafted and
revised the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Blood sample collections were performed under the
approved guidelines by the Institutional Committees on Research
Involving Human Subjects and approval of the Faculty of Associated
Medical Sciences, Chiang Mai University (approval no.
AMSEC-62EM-002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rithidech KN, Golightly M and Whorton E:
Analysis of cell cycle in mouse bone marrow cells following acute
in vivo exposure to 56Fe ions. J Radiat Res. 49:437–443.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jelveh S, Kaspler P, Bhogal N, Mahmood J,
Lindsay PE, Okunieff P, Doctrow SR, Bristow RG and Hill RP:
Investigations of antioxidant-mediated protection and mitigation of
radiation-induced DNA damage and lipid peroxidation in murine skin.
Int J Radiat Biol. 89:618–627. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Villani P, Fresegna AM, Ranaldi R,
Eleuteri P, Paris L, Pacchierotti F and Cordelli E: X-ray induced
DNA damage and repair in germ cells of PARP1(-/-) male mice. Int J
Mol Sci. 14:18078–18092. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tungjai M, Whorton EB and Rithidech KN:
Persistence of apoptosis and inflammatory responses in the heart
and bone marrow of mice following whole-body exposure to
28Silicon (28Si) ions. Radiat Environ
Biophys. 52:339–350. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Olivieri G, Bodycote J and Wolff S:
Adaptive response of human lymphocytes to low concentrations of
radioactive thymidine. Science. 223:594–597. 1984.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bond VP, Benary V and Sondhaus CA: A
different perception of the linear, nonthreshold hypothesis for
low-dose irradiation. Proc Natl Acad Sci USA. 88:8666–8670.
1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Azzam EI, de Toledo SM, Raaphorst GP and
Mitchel RE: Low-dose ionizing radiation decreases the frequency of
neoplastic transformation to a level below the spontaneous rate in
C3H 10T1/2 cells. Radiat Res. 146:369–373. 1996.PubMed/NCBI
|
|
8
|
Wolff S: The adaptive response in
radiobiology: Evolving insights and implications. Environ Health
Perspect. 106 (Suppl 1):277–283. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Redpath JL, Liang D, Taylor TH, Christie C
and Elmore E: The shape of the dose-response curve for
radiation-induced neoplastic transformation in vitro: Evidence for
an adaptive response against neoplastic transformation at low doses
of low-LET radiation. Radiat Res. 156:700–707. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Feinendegen LE: Evidence for beneficial
low level radiation effects and radiation hormesis. Br J Radiol.
78:3–7. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Scott BR and Di Palma J: Sparsely ionizing
diagnostic and natural background radiations are likely preventing
cancer and other genomic-instability-associated diseases. Dose
Response. 5:230–255. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Elmore E, Lao XY, Kapadia R, Giedzinski E,
Limoli C and Redpath JL: Low doses of very low-dose-rate low-LET
radiation suppress radiation-induced neoplastic transformation in
vitro and induce an adaptive response. Radiat Res. 169:311–318.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Rithidech KN and Scott BR: Evidence for
radiation hormesis after in vitro exposure of human lymphocytes to
low doses of ionizing radiation. Dose Response. 6:252–271.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mitchel RE: The dose window for
radiation-induced protective adaptive responses. Dose Response.
8:192–208. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rithidech KN, Udomtanakunchai C, Honikel L
and Whorton E: Lack of genomic instability in bone marrow cells of
SCID mice exposed whole-body to low-dose radiation. Int J Environ
Res Public Health. 10:1356–1377. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rithidech KN, Udomtanakunchai C, Honikel
LM and Whorton EB: No evidence for the in vivo induction of genomic
instability by low doses of CS gamma-rays in bone marrow cells of
BALB/CJ and C57BL/6J mice. Dose Response. 10:11–36. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu HS, Liu ZM, Yu XY, Song AQ, Liu N and
Wang H: Low-dose radiation induces antitumor effects and
erythrocyte system hormesis. Asian Pac J Cancer Prev. 14:4121–4126.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dai X, Tao D, Wu H and Cheng J: Low dose
hyper-radiosensitivity in human lung cancer cell line A549 and its
possible mechanisms. J Huazhong Univ Sci Technolog Med Sci.
29:101–106. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Enns L, Bogen KT, Wizniak J, Murtha AD and
Weinfeld M: Low-dose radiation hypersensitivity is associated with
p53-dependent apoptosis. Mol Cancer Res. 2:557–566. 2004.PubMed/NCBI
|
|
20
|
Short SC, Woodcock M, Marples B and Joiner
MC: Effects of cell cycle phase on low-dose hyper-radiosensitivity.
Int J Radiat Biol. 79:99–105. 2003.PubMed/NCBI
|
|
21
|
Smith JT, Willey NJ and Hancock JT: Low
dose ionizing radiation produces too few reactive oxygen species to
directly affect antioxidant concentrations in cells. Biol Lett.
8:594–597. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rithidech KN, Tungjai M and Whorton EB:
Protective effect of apigenin on radiation-induced chromosomal
damage in human lymphocytes. Mutat Res. 585:96–104. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Betteridge DJ: What is oxidative stress?
Metabolism. 49 (Suppl 1):3–8. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Feinendegen LE, Pollycove M and Sondhaus
CA: Responses to low doses of ionizing radiation in biological
systems. Nonlinearity Biol Toxicol Med. 2:143–171. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Spitz DR, Azzam EI, Li JJ and Gius D:
Metabolic oxidation/reduction reactions and cellular responses to
ionizing radiation: A unifying concept in stress response biology.
Cancer Metastasis Rev. 23:311–322. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zbikowska HM, Antosik A, Szejk M, Bijak M
and Nowak P: A moderate protective effect of quercetin against
γ-irradiation- and storage-induced oxidative damage in red blood
cells for transfusion. Int J Radiat Biol. 90:1201–1210.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zbikowska HM and Antosik A: Irradiation
dose-dependent oxidative changes in red blood cells for
transfusion. Int J Radiat Biol. 88:654–660. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Diederich L, Suvorava T, Sansone R, Keller
TC IV, Barbarino F, Sutton TR, Kramer CM, Lückstädt W, Isakson BE,
Gohlke H, et al: On the Effects of Reactive Oxygen Species and
Nitric Oxide on Red Blood Cell Deformability. Front Physiol.
9(332)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Baroni F, Marraccini C, Merolle L,
Piccagli V, Lambertini D, Iori M, Fasano T, Casali E, Spisni A,
Baricchi R, et al: Red blood cells metabolome changes upon
treatment with different X-ray irradiation doses. Ann Hematol.
97:1909–1917. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tungjai M, Phathakanon N, Ketnuam P,
Tinlapat J and Kothan S: Determination of hemolysis, osmotic
fragility and fluorescence anisotropy on irradiated red blood cells
as a function of kV of medical diagnostic X-rays. J Radiat Res.
16:123–127. 2018.
|
|
31
|
Tungjai M, Sopapang J, Tasri N,
Osothsongkroh C, Jantarato A and Kothan S: The effects of medical
diagnostic low dose X-rays after in vitro exposure of human red
blood cells: Hemolysis and osmotic fragility. Toxicol Environ
Health Sci. 11:237–243. 2019.
|
|
32
|
International Atomic Energy Agency:
Effects of ionizing radiation on blood and blood components: A
survey. IAEA-TECDOC-934, Vienna, 1997.
|
|
33
|
Tungjai M, Phathakanon N and Rithidech KN:
Effects of medical diagnostic low-dose X rays on human lymphocytes:
mitochondrial membrane potential, apoptosis and cell cycle. Health
Phys. 112:458–464. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
El-Shanshoury H, El-Shanshoury G and Abaza
A: Evaluation of low dose ionizing radiation effect on some blood
components in animal model. J Radiat Res Appl Sci. 9:282–293.
2016.
|
|
35
|
Antosik A, Czubak K, Gajek A, Marczak A,
Glowacki R, Borowczyk K and Zbikowska HM: Influence of pre-storage
irradiation on the oxidative stress markers, membrane integrity,
size and shape of the cold stored red blood cells. Transfus Med
Hemother. 42:140–148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dumaswala UJ, Zhuo L, Jacobsen DW, Jain SK
and Sukalski KA: Protein and lipid oxidation of banked human
erythrocytes: Role of glutathione. Free Radic Biol Med.
27:1041–1049. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Anand AJ, Dzik WH, Imam A and Sadrzadeh
SM: Radiation-induced red cell damage: Role of reactive oxygen
species. Transfusion. 37:160–165. 1997.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Joiner MC, Marples B, Lambin P, Short SC
and Turesson I: Low-dose hypersensitivity: Current status and
possible mechanisms. Int J Radiat Oncol Biol Phys. 49:379–389.
2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wouters BG and Skarsgard LD: The response
of a human tumor cell line to low radiation doses: Evidence of
enhanced sensitivity. Radiat Res. 138 (Suppl 1):S76–S80.
1994.PubMed/NCBI
|
|
40
|
Skarsgard LD, Skwarchuk MW, Wouters BG and
Durand RE: Substructure in the radiation survival response at low
dose in cells of human tumor cell lines. Radiat Res. 146:388–398.
1996.PubMed/NCBI
|
|
41
|
Wouters BG, Sy AM and Skarsgard LD:
Low-dose hypersensitivity and increased radioresistance in a panel
of human tumor cell lines with different radiosensitivity. Radiat
Res. 146:399–413. 1996.PubMed/NCBI
|
|
42
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kim W, Lee S, Seo D, Kim D, Kim K, Kim E,
Kang J, Seong KM, Youn H and Youn B: Cellular Stress Responses in
Radiotherapy. Cells. 8(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kawamura K, Qi F and Kobayashi J:
Potential relationship between the biological effects of low-dose
irradiation and mitochondrial ROS production. J Radiat Res. 59
(Suppl 2):ii91–ii97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang JS, Wang HJ and Qian HL: Biological
effects of radiation on cancer cells. Mil Med Res.
5(20)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Walsh DW, Siebenwirth C, Greubel C, Ilicic
K, Reindl J, Girst S, Muggiolu G, Simon M, Barberet P, Seznec H, et
al: Live cell imaging of mitochondria following targeted
irradiation in situ reveals rapid and highly localized loss of
membrane potential. Sci Rep. 7(46684)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Leach JK, Van Tuyle G, Lin PS,
Schmidt-Ullrich R and Mikkelsen RB: Ionizing radiation-induced,
mitochondria-dependent generation of reactive oxygen/nitrogen.
Cancer Res. 61:3894–3901. 2001.PubMed/NCBI
|