Introduction
Brain tumor statistics in the USA and Germany has
indicated that glioblastoma (GBM) during childhood is relatively
rare and accounts for less than 3% of childhood brain tumors
(1,2). Genetic characteristics differ between
pediatric GBM (pGBM) and adult GBM (aGBM), and there are
differences in five-year survival rates and sensitivity to
chemotherapy (2-4).
It is possible that pGBM and aGBM, although histologically similar,
are different tumors. Also, GBM sometimes has multiple contrast
lesions (5,6). In this case, it is classified as
multifocal GBM or multicentric GBM depending on whether the
contrast area is continuous or independent in high signal areas in
T2-weighted images (7). Multiple
foci of enhancement embedded within a larger region of T2-weighted
signal abnormality is defined as multifocal GBM, and discrete
enhancing regions without evidence of connecting tumor is defined
as multicentric GBM (7). Those with
multiple lesions have shorter survival time compared with those
with a single lesion (7). Here we
report a case of childhood onset and multicentric GBM.
Case report
The patient was a 4-year-old female, who was born
full term and had no problems with growth and development. She had
no family history of hereditary disease. One day, she felt a lack
of vigor and experienced appetite loss. Two days later, headache
and vomiting appeared. After two more days, the vomiting continued
and she gradually became drowsy, so she was taken to a nearby
doctor. Computed tomography (CT) was performed and revealed an
intracranial abnormality, and the patient was transferred to our
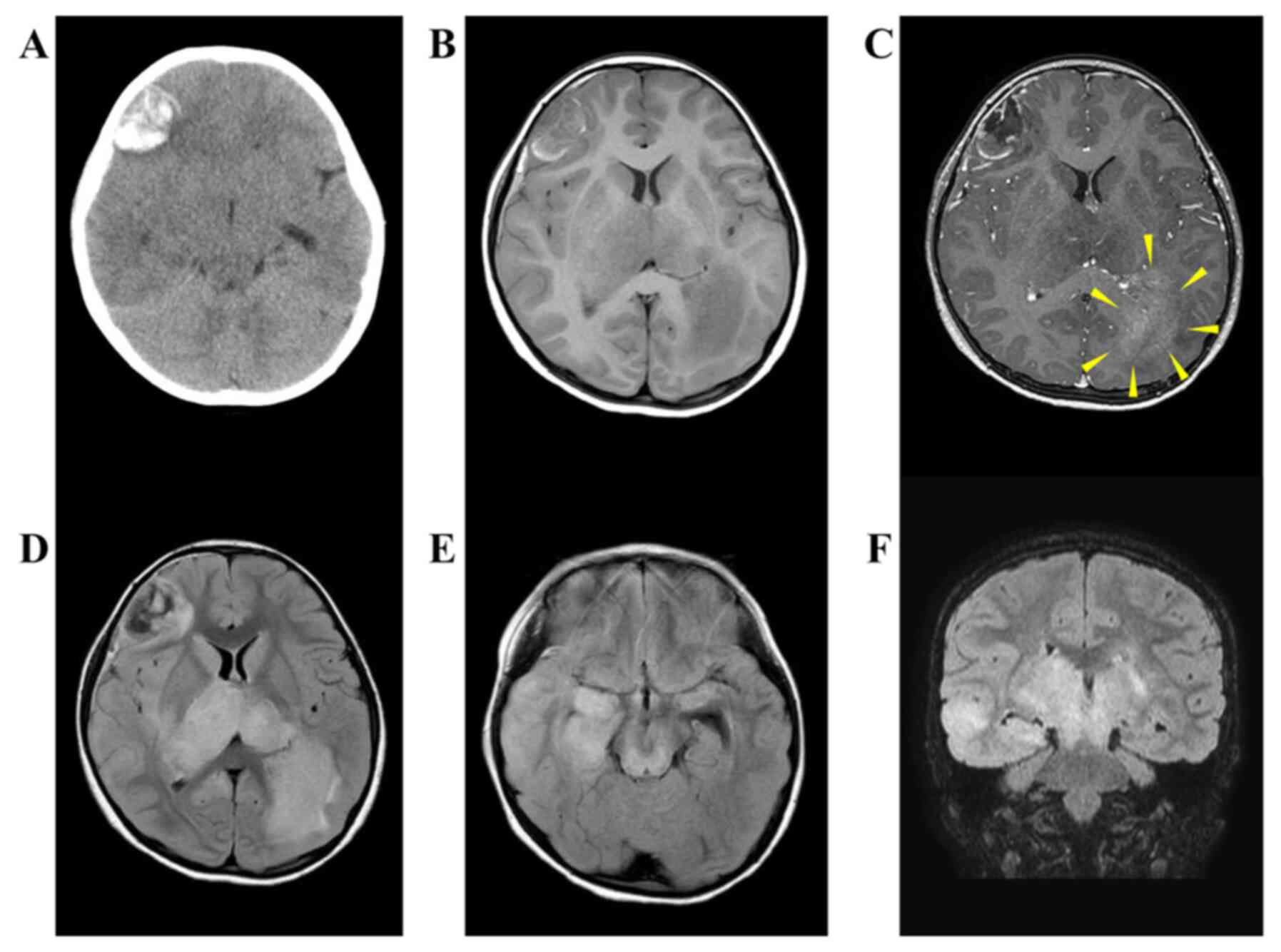
hospital. On the plain head CT, a 3.5 cm high-density area was
observed and found to be an acute hemorrhagic lesion (Fig. 1A). The bilateral thalamus, right
temporal lobe, and left occipital lobe showed low density. On the
magnetic resonance image (MRI), the right frontal lobe lesion
showed a low signal on a T1-weighted image and a slightly high
signal at the edge (Fig. 1B).
fluid-attenuated inversion recovery (FLAIR) images revealed regions
of high-intensity signal inside the lesion (Fig. 1D). T1-gadolinium (T1-Gd) imaging
showed an enhancing effect along the bleeding lesion (Fig. 1C). The bilateral thalamus, right
temporal lobe, and left occipital lobe continued with high FLAIR
signal (Fig. 1D-F). Within the
FLAIR hyperintense region of the left occipital lobe, we found a
lesion with a reticulated enhanced effect by T1-Gd imaging
(Fig. 1C). Therefore, the lesion
was considered to be multiple GBM configured frontal and right
temporal-bilateral thalamus-left occipital that caused intratumoral
hemorrhage in part, and a craniotomy was performed on the right
frontal lobe lesion for the purpose of decompression, diagnosis and
preventing rebleeding. Preoperative Karnofsky Performance status
(KPS) was 80. The patient was placed in the supine position with
her head rotated to the left 40 degrees and supported with a
Mayfield headframe. A standard preauricular question mark incision
was made. The navigation was used to craniotomy just above the
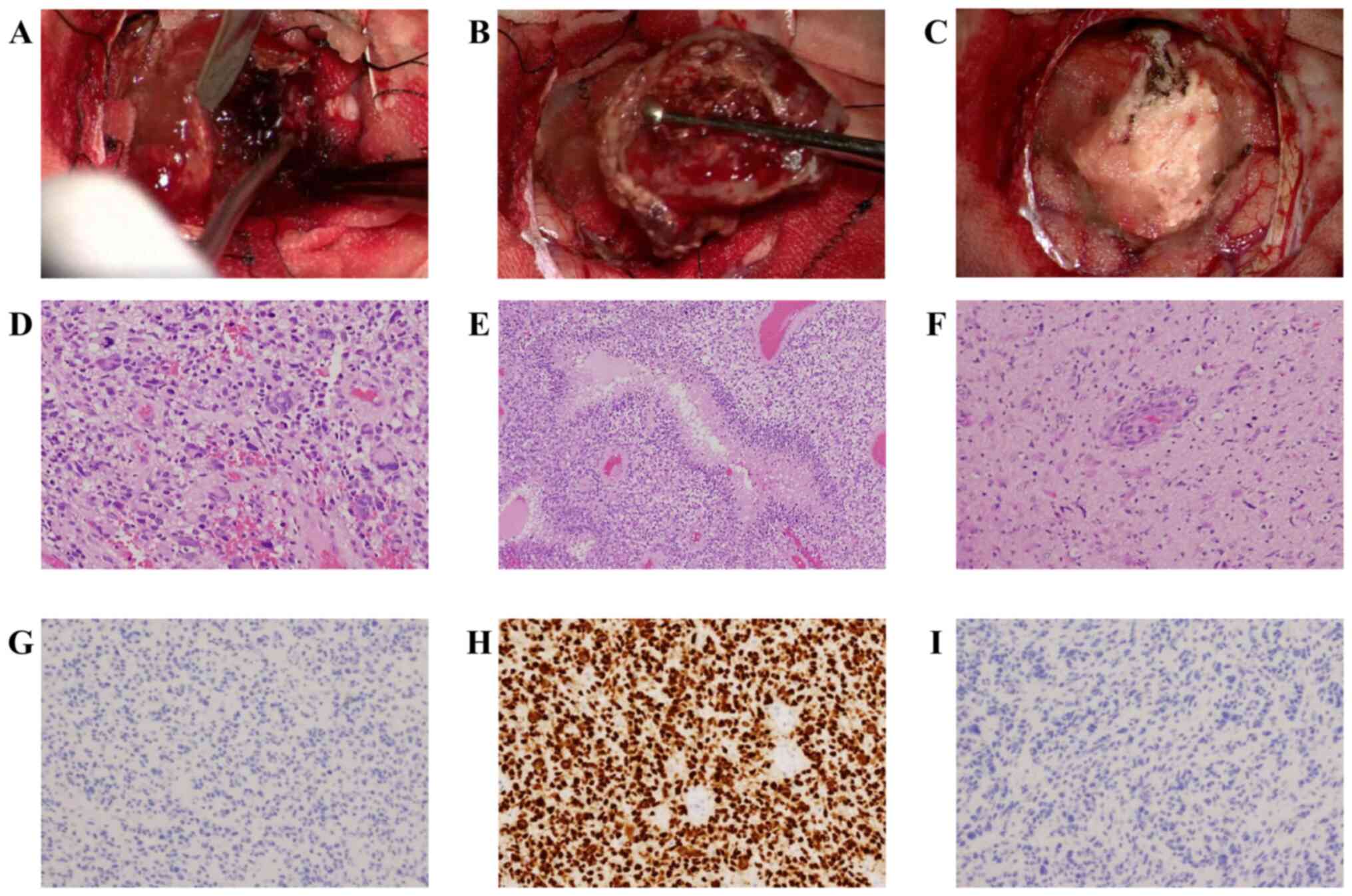
tumor. When the dura mater was incised, we observed a reddish-brown
neoplastic lesion. The tumor was hemorrhagic, and a hematoma was
found inside (Fig. 2A). The tumor
was clearly demarcated from the surrounding area and was removed as
en bloc (Fig. 2B and
C). No surgery was performed on the
left occipital lobe lesion. The reason is as follows (1). The lesion responsible for the symptom
was considered to be a bleeding right frontal lobe lesion (2). The purpose of surgery included
decompress, prevention of rebleeding, and pathological
diagnosis.
Histopathological and molecular
findings
Histopathologically, the tumor samples revealed
moderate to high cellularity, and tumor cells had pleomorphic,
hyperchromatic nuclei. In addition, we also observed large, highly
atypical, multinucleated cells (Fig.
2D). We observed mitotic figures occasionally and palisading
necrosis and mild microvascular proliferation (Fig. 2E and F). Immunohistochemically, the tumor cells
were negative for IDH-1 (Fig. 2G)
and positive for ATRX. P53 was diffusely positive (Fig. 2H) and H3K27M was negative (Fig. 2I). To determine H3F3A, H3K27M,
H3G34R, AND H3G34V mutation status we performed QP
(QProbe/Qprimer)-Polymerase Chain Reaction using a fully-automated
genetic analysis system, the i-densy IS-5320 (Arkray, Inc., Kyoto,
Japan). However, no mutations were found. Based on these findings,
the tumor was diagnosed as GBM, IDH-wildtype, WHO grade IV.
Confirmation of MGMT methylation status of postoperative
chemotherapy was performed by immunostaining. The number of cells
stained was about <1%, and Temozolomide was considered to be
effective.
Postoperative course
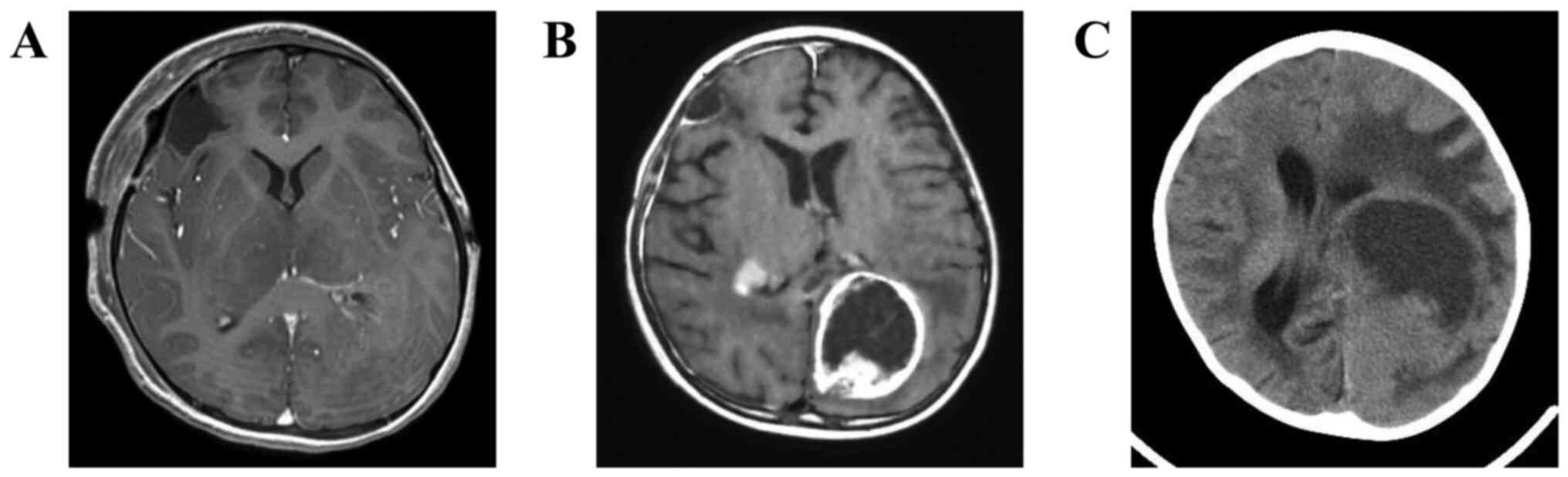
Head MRI performed the day after surgery confirmed
the total removal of the right frontal lobe lesion (Fig. 3A). The postoperative KPS was 90
because the symptoms of increased intracranial pressure improved.
We performed postoperative treatment with temozolomide (TMZ) and
radiation according to the Stupp regimen (8). The radiation dose was reduced to 40
Gy/20 Fr for the child and TMZ was increased from the third-course
cycle up to 200 mg/m2. She was given four courses of
chemotherapy. At six months after surgery, the FLAIR high signal
area and the reticular contrast lesion in the left occipital lobe
were both enlarged (Fig. 3B). KPS
fell to 40, we stopped chemotherapy and started to palliative care.
At seven months after surgery, plane CT revealed further enlarged
tumor (Fig. 3C), and she passed
away 11 months after the surgery.
Discussion
This case report is unique in that the patient was a
four-year-old suffering from GBM with multiple lesions. pGBM is
often associated with neurofibromatosis type 1, Turcot syndrome,
and Li-Fraumeni syndrome (9).
Although this case did not undergo genetic testing, it was
considered to be a relatively rare sporadic case with no family
history. GBM has a different prognosis depending on the age of
onset, and the five-year survival rate is less than 6% at 45 years
of age or older, but there was a report that the five-year survival
rate is 29.8% in patients younger than five years old (2). In a past study, a subset of pGBM cases
that were diagnosed by histopathology more closely resembled
pleomorphic xanthoastrocytoma or low-grade glioma in hierarchical
clustering of genome-wide DNA methylation (10). Cases in this subset had the BRAF
V600E mutation and 9p21 homozygous deletion, or generally balanced
genomes in terms of copy number, and long-term survival was
observed (10). Therefore, for
those cases of pGBM that showed long-term survival, it is possible
that detailed examination could reveal biologically distinct
characteristics from other, common pGBM cases. When GBM is
diagnosed in children, long-term survival is considered difficult
if the children do not have special molecular features. Most
recently, genomic characterizations have revealed highly prevalent
driver mutations in pGBM, including mutations involving histone
H3.3, other chromatin remodeling genes, and ACVR1 genes (11). In aGBM, deletion of PTEN and
amplification of EGFR are more common but less common in
pGBM, and PTEN deletion is considered to be a poor
prognostic factor in pGBM (12).
IDH1 wildtype and TP53 mutation are also associated
with poor prognosis in pGBM (10,13,14).
In regards to p53 expression, there are two contradictory reports
on whether or not p53 expression is associated with poor prognosis
(13,14). For the pGBM case reported in this
study, we do not know if there was a deletion of PTEN, but
immunohistochemically, IDH1 was negative and p53 was highly
expressed. Therefore, it may be possible that the prognosis was
genotypically poor.
In addition, this case showed multiple contrast
lesions that did not show continuity in high FLAIR signal and
presented findings of multicentric GBM. There is no consolidated
report on pediatric multicentric GBM, and there is no established
treatment. In this case, postoperative radiotherapy and TMZ
chemotherapy were performed according to the Stupp regimen. TMZ has
an effect of prolonging progression-free survival in pGBM (3). Although it was examined in adult
cases, TMZ also prolongs overall survival in multicentric GBM
(15), and it was considered to be
effective also in this case. Regarding radiation therapy, although
there was a report that no significant effect was obtained at age
five years or less for pGBM (16),
radiation therapy was performed at a reduced dose, because only
frontal lobe lesions could be removed in our case. The tumor
progressed five months after surgery, and this case was considered
to be incurable by conventional treatment alone. Recently, an
association between pGBM and Biallelic mismatch repair deficiency
syndrome and the effectiveness of Nivolumab have been reported
(17). For intractable pGBM,
further examination of genetic tests and examination of treatment
methods are needed in the future.
We experienced a case of pediatric multicentric GBM
with intra-tumor hemorrhage. The patient had characteristics of
both pediatric and multiple lesion GBM, and the malignancy was
difficult to treat. This disease has a poor prognosis, and
additional cases need to be accumulated and evaluated to establish
an effective treatment regimen.
Acknowledgements
Not applicable.
Funding
No funding are received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the present
study.
Authors' contributions
TE drafted the manuscript. MA and KN supervised and
reviewed the manuscript. TE, MA and KN performed the histological
examination. TK, MT, HA, TI, YN and SH treated the patient. YN and
SH are treating pediatrician. The authenticity of all the raw data
have been assessed by TE, MA and KN to ensure its legitimacy. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Consent was obtained from family members at the time
of surgery for publication of patient data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaatsch P, Rickert CH, Kühl J, Schüz J and
Michaelis J: Population-based epidemiologic data on brain tumors in
German children. Cancer. 92:3155–3164. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barmholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 15
(Suppl 2):ii1–ii56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walston S, Hamstra DA, Oh K, Woods G,
Guiou M, Olshefski RS, Chakravarti A and Williams TM: A
multi-institutional experience in pediatric high-grade glioma.
Front Oncol. 5(28)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
MacDonald TJ, Aguilera D and Kramm CM:
Treatment of high-grade glioma in children and adolescents. Neuro
Oncol. 13:1049–1058. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Giannopoulos S and Kyritsis AP: Diagnosis
and management of multifocal gliomas. Oncology. 79:306–312.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hassaneen W, Levine NB, Suki D, Salaskar
AL, de Moura Lima A, McCutcheon IE, Prabhu SS, Lang FF, DeMonte F,
Rao G, et al: Multiple craniotomies in the management of multifocal
and multicentric glioblastoma. Clinical article. J Neurosurg.
114:576–584. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lasocki A, Gaillard F, Tacey M, Drummond K
and Stuckey S: Multifocal and multicentric glioblastoma: Improved
characterisation with FLAIR imaging and prognostic implications. J
Clin Neurosci. 31:92–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tamber MS and Rutka JT: Pediatric
supratentorial high-grade gliomas. Neurosurg Focus.
14(e1)2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Korshunov A, Ryzhova M, Hovestadt V,
Bender S, Strum D, Capper D, Meyer J, Schrimpf D, Kool M, Northcott
PA, et al: Integrated analysis of pediatric glioblastoma reveals a
subset of biologically favorable tumors with associated molecular
prognostic markers. Acta Neuropathol. 129:669–678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lam S, Lin Y, Zinn P, Su J and Pan IW:
Patient and treatment factors associated with survival among
pediatric glioblastoma patients: A surveillance, epidemiology, and
end results study. J Clin Neurosci. 47:285–293. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pollack IF, Hamilton RL, Burger PC, Brat
DJ, Rosenblum MK, Murdoch GH, Nikiforova MN, Holmes EJ, Zhou T,
Cohen KJ, et al: Akt activation is a common event in pediatric
malignant gliomas and a potential adverse prognostic marker: A
report from the children's oncology group. J Neurooncol.
99:155–163. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antonelli M, Buttarelli FR, Arcella A,
Nobusawa S, Donofrio V, Oghaki H and Giangaspero F: Prognostic
significance of histological grading, p53 status, YKL-40
expression, and IDH1 mutations in pediatric high-grade gliomas. J
Neurooncol. 99:209–215. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pollack IF, Finkelstein SD, Woods J,
Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL
and Sposto R: Children's Cancer Group. Expression of p53 and
prognosis in children with malignant gliomas. N Engl J Med.
346:420–427. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Syed M, Liermann J, Verma V, Bernhardt D,
Bougatf N, Paul A, Rieken S, Debus J and Adeberg S: Survival and
recurrence patterns of multifocal glioblastoma after radiation
therapy. Cancer Manag Res. 10:4229–4235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu M, Thakkar JP, Garcia CR, Dolecek TA,
Wagner LM, Dressler EVM and Villano JL: National cancer database
analysis of outcomes in pediatric glioblastoma. Cancer Med.
7:1151–1159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bouffet E, Larouche V, Campbell BB, Merico
D, de Borja R, Aronson M, Durno C, Krueger J, Cabric V, Ramaswamy
V, et al: Immune checkpoint inhibition for hypermutant glioblastoma
multiforme resulting from germline biallelic mismatch repair
deficiency. J Clin Oncol. 34:2206–2211. 2016.PubMed/NCBI View Article : Google Scholar
|