Introduction
Colorectal cancer (CRC) is the third most common
malignant tumor and the second leading cause of cancer-associated
mortality in Western countries. Currently, ~25% of patients present
with liver metastases at the time of diagnosis, and 80% of cases
are bilateral and multiple (1,2). This
is a very heterogeneous group of patients. Furthermore, associated
comorbidity is often relevant, and thus could determine the most
appropriate treatment for each patient. The survival prognosis is
poor, and the treatment is usually palliative.
Primary tumor resection (PTR), followed by
chemotherapy, has traditionally been considered the first
therapeutic option, which aims to decrease the tumor burden and
avoid potential complications of the primary tumor. Following
intervention, the start of chemotherapy can be delayed or suspended
in patients who develop complications after surgery, which occurs
in up to 25% of patients (3,4).
Historically, the median survival for patients with
unresectable simultaneous liver metastases was <8 months. In the
last decade, there has been a substantial improvement in the
prognosis, with the median survival currently increased to 24
months (2). This has been possible
due to the implantation of second-generation chemotherapeutic
agents (5) and monoclonal
antibodies against specific targets, such as the epidermal growth
factor receptor (EGFR). Furthermore, biological markers, such as
the KRAS oncogene, help in the selection of chemotherapeutic agents
(6).
The improvement in therapeutic results has motivated
the introduction of changes in treatment schemes (7,8). The
benefit of surgical resection of the primary tumor on survival and
quality of life has been controversial. Due to the fact that it is
the degree of liver involvement what determines the survival of
these patients (8), surgery
treatment on the primary tumor could be avoided in patients with
uncomplicated tumors (obstruction, perforation or hemorrhage)
(9,10).
However, the treatment of choice for primary tumors
remain debatable. To the best of our knowledge, no randomized
controlled trials have yet addressed this clinical question. The
results currently published in meta-analyses, clinical series and
population-based studies provide unclear conclusions (11-18).
The lack of homogeneity of the compared groups and in the control
of clinicopathological variables that affect the outcome have
limited the conclusions. Therefore, it is necessary to identify
prognostic factors that allow to selectively indicate therapeutic
strategies.
The present study analyzed the survival of patients
with stage IV CRC with bilateral liver metastases. A homogeneous
group of patients with good functional status and without
multifocal carcinomatosis has been analyzed. The aim was to
identify the clinical and histopathological prognostic factors that
influenced survival.
Materials and methods
Retrospective cohort study
Patients diagnosed with stage IV CRC with multiple
(>3 metastases), bilateral and synchronous liver metastases,
treated between January 2013 and December 2018, were included in
the present study. The patients were selected from the data
collected in the computerized file of the Coloproctology Unit of
Príncipe de Asturias Teaching Hospital, Alcalá de Henares, Spain,
which was performed prospectively during this period of time. The
present study was approved by the Ethics Committee of the Príncipe
de Asturias Teaching Hospital.
The inclusion criteria were as follows: i) Patients
aged between 18-75 years; ii) primary tumor with histopathology of
colorectal adenocarcinoma, without previous oncological treatment;
iii) resectable primary tumor; iv) presence of liver metastases
detected by CT or MRI scans, bilateral and multiple, which ensured
that the tumor was not surgically resectable; v) absence of
peritoneal carcinomatosis, central nervous system or bone
metastases; and vi) Eastern Cooperative Oncology Group (ECOG)
performance status 0-2. Those patients who presented with
locoregional recurrence and those who developed liver metastases
during follow-up were excluded.
The diagnosis of colorectal adenocarcinoma was made
by histopathological examination of the biopsy obtained via
colonoscopy. Levels of carcinoembryonic antigen (CEA) and CA 19-9,
and the biochemical parameters, were determined at the time of
diagnosis. Thoracic, abdominal and pelvic CT were performed for the
staging of distant metastases. Rectal tumors were staged after MRI
and endorectal ultrasound. Each patient was discussed by the
multidisciplinary team and the metastatic disease was considered to
be unresectable. KRAS mutational status (codons 12,13) was assessed
in the biopsies of tumor samples obtained by colonoscopy or in the
surgically resected tumors, and the results were categorized into
two groups: Wild-type KRAS (WT-KRAS) and mutant-type KRAS
(MT-KRAS).
Treatment
Patients with asymptomatic tumors or stenosing
tumors, but in whom a stent was implanted previously or a
derivative colostomy was performed, were initially treated with
chemotherapy. Cisplatin/irinotecan-based therapy (FOLFOX/FOLFIRI)
and bevacizumab or anti-epidermal growth factor receptor
(anti-EGFR) antibodies (cetuximab or panitumab) were administered
based on the KRAS mutation status in the tumor biopsy. Next, six
cycles of chemotherapy were programmed according with the cited
scheme. Once treatment was finished, the response of primary tumor
and metastases were evaluated via a CT scan. According to RECIST
criteria (Version 1.1) (19). In
the case of stability or partial response, PTR was performed. After
PTR, resection (segmental/lobar hepatectomy) or local non-surgical
treatment (radiofrequency, microwave ablation) of hepatic
metastases was evaluated.
Patients with tumors where it was not possible to
pass the colonoscopy beyond the primary tumor and therefore could
not implant a stent, were treated with PTR as the first measure,
followed by chemotherapy. Patients with asymptomatic tumors were
also included in this group, in which the patient and the
multidisciplinary medical team decided this measure. After surgery,
chemotherapy and follow-up were scheduled as per the previous
group.
Statistical analysis
Variables were collected in a spreadsheet of
Microsoft Excel 2019 (v.19). Statistical analysis was
performed by SPSS program (v.23) (IBM, Armonk). The present
study first described survival time in the CRC cohort of patients.
Follow-up was defined as the time between diagnosis and death, or
the last time of medical appointment. Overall survival (OS) ≤3
years after diagnosis and median survival were estimated (with 95%
CI) with each variable by using the Kaplan-Meir test. Survival
curves were compared by log-rank test. Next, the present study
focused on the association between the KRAS mutation status and
patient survival. The distribution of patient and tumor
characteristics between groups of KRAS mutations was compared using
the Kruskal-Wallis test. Finally, the effect of the KRAS mutation
on survival adjusted for these characteristics was evaluated using
a Cox proportional hazard regression. P<0.05 was considered to
indicate statistically significant.
Results
Patient characteristics
A total of 104 patients were included in the present
study, 43 women (41.3%) and 61 men (58.7%), with a mean age of
63±10 years. The clinicopathological characteristics are presented
in Table I.
 | Table IPatient and tumor characteristics and
survival estimates at 36 months after diagnosis. |
Table I
Patient and tumor characteristics and
survival estimates at 36 months after diagnosis.
| Characteristic | Patients, n (%) | Cumulative survival
at 36 months | Median survival time,
months | P-value | HR | 95% CI |
|---|
| Sex | | | | 0.005 | | |
|
Male | 61 (58.7) | 34 | 28 | | 1 | |
|
Female | 43 (41.3) | 9 | 21 | | 1.96 | 1.21-3.20 |
| Age, years | | | | 0.190 | | |
|
<60 | 34 (32.7) | 29 | 27 | | 1 | |
|
≥60 | 70 (67.3) | 21 | 23 | | 1.41 | 0.82-2.45 |
| ECOG | | | | 0.001 | | |
|
0 | 38 (36.5) | 42 | 35 | | 1 | |
|
1-2 | 66 (63.5) | 16 | 21 | | 2.77 | 1.5-5.10 |
| Localization | | | | 0.025 | | |
|
Right
colon | 20 (19.2) | 13 | 17 | | 1.96 | 1.03-3.72 |
|
Left
colon | 48 (46.2) | 28 | 25 | | 0.90 | 0.51-1.59 |
|
Rectum | 36 (34.6) | 24 | 27 | | 1 | 1 |
| Charlson index | | | | 0.370 | | |
|
≤8 | 53(51) | 24 | 27 | | 1 | |
|
>8 | 51(49) | 23 | 21 | | 1.41 | 0.82-2.41 |
| T stage | | | | 0.030 | | |
|
T2-3 | 70 (67.3) | 27 | 28 | | 1 | |
|
T4 | 34 (32.7) | 16 | 20 | | 1.7 | 1.03-2.90 |
| N stage | | | | 0.470 | | |
|
N0 | 19 (18.3) | 29 | 27 | | 1 | |
|
N1-2 | 85 (81.7) | 22 | 24 | | 1.25 | 0.65-2.40 |
| Therapeutic
program | | | | 0.510 | | |
|
Surgery
first | 44 (42.3) | 19 | 23 | | 1 | |
|
Chemotherapy
first | 60 (57.7) | 29 | 25 | | 0.85 | 0.52-1.38 |
| Resection primary
tumor | | | | 0.420 | | |
|
Yes | 74 (71.2) | 25 | 25 | | 1 | |
|
No | 30 (28.8) | 21 | 21 | | 1.23 | 0.72-2.09 |
| Lung
metastases | | | | 0.990 | | |
|
Absent | 85 (81.7) | 22 | 24 | | 1 | |
|
Present | 19 (18.3) | 27 | 26 | | 0.99 | 0.54-1.83 |
| Resection/local
treatment | | | | 0.001 | | |
| hepatic
metastases | | | | | | |
|
Yes | 28 (29.9) | 46 | 35 | | 0.32 | 0.15-0.65 |
|
No | 76 (73.1) | 17 | 21 | | 1 | |
| KRAS status | | | | 0.001 | | |
|
Native | 53(51) | 42 | 30 | | 1 | |
|
Mutated | 51(49) | 9 | 21 | | 2.33 | 1.39-3.9 |
| Grade of
differentiation | | | | 0.290 | | |
|
Poorly
differentiated | 19 (19.3) | 23 | 17 | | 1 | |
|
Well-moderately
differentiated | 85 (81.7) | 24 | 23 | | 1.39 | 0.74-2.60 |
| Histologic
type | | | | 0.190 | | |
|
Classical
adenocarcinoma | 96 (92.3) | 24 | 25 | | 1 | |
|
Mucinous | 6 (7.7) | 19 | 17 | | 0.55 | 0.22-1.39 |
| CEA,ng/ml | | | | 0.110 | | |
|
≤10 | 30 (28.8) | 36 | 30 | | 1 | |
|
>10 | 74 (71.2) | 19 | 23 | | 1.59 | 0.86-2.93 |
| CA19-9, U/ml | | | | 0.030 | | |
|
≤37 | 48 (46.2) | 35 | 30 | | 1 | |
|
>37 | 56 (53.8) | 15 | 21 | | 1.67 | 1.02-2.30 |
| GOT, U/l | | | | 0.053 | | |
|
≤34 | 63 (60.6) | 28 | 28 | | 1 | |
|
>34 | 35 (39.4) | 18 | 21 | | 1.59 | 0.97-2.60 |
| AF, U/l | | | | 0.120 | | |
|
≤120 | 55 (52.9) | 30 | 27 | | 1 | |
|
>120 | 49 (46.2) | 17 | 24 | | 1.12 | 0.88-2.36 |
Of these patients, 60 (57.7%) were treated with
chemotherapy as the first measure, and 44 (42.3%) with RTP as first
measure. In total, PTR was performed on 74 patients (71.1%) and 30
(28.9%) were treated with chemotherapy only. The location of the
primary tumor was in the rectum in 36 patients (34.6%), in the left
colon in 48 patients (46.2%) and in the right colon in 20 patients
(19.2%). A mutation in the KRAS gene was detected in 51 patients
(49%). In 38 cases (36.5%), the ECOG Index was 0. The Charlson
Comorbidity Index was >8 in 51 (49%) patients. Resection/local
treatment of liver metastases (THM) could be performed in 28
patients (26.9%).
Patients initially treated with
chemotherapy
In this group, which was composed of 60 patients,
response/stabilization of metastatic disease was recorded in 38
patients (63.3%) and progression/appearance of new metastases in 22
(36.7%). The 38 patients who showed a response to chemotherapy were
evaluated to perform PTR, and 33 were finally operated on. Of
these, five patients were not operated on; one due to complete
response of the primary tumor; three for serious comorbidities
associated with their disease (pulmonary hypertension, ischemic
heart disease and progressive deterioration of functional status);
and one of them for presenting with serious complications to the
chemotherapy (ischemic stroke) (in this patient, a colostomy was
subsequently performed due to intestinal obstruction). Among the 33
patients that received surgery, 5 presented with unresectable local
disease at the time of the intervention.
The 22 patients in whom progression was recorded
were treated with new lines of chemotherapy. During follow-up, 8 of
them developed intestinal obstruction, 5 of them had a colostomy
performed, 1 had a stent implanted and 2 had resection of the
primary tumor performed.
Overall, in this group of patients, PTR was
performed in 30 patients and another 30 were treated with
chemotherapy alone. A total of 9 patients (30%) developed morbidity
to some extent following surgery: Two cases of intra-abdominal
infection and 7 of wound infection. No postoperative death was
observed.
THM was subsequently performed in 15 patients (25%);
3 received lobectomy and radiofrequency; 4 received segmental
resection; 6 received radiofrequency and 2 received
chemoembolization and thermoablation.
Patients initially treated by PTR
Following PTR, 14 of the 37 patients in this group
(37%) developed some complication. Of these, one died from
intra-abdominal infection and peritonitis; 6 presented with
anastomotic fistula; one with evisceration; one with prolonged
ileus and one with pneumonia; and six presented with wound
infection. In addition, 3 patients were unable to receive the
chemotherapy initially planned; one had died after the intervention
and two developed serious sequelae of post-surgical complications.
In two other patients, the start of treatment was delayed >3
months after surgery due to surgical complications. THM was
subsequently performed in 13 patients (35%); 2 received lobectomy,
2 received segmentectomy, 7 received radiofrequency and 2 received
chemoembolization.
Long-term survival
Survival, at 36 and 60 months after diagnosis, was
29 and 8%, respectively (median, 25 months) (95% CI, 21-28). The
results of the univariate analysis of survival, performed at 36
months after diagnosis by patients and tumor characteristics, is
presented in Table I. Survival was
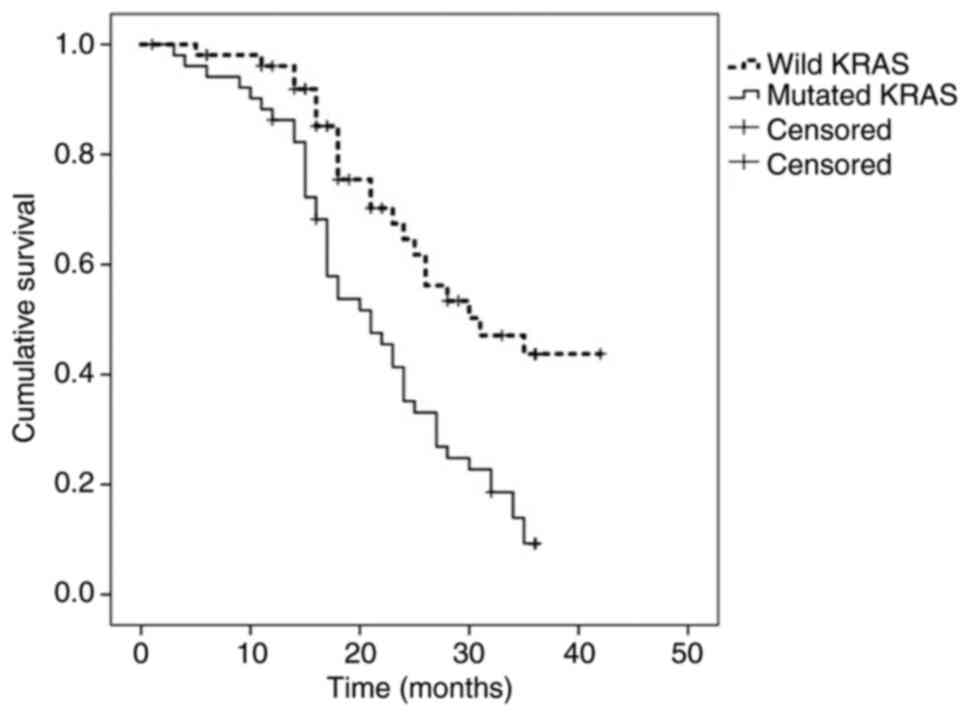
higher in patients with WT-KRAS tumors (42 vs. 9%; median, 30 vs.
21 months; P=0.001) (Fig. 1),
patients in whom THM could be performed (46 vs. 17%; median, 35 vs.
21 months; P=0.001), T2-3 tumors (27 vs. 16%; median, 28 vs. 20
months; P=0.03) and in ECOG 0 patients (42 vs. 16%; median, 35 vs.
21 months; P=0.001).
The risk of dying was significantly higher in women
(HR, 1.96), patients with ECOG Index 1-2 (HR, 2.77), tumors of the
right colon (HR, 1.96), T4 tumors (HR, 1.7), MT-KRAS tumors (HR,
2.33) and patients with CA 19-9 levels >37 ng/ml (HR, 1.67). The
risk of dying was lower when THM was performed (HR, 0.32).
Survival was not influenced by the type of treatment
(surgery versus chemotherapy). Survival in the group of patients
initially treated with chemotherapy was 29% (median, 25 months),
and was 19% among those treated with PTR as first step (median, 23
months) (P=0.51). There were also no differences in survival
regarding the action on the primary tumor; when the tumor was
resected, survival was 25% (median, 25) vs. 21% among those treated
with chemotherapy only (median, 21) (P=0.42).
Variation of survival results is
observed depending on the KRAS status
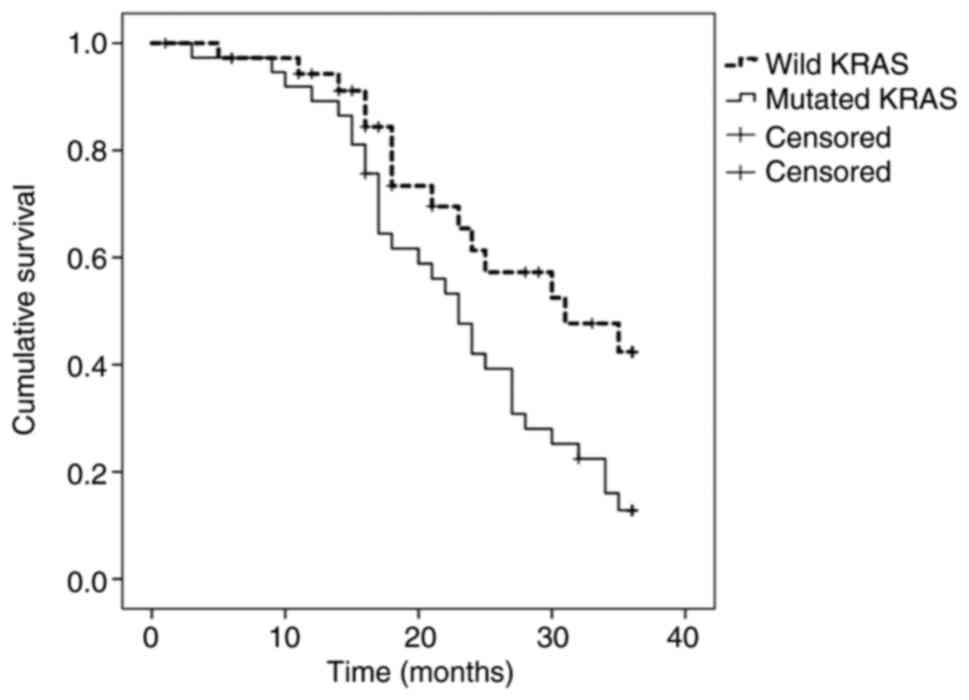
When PTR was performed, survival was 13% (median, 23
months) in MT-KRAS tumors, compared with 42% (median, 31 months) in
WT-KRAS tumors (P=0.032) (Fig. 2).
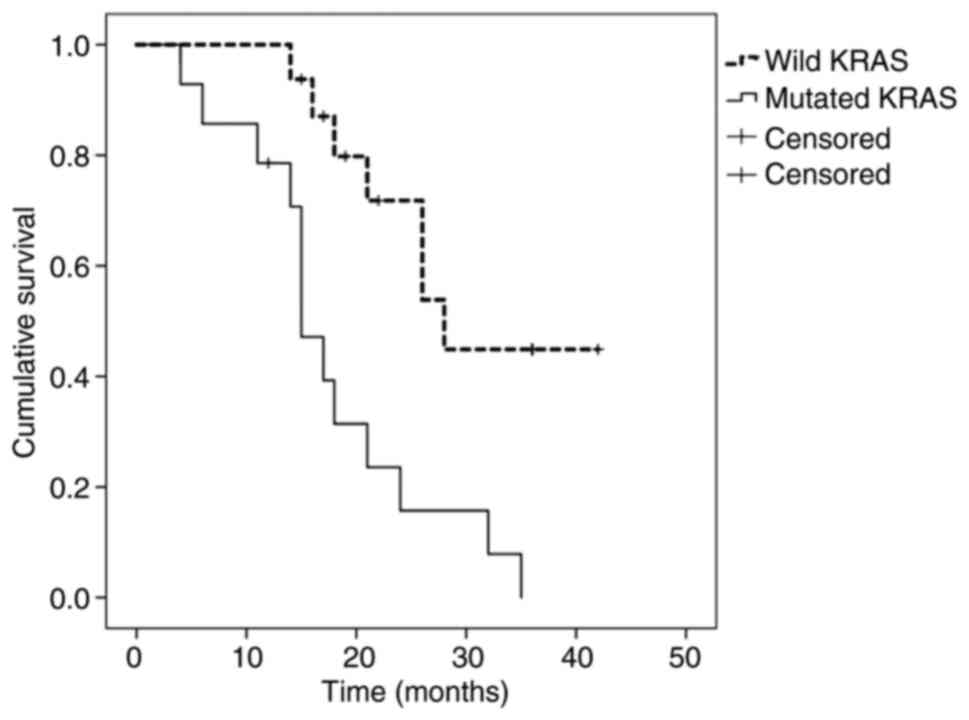
Among those treated with chemotherapy only, survival was 0%
(median, 15 months) in MT-KRAS tumors and 44% (median, 28 months)
in WT-KRAS tumors (P=0.002) (Fig.
3).
Mutation frequency in KRAS was analyzed with regard
to clinical and histopathological factors (Table II). The presence of the mutation
was more frequent in tumors of the right colon (P=0.001), in women
(P=0.01) and in patients in whom THM could be performed
(P=0.016).
 | Table IIKRAS mutation status according to
patient and tumor characteristics. |
Table II
KRAS mutation status according to
patient and tumor characteristics.
| Characteristic | Total, n | WT KRAS, n (%;
n=53) | MT KRAS, n (%;
n=51) | P-value |
|---|
| Sex | | | | 0.015 |
|
Male | 61 | 37 (60.6) | 24 (39.4) | |
|
Female | 43 | 16 (37.2) | 27 (62.8) | |
| Age, years | | | | 0.180 |
|
<60 | 34 | 20 (58.8) | 14 (41.2) | |
|
≥60 | 70 | 33 (47.2) | 37 (52.8) | |
| ECOG | | | | 0.069 |
|
0 | 38 | 24 (63.2) | 14 (36.8) | |
|
1-2 | 66 | 29 (43.9) | 37 (50.1) | |
| Tumor
localization | | | | 0.001 |
|
Right
colon | 20 | 3(15) | 17(85) | |
|
Left
colon | 48 | 27 (56.2) | 21 (43.8) | |
|
Rectum | 36 | 23 (63.9) | 13 (36.1) | |
| Charlson index | | | | 0.550 |
|
≤8 | 53 | 29 (54.7) | 24 (45.3) | |
|
>8 | 51 | 24(47) | 27(53) | |
| T stage | | | | 0.310 |
|
T2-3 | 70 | 34 (48.6) | 36 (51.4) | |
|
T4 | 34 | 19 (55.9) | 15 (44.1) | |
| N stage | | | | 0.270 |
|
N
negative | 19 | 8 (42.1) | 11 (57.9) | |
|
N
positive | 85 | 45(53) | 40(47) | |
| Treatment
program | | | | 0.020 |
|
Surgery
first | 60 | 36(60) | 24(40) | |
|
Chemotherapy
first | 44 | 17 (38.6) | 27 (61.4) | |
| Primary tumor
resection | | | | 0.460 |
|
Yes | 74 | 37(50) | 37(50) | |
|
No | 30 | 16 (53.3) | 14 (46.7) | |
| Lung
metastases | | | | 0.530 |
|
Absent | 85 | 43 (50.6) | 42 (49.4) | |
|
Present | 19 | 10 (52.6) | 9 (47.4) | |
| Resection/local
treatment hepatic metastases | | | | 0.016 |
|
No | 76 | 35(46) | 41(54) | |
|
Yes | 28 | 19 (67.9) | 9 (32.1) | |
| Grade of
differentiation | | | | 0.460 |
|
Well-moderately
differentiated | 85 | 44 (52.8) | 41 (48.2) | |
|
Poorly
differentiated | 19 | 9 (47.3) | 10 (52.7) | |
| CEA, ng/ml | | | | 0.300 |
|
≤10 | 30 | 17 (56.6) | 13 (43.4) | |
|
>10 | 74 | 36 (48.7) | 38 (51.3) | |
| CA19-9, U/l | | | | 0.009 |
|
≤37 | 48 | 31 (64.5) | 17 (35.5) | |
|
>37 | 56 | 22 (39.3) | 34 (60.7) | |
| GOT, U/l | | | | 0.280 |
|
<34 | 63 | 34(54) | 29(46) | |
|
>34 | 41 | 19 (46.3) | 22 (53.7) | |
| AF, U/l | | | | 0.420 |
|
≤120 | 55 | 29 (52.7) | 26 (47.3) | |
|
>120 | 49 | 24(49) | 25(51) | |
In the multiple regression analysis (Table III), the factors that showed an
independent predictive value were: The presence of the KRAS
mutation (HR, 2.484; 95% CI, 1.472-4.192), T4 tumors (HR, 1.795;
95% CI, 1.045-3.084), THM (HR, 0.447; 95% CI, 0.222-0.901) and ECOG
1-2 (HR, 1.632; 95% CI, 1.182-2.254).
 | Table IIIPredictive factors of survival
analyzed using Cox's proportional hazards model. |
Table III
Predictive factors of survival
analyzed using Cox's proportional hazards model.
| | 95.0% confidence
interval |
|---|
| Factor | P-value | Hazard ratio | Inferior | Superior |
|---|
| KRAS mutation | 0.001 | 2.484 | 1.472 | 4.192 |
| Resection/local
treatment of hepatic metastases | 0.024 | 0.447 | 0.222 | 0.901 |
| T4 stage | 0.034 | 1.795 | 1.045 | 3.084 |
| ECOG Performance
1-2 | 0.003 | 1.632 | 1.182 | 2.254 |
Discussion
There is no consensus on the indication of PTR in
patients with stage IV CRC with bilateral and synchronous liver
metastasis. This controversy comes from the absence of reliable
prognostic factors that allow to selectively indicate therapeutic
strategies (3,13). In the present study, the two types
of treatment issued, chemotherapy or PTR, offered similar influence
on survival.
The present study indicated that KRAS mutation
status, T4 stage, THM performance and ECOG Index, have independent
predictive values on the survival of patients with stage IV CRC.
Among them, the KRAS mutation exhibited greater predictive
importance. Patients with MT-RAS tumors had a 2.48-times higher
risk of dying than patients with WT-RAS tumors, and the OS at 36
months was lower (9 vs. 42%) (P=0.001). However, the most important
data in our study was the finding that the results of the treatment
varied depending on the KRAS status. When RPT was performed, the
survival at 36 months was 13% in MT-KRAS tumors versus 42% in WT
KRAS tumors (P=0.032). Likewise, among those treated with
chemotherapy only, the 36-month survival was 0% in MT-RAS tumors
and 44% in WT-KRAS tumors (P=0.002).
KRAS is a key component of the EGFR signal
transduction pathway, affecting the proliferation and angiogenesis
of the tumor cells. KRAS mutations are present in ~40% of CRC
cases. A mutated KRAS gene is constitutively activated, enabling
the downstream effects of the EGFR signaling pathway independent of
EGFR activation by the ligand, thus leading to uninterrupted
proliferation (17). Identification
of KRAS status is decisive to indicate EGFR-targeted agents
(18).
Previous publications have also reported that the
presence of the KRAS mutation is significantly associated with
decreased survival in stage IV CRC patients (20-22).
The results of those studies indicate that the worst prognosis for
patients with MT-KRAS tumors was independent of chemotherapy
treatment and was due to more aggressive tumor behavior.
In the present study, the KRAS mutation was more
frequent in women and in tumors located in the right colon. These
findings are in line with data published in previous studies. In
addition, some studies have found that survival in patients with
tumors of the right colon is lower than that observed in patients
with tumors of the left colon and rectum, and this difference is
particularly significant in patients with stage IV disease
(23,24). It is thought that the worst
progression of proximal tumors could be attributed in part to the
higher frequency with which genetic alterations, such as BRAF and
KRAS mutations, microsatellite instability and CpG island
methylator phenotype, are detected (25,26).
The choice of treatment in patients with stage IV
CRC with bilateral and synchronous liver metastasis must be
individualized. It seems appropriate to indicate PTR as the first
therapeutic measure for patients with symptomatic tumors
(obstruction, perforation or bleeding). In other cases, the choice
must be based on other aspects. Tumor burden, comorbidities and
patient functional status are objective and useful parameters.
However, the data from the present study indicate that the genetic
profile of the tumor must also be taken into account, particularly
the mutation status of the KRAS gene. The negative influence on the
prognostic of survival that MT-RAS has demonstrated, may have a
practical application to advise on the survival that can be
expected in each patient, and thus it may be an aid to provide a
specific treatment. Our data indicate that KRAS status may help to
differentiate subgroups of patients with different prognostic of
survival. This information would be useful to adjust the
therapeutic effort in each patient. Hypothetically, traumatic RPT
surgery could be avoided in asymptomatic MT-RAS tumors, since this
act will not provide an improvement in survival.
The main limitations of the present study are the
small sample size and the retrospective design. We know that it is
impossible to obtain firm conclusions with such a reduced number of
patients, although the homogeneity of the population studied is in
its favor. But we think that the results obtained are clinically
significant. If MT-KRAS has shown a strong predictive effect with a
reduced sample of patients, it is indicative of the real value that
it owns as prognostic factor of survival. However, it is necessary
to perform prospective studies with a higher number of
patients.
The results of the present study indicate that the
mutational status of the KRAS gene is connected with survival and
may be a useful prognostic parameter in patients with stage IV CRC
with multiple and synchronous liver metastases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MDA and FMM were involved in study concept and
design. MDA, FMM, LJA and ON have contributed to the analysis of
data, and the preparation and drafting of the manuscript. ABM, ASG,
ASJ, BMG, RM and AGC made substantial contributions to acquisition
of data and database compilation. MDA and FMM were responsible for
assessing the authenticity of all raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics and
Clinical Trials Committee of the Príncipe de Asturias Teaching
Hospital (Madrid, Spain).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leporrier J, Maurel J, Chiche L, Bara S,
Segol P and Launoy G: A population-based study of the incidence,
management and prognosis of hepatic metastases from colorectal
cáncer. Br J Surg. 93:464–474. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Krell RW and D'Angelica M: Treatment
sequencing for simultaneous colorectal liver metastases. J Surg
Oncol. 119:583–593. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cirocchi R, Trastulli S, Abraha I,
Vettoretto N, Boselli C, Montedori A, Parisi A, Noya G and Platell
C: Nonresection versus resection for asymptomatic primary tumour in
patients with unresectable stage IV colorectal cáncer. Cochrane
Database Syst Rev. 8(CD008997)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aslam MI, Kelkar A, Sharpe D and Jameson
JS: Ten years' experience of managing the primary tumours in
patients with stage IV colorectal cancers. Int J Surg. 8:305–313.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sanoff HK, Sargent DJ, Campbell ME, Morton
RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC
and Goldberg RM: Five-year data and prognostic factor analysis of
oxaliplatin and irinotecan combinations for advanced colorectal
cáncer: N9741. J Clin Oncol. 26:5721–5727. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initialtreatment for
metastatic colorectal cáncer. N Engl J Med. 360:1408–1417.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahmed S, Shahid RK, Leis A, Haider K,
Kanthan S, Reeder B and Pahwa P: Shoudnoncurative resection of the
primary tumor be performed in patients with stage IV colorectal
cáncer? A systematic review and meta-analysis. Curr Oncol.
20:e420–441. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stillwell AP, Buettner PG and Ho YH:
Meta-analysis of survival of patients with stage IV colorectal
cáncer managed with surgical resección versus chemotherapy alone.
World J Surg. 34:797–807. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cáncer. Ann Oncol.
27:1386–1422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Messersmith WA: NCCN guidelines updates:
Management of metastatic colorectal cancer. J NatlCompr Cancer
Netw. 17:599–601. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alawadi Z, Phatak UR, Hu CY, Bailey CE,
You YN, Kao LS, Massarweh NN, Feig BW, Rodriguez-Bigas MA, Skibber
JM and Chang GJ: Comparative effectiveness of primary tumor
resection in patients with stage IV colon cáncer. Cancer.
123:1124–1133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee K, Ou Y, Hu WH, Liu CC and Chen HH:
Meta-analysis of outcomes of patients with stage IV colorectal
cáncer managed with chemotherapy/radiochemotherapy with and without
primary tumor resection. Onco Targets Ther. 9:7059–7069.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu CY, Bailey CE, You YN, Skibber JM,
Rodriguez-Bigas MA, Feig BW and Chang GJ: Time trend analysis of
primary tumor resection for stage IV colorectal cáncer: Less
surgery, improved survival. JAMA Surg. 150:245–251. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tarantino I, Warschkow R and Güller U:
Palliative primary tumor resection in patients with metastatic
colorectal cancer: For whom and when? Ann Surg. 265:e59–e60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mehta HB, Vargas GM, Adhikari D, Dimou F
and Riall TS: Comparative effectiveness of chemotherapy vs
resection of the primary tumour as the initial treatment in older
patients with stage IV colorectal cáncer. Colorectal Dis.
19:O210–O218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tominaga T, Nonaka T, Shiraisi T, Hamada
K, Noda K, Takeshita H, Maruyama K, Fukuoka H, Wada H, Hashimoto S,
et al: Factors related to short-term outcomes and delayed systemic
treatment following primary tumor resection for asymptomatic stage
IV colorectal cáncer. Int J Colorectal Dis. 35:837–846.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rehman A, Jones RP and Poston G:
Prognostic and predictive markers in liver limited stage IV
colorectal cáncer. Eur J Surg Oncol. 45:2251–2256. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yarom N, Gresham G, Boame N and Jonker D:
KRAS status as a predictor of chemotherapy activity in patients
with metastatic colorectal cancer. Clin Colorectal Cancer.
18:e309–e315. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yeom SS, Lee SY, Kwak HD, Kim CH, Kim YJ
and Kim HR: The outcome of primary tumor resection in the
unresectable stage IV colorectal cáncer patients who received the
bevacizumab-containing chemotherapy. Medicine (Baltimore).
99(e19258)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Andreyev HJ, Norman AR, Cunningham D,
Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N,
et al: Kirsten ras mutations in patients with colorectal cáncer:
The RASCAL II study. Br J Cancer. 85:692–696. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Phipps AI, Buchanan DD, Makar KW, Win AK,
Baron JA, Lindor NM, Potter JD and Newcomb PA: KRAS-mutation status
in relation to colorectal cancer survival: The joint impact of
correlated tumour markers. Br J Cancer. 108:1757–1764.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tapia Rico G, Price T, Tebbutt N,
Hardinghamm J, Lee C, Buizen L, Wilson K, Gebski V and Townsend A:
Right or left primary site of colorectal cancer: Outcomes from the
molecular analysis of the AGITG MAX trial. Clin Colorectal Cancer.
18:141–148. 2018.
|
|
24
|
Ulanja MB, Rishi M, Beutler BD, Sharma M,
Patterson DR, Gullapalli N and Ambika S: Colon cancer sidedness,
presentation and survival at different stages. J Oncol.
2019(4315032)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Imamura Y, Morikawa T, Liao X, Lochhead P,
Kuchiba A, Yamauchi M, Qian ZR, Nishihara R, Meyerhardt JA, Haigis
KM, et al: Specific mutations in KRAS codons 12 and 13, and patient
prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer
Res. 18:4753–4763. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rasmy A, Fayed A, Omar A and Fahmy N:
Effect of KRAS mutational status on disease behavior and
treatment outcome in patients with metastatic colorectal cáncer:
Intratumor heterogeneity and mutational status. J
GastrointestOncol. 10:886–895. 2019.PubMed/NCBI View Article : Google Scholar
|