Introduction
Breast cancer is one of the most common cancers in
women worldwide (1,2). Triple-negative breast cancer (TNBC) is
characterized by the lack of estrogen receptor (ER), progesterone
receptor (PgR), and human epidermal growth factor receptor-2 (HER2)
expression. TNBC is reported to account for 10-15% of all sporadic
breast cancers. Compared to non-TNBCs, they are generally larger,
show higher grade, have lymph node involvement at the time of
diagnosis, and are biologically more aggressive (3-5).
In previous studies, 20-56% of patients with TNBC were reported to
achieve a pathological complete response (pCR) after neoadjuvant
chemotherapy (NAC). Despite higher response rates to NAC, the
patients with TNBC who did not achieve pCR had a higher rate of
distant recurrence and poorer prognosis compared to the non-TNBC
group. Disease-free survival (DFS) of patients with TNBC was
reported to be 5060% (6-13).
However, the median follow-up periods in those reports were
relatively short (3-6 years), and the long-term overall survival of
patients with TNBC who did not achieve pCR have not been reported.
Furthermore, reports of a pooled analysis included various regimens
for NAC.
Gene expression profiling has established several
distinct breast cancer molecular subtypes, including luminal A and
B, HER2-positive, basal-like, and claudin-low (14-16).
TNBCs account for 39-54% of basal-like and 25-39% of claudin-low
cases. Each breast cancer is associated with different clinical
outcomes, biological features, and treatment responses (17). Epithelial-derived cancers often show
high or low expression of claudins. These proteins are the most
important structural and functional components of tight junction
integral membrane proteins. However, the association between
claudin expression and prognosis is unknown (18).
In this study, we retrospectively investigated the
long-term overall survival of patients with TNBC who received NAC
and their prognostic factors including basal marker and claudin
expression.
Patients and methods
Patients and treatment
We retrospectively reviewed the records of 323
consecutive breast cancer patients who received NAC at the Jikei
University Hospital between November 2005 and March 2012. This
study was conducted in accordance with the Helsinki Declaration of
1975, as revised in 1983, and was approved by the Ethics Committee
of the Jikei University School of Medicine; patient consent was
also obtained. We evaluated the age of patients,
clinicopathological characteristics such as clinical tumor size and
clinical lymph node status before NAC, ER, PgR, and HER2
expression, pathological tumor size, pathological lymph node
status, lymphovascular infiltration, epidermal growth factor
receptor (EGFR), cytokeratin (CK) 5/6 as the basal marker,
claudin-3 expression, and survival data for the patients.
Lymphovascular infiltration was evaluated with the operative sample
after NAC. A clinically node-positive axilla was defined as the
presence of palpable mass in the nodal basin and, when assessed
using ultrasound, magnetic resonance imaging, or computed
tomography images, the presence of abnormal lymph nodes. When these
had a suspected cancerous appearance on images, positivity was
confirmed via fine needle aspiration.
All patients received NAC with four cycles of
epirubicin (100 mg/m2), 5-fluorouracil (500
mg/m2), and cyclophosphamide (500 mg/m2),
followed by four cycles of docetaxel (100 or 75 mg/m2).
If patients were HER2-positive, trastuzumab was administered
concurrently with docetaxel and adjuvant trastuzumab for one-year
duration. A five-year adjuvant hormonal therapy was followed if the
patients were hormone receptor positive. Patients who underwent
breast conserving surgery received whole breast radiotherapy, and
regional lymph node radiotherapy was used for patients with ≥4
-positive nodes. Post-mastectomy radiation therapy was administered
to patients with initial tumors ≥5 cm or those with ≥4 positive
nodes.
Systemic and breast examinations were performed
before neoadjuvant chemotherapy, before surgery, and every 12
months postoperatively using chest and abdominal computed
tomography, mammograms, breast ultrasonography, brain magnetic
resonance imaging, and bone scans.
Pathology assessment
Immunohistochemistry (IHC) was evaluated using core
needle samples before NAC and to ensure accuracy we used positive
staining tissue as a control. IHC was performed according to the
standard protocol on 3 µm sections of formalin-fixed paraffin
embedded (FFPE) tissues using CONFIRM anti-ER rabbit monoclonal
antibody (SP1; Roche Diagnostics Ltd.) for ER staining and CONFIRM
anti-PGR rabbit monoclonal antibody (SP1; Roche Diagnostics Ltd.)
for PgR staining. Nuclear staining ≥1% was considered positive.
HER2 expression was determined using IHC with a rabbit polyclonal
antibody (Agilent Technologies) on 4 µm sections of
paraffin-embedded tissue. A staining score of 3+
according to the HercepTest criteria was considered positive; a
result of 2+ was considered positive only if confirmed
by fluorescence in situ hybridization with an amplification
ratio of ≥2.0. Furthermore, on 3 µm FFPE tissue sections, the EGFR
and CK5/6 expression were determined using IHC with mouse
monoclonal antibodies (EGFR; dilution, 1:10; Leica Biosystems, cat.
no. EGFR-L-CE, Wetzlar, CK5/6; dilution, 1:50; Agilent
Technologies, cat. no. M7273). The expression of claudin-3 was
determined using IHC with a rabbit polyclonal antibody (dilution,
1:100; Invitrogen; Thermo Fisher Scientific, Inc. cat. no. 18-7340)
on 5 µm FFPE tissue sections. EGFR, CK5/6 and claudin-3 staining
results were considered positive if any cytoplasmic and/or
membranous invasive carcinoma cell staining was observed. If EGFR
and/or CK5/6 were positive, the sample was considered basal marker
positive.
pCR was defined as no evidence of residual tumor
cells in the breast and in the lymph nodes.
Statistical analysis
We conducted Fisher's exact test to assess the
association of clinicopathological characteristics and pCR between
the patients with and without TNBC and that between basal marker
and claudin expressions and pCR in the TNBC group. DFS was measured
from the date of surgery to the date of any recurrence or the last
follow-up. Overall survival (OS) was measured from the date of
diagnosis to the date of death or the last follow-up. The
Kaplan-Meier method was used to generate survival curves and the
cumulative incidence of events. Cramér-von Mises test was used to
assess the differences in Kaplan-Meier curves. The Cox regression
model was used to identify the potential prognostic and predictive
indicators. All significant tests were two-sided, a P-value ≤0.05
was considered statistically significant. All analyses were
performed using Stata statistical software (Stata SE 10; StataCorp
LP).
Results
Patients and tumor
characteristics
The median patients age was 53 years (range; 24-75
years). Table I shows the patient
details and tumor characteristics. TNBC accounted for 20.4% of the
total breast cancer. Age, clinical tumor size and node status
before NAC, pathological tumor size and node status, and
lymphovascular infiltration were not significantly different
between TNBC and non-TNBC patients. Among the 323 patients, 26
patients (8%) achieved a pCR including 13 patients (19.7%) with
TNBC and 13 (5.1%) without TNBC. The pCR rate of TNBC was
significantly higher than that of the non-TNBC group (P<0.001).
The reduction in tumor status was significant in patients with TNBC
(P=0.001).
 | Table IPatient and tumor characteristics by
subtype. |
Table I
Patient and tumor characteristics by
subtype.
| Characteristics | Triple-negative
(n=66) | Non Triple-negative
(n=257) | P-value |
|---|
| Age (years) | | | 0.395 |
|
<50 | 22 | 103 | |
|
≥50 | 44 | 154 | |
| Clinical tumor size
before NAC (cm) | | | 0.625 |
|
≤5 | 49 | 199 | |
|
>5 | 17 | 58 | |
| Clinical node status
before NAC | | | >0.999 |
|
Negative | 38 | 147 | |
|
Positive | 28 | 110 | |
| Clinical stage before
NAC | | | 0.285 |
|
I | 8 | 18 | |
|
II | 41 | 181 | |
|
III | 17 | 58 | |
| Estrogen
receptor | | | <0.001 |
|
Negative | 66 | 38 | |
|
Positive | 0 | 219 | |
| Progesterone
receptor | | | <0.001 |
|
Negative | 66 | 95 | |
|
Positive | 0 | 162 | |
| HER2 | | | <0.001 |
|
Negative | 66 | 178 | |
|
Positive | 0 | 79 | |
| Pathological tumor
size after NAC (cm) | | | 0.311 |
|
≤5 | 55 | 226 | |
|
>5 | 11 | 31 | |
| Pathological node
status after NAC | | | 0.249 |
|
Negative | 47 | 161 | |
|
Positive | 19 | 96 | |
| Lymphovasculer
infiltration after NAC | | | >0.999 |
|
Negative | 58 | 226 | |
|
Positive | 8 | 31 | |
| pCR | | | <0.001 |
|
Yes | 13 | 13 | |
|
No | 53 | 244 | |
| Tumor status | | | 0.001 |
|
Downstaging | 50 | 138 | |
|
Stable | 16 | 119 | |
| Nodal status | | | 0.297 |
|
Downstaging | 16 | 47 | |
|
Stable | 50 | 210 | |
Correlation between pCR and expression
of basal marker and claudin in TNBC
Core needle samples before NAC were available for
basal marker and claudin staining for 43 patients with TNBC. We
observed basal marker positivity in 25 (58.1%) cases of TNBCs and
claudin-3 positivity in 21 (48%) cases with TNBC. Table II shows the associations between
basal marker and claudin expressions and pCR. The pCR rate of basal
marker-positive tumors was 24% and that of claudin-positive tumors
was 25%. Basal marker-negative and claudin-negative tumors had the
lowest pCR rate (0%) whereas basal marker-negative and
claudin-positive tumors had the highest pCR rate (33.3%). The
association between the basal marker and/or claudin expression and
pCR rate were not statistically significant (P=0.134).
 | Table IIExpression of basal marker and
claudin, and the association between pathological complete response
rate and their expression. |
Table II
Expression of basal marker and
claudin, and the association between pathological complete response
rate and their expression.
| Marker
expression | N | pCR, n (%) | P-value |
|---|
| Basal marker | | | 0.712 |
|
Positive | 25 | 6 (24.0) | |
|
Negative | 18 | 3 (16.7) | |
| Claudin | | | 0.708 |
|
Positive | 21 | 6 (25.0) | |
|
Negative | 22 | 3 (15.8) | |
| Basal marker and
Claudin | | | 0.134 |
| Basal marker
positive, claudin positive | 15 | 3 (20.0) | |
| Basal marker
positive, claudin negative | 10 | 3 (30.0) | |
| Basal marker
negative, claudin positive | 9 | 3 (33.3) | |
| Basal marker
negative, claudin negative | 9 | 0 (0.0) | |
Survival
After a median follow-up time of 111.5 (range:
6.8-170.2) months, 23 patients showed local recurrence and 47
patients showed distant recurrence. Breast cancer related-disease
occurred in 13 (19.7%) patients with TNBC (5-year DFS: 80.3%, 95%
CI: 0.68-0.88; 10-year DFS: 80.3%, 95% CI: 0.68-0.88) and in 45
(17.5%) patients with non-TNBC (5-year DFS: 87.5%, 95% CI:
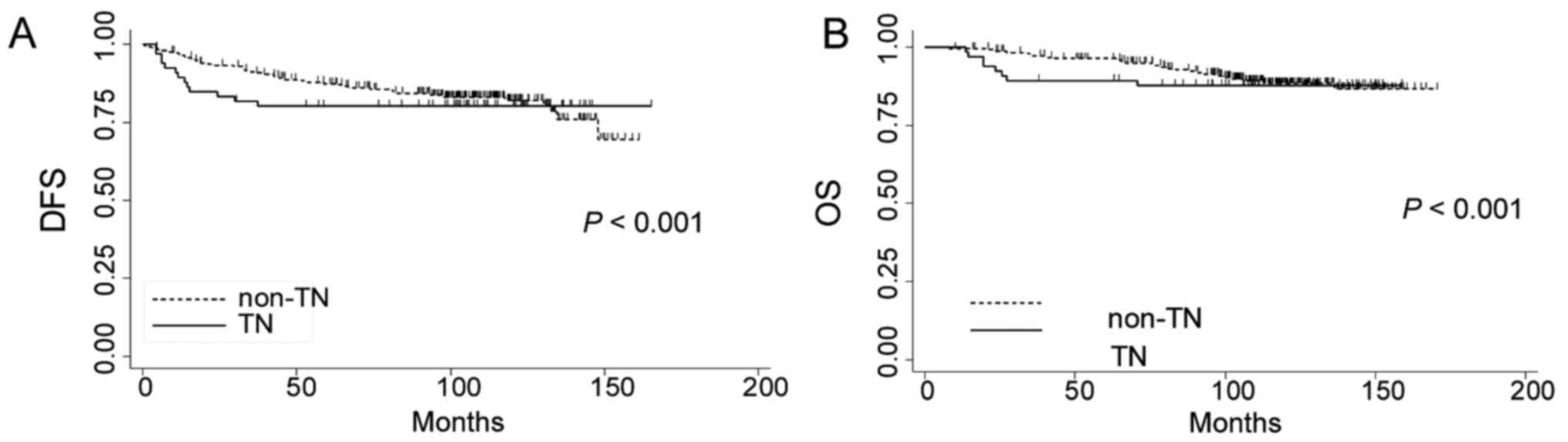
0.83-0.91; 10-year DFS 82.1%, 95% CI: 0.76-0.87). Fig. 1A shows the cumulative DFS by TNBC
versus non-TNBC (Cramér-von Mises P<0.001). Overall, 8 (12.1%)
patients with TNBC (5-year OS: 86.8%, 95% CI: 0.74-0.93; 10-year
OS: 84.8%, 95% CI: 0.72-0.92) and 26 (10.1%) patients with non-TNBC
died (5-year OS: 96.2%, 95% CI: 0.93-0.98; 10-year OS: 88.6%, 95%
CI: 0.83-0.92). Fig. 1B shows the
cumulative OS by TNBC versus non-TNBC (Cramér-von Mises
P<0.001). Table III summarizes
the results of univariate and multivariate analyses for survival.
In the univariate analysis for DFS and OS, clinical tumor size,
node status before NAC, pathological tumor size, pathological node
status, and lymphovascular infiltration were statistically
significant prognostic factors. In the multivariate analysis for
DFS, clinical and pathological tumor size, and lymphovascular
infiltration were independent prognostic factors. In the
multivariate analysis for OS, pathological node-positive and
lymphovascular infiltration were independent worse prognostic
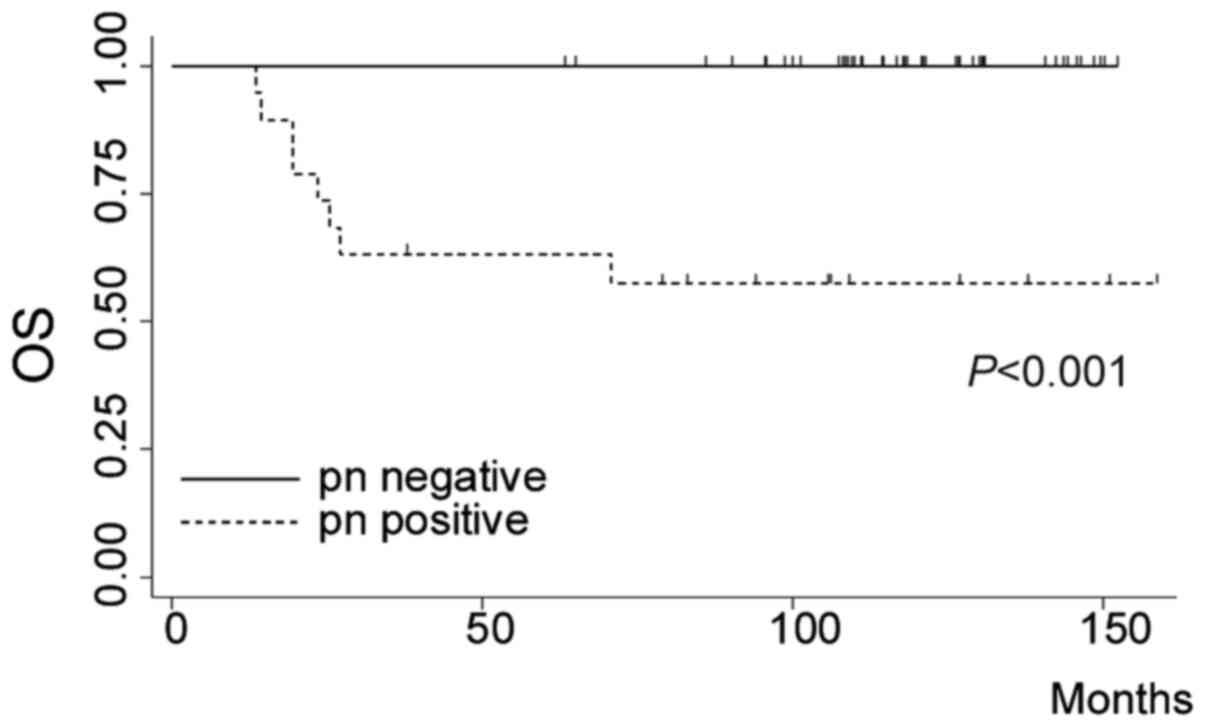
factors. Fig. 2 shows the
cumulative survival of patients with TNBC. The patients with
pathological node-negative status showed significantly good
prognosis. (log rank P<0.001). Among TNBC patients with
pathological node-positive status, negative lymphovascular
infiltration was a significant favorable prognostic factor
(Table IV).
 | Table IIIUnivariate and multivariate analyses
of patient overall survival. |
Table III
Univariate and multivariate analyses
of patient overall survival.
| | Disease-free
survival | Overall
survival |
|---|
| | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
| Parameter | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
|---|
| Age <50 vs. ≥50
years | 0.91 | 0.53-1.53 | | | 0.91 | 0.46-1.80 | | |
| Clinical tumor size
>5 vs. ≤5 cm | 3.49 | 2.08-5.85 | 2.31 | 1.30-4.10 | 3.19 | 1.62-6.26 | 2.01 | 0.96-4.21 |
| Clinical node
status positive vs. negative | 2.37 | 1.40-4.02 | 1.66 | 0.96-2.88 | 2.69 | 1.32-5.43 | 1.69 | 0.81-3.50 |
| Estrogen receptor
positive vs. negative | 0.70 | 0.41-1.20 | | | 0.57 | 0.29-1.13 | | |
| Progesterone
receptor positive vs. negative | 0.73 | 0.44-1.23 | | | 0.50 | 0.25-1.02 | | |
| HER2 positive vs.
negative | 1.33 | 0.75-2.38 | | | 1.85 | 0.91-3.74 | | |
| Subtype TN vs.
non-TN | 1.17 | 0.63-2.17 | | | 1.19 | 0.54-2.64 | | |
| pTumor size >5
vs. ≤5 cm | 4.18 | 2.41-7.27 | 2.18 | 1.18-4.03 | 4.22 | 2.09-8.54 | 1.93 | 0.89-4.16 |
| pNode status
positive vs. negative | 2.42 | 1.44-4.07 | 1.39 | 0.82-2.42 | 3.90 | 1.90-8.01 | 2.20 | 1.04-4.66 |
| Pathological
response pCR vs. non-pCR | 0.38 | 0.09-1.57 | | | 0.33 | 0.04-2.40 | | |
| Lymphovascular
infiltration positive vs. negative | 2.64 | 1.43-4.87 | 4.21 | 2.05-8.66 | 6.05 | 3.05-11.99 | 4.63 | 2.29-9.35 |
| Tumor status down
vs. stable | 0.87 | 0.52-1.47 | | | 0.79 | 0.40-1.55 | | |
| Node status down
vs. stable | 1.03 | 0.53-2.00 | | | 0.92 | 0.38-2.23 | | |
 | Table IVUnivariate analysis for the overall
survival of patients with node-positive triple-negative breast
cancer. |
Table IV
Univariate analysis for the overall
survival of patients with node-positive triple-negative breast
cancer.
| | Univariate
analysis |
|---|
| Parameter | Hazard ratio | 95% CI |
|---|
| Age <50 vs. ≥50
years | 0.98 | 0.93-1.04 |
| Clinical tumor size
≤5 vs. >5 cm | 1.22 | 0.29-5.11 |
| Pathological tumor
size ≤5 vs. >5 cm | 7.01 | 0.85-57.5 |
| Lymphovascular
infiltration negative vs. positive | 15.68 | 3.00-81.97 |
| Basal marker
expression negative vs. positive | 0.24 | 0.04-1.49 |
| Claudin expression
negative vs. positive | 0.79 | 0.13-4.74 |
Discussion
Our study showed that the long-term overall survival
of patients with TNBC who received NAC containing anthracycline and
taxane was favorable compared to that in non-TNBC patients.
Especially, patients with TNBC who achieved pathological
node-negative status after NAC survived during the median follow-up
of 111.5 months.
A previous study using PAM 50 showed that the new
subtype ‘claudin-low’ had a preponderance for low to absent
expressions of E-cadherin and claudin-3, and almost all TNBC cases
were either basal-like or claudin-low (16). They showed different prognosis among
subtypes. In this study, we evaluated the efficiency of IHC for
claudin-3 and basal markers such as EGFR and CK5/6 to predict pCR
and prognosis instead of gene profiling. The expression of
claudin-3 and basal markers was not associated with prognosis and
the response to neoadjuvant chemotherapy. IHC is an inexpensive
technique; however, it failed to substitute for the gene
profiles.
On the contrary, Lehman and colleagues reported that
TNBC could be classified into seven subtypes. These seven TNBC
subtypes were characterized based on gene ontologies and
differential gene expressions and were labeled as basal-like 1,
basal-like 2, immunomodulatory, mesenchymal, mesenchymal stem-like,
luminal androgen receptor, and unstable (19). They also reported the survival
analysis and chemotherapy response (20), and advocated the implications for
NAC according to the seven subtypes (21). In their study, basal-like 1 and
basal-like 2 showed different prognosis and response to standard
chemotherapy. The basal-like 2 subtype had unique ontologies
involving growth factor signaling and new therapeutic applications
were required.
In our study, the multivariate analyses showed that
pathological node status was an independent prognostic factor for
overall survival but not for disease-free survival. On the other
hand, clinical and pathological tumor statuses were independent
prognostic factors for disease-free survival but not for overall
survival. These contradictory results might stem from the fact that
patients who had only locoregional lymph node metastasis or breast
recurrence without distant metastasis were alive for long periods,
and that such patients had large tumors and negative lymph
nodes.
Our study showed that patients with TNBC without any
recurrence within 4 years had an excellent prognosis. For patients
with TNBC, achievement of a pathological node-negative status was
the most desirable result for improving prognosis. Pathological
node-positive status or positive lymphovascular infiltration after
NAC might be observed because of resistance to chemotherapy and may
lead to worse prognosis. Hence, we need to predict chemosensitivity
and develop specific treatments. A recent study has shown that
adjuvant capecitabine therapy improved the outcomes for patients
with TNBC without a pCR after standard NAC with anthracycline and
taxane (22). Furthermore, various
studies and clinical trials including targeted therapies, such as
tyrosine kinase inhibitors, poly ADP-ribose polymerase-1
inhibitors, immune checkpoints, anti-androgens, and histone
deacetylase inhibitors, have been conducted to improve the
prognosis of patients with TNBC (23-26).
The limitations of this study need to be considered
carefully. This is a retrospective study conducted at a single
institute and the number of patients was limited, especially in the
TNBC group. The strength of this study lies in the use of a single
regimen as the NAC and the long follow-up periods for evaluating
the survival of patients who received NAC.
In conclusion, patients with TNBC who showed no
distant recurrence within 4 years after surgery had a good
prognosis and their survival curve crossed with that of the
non-TNBC group. For the patients with TNBC, pathological
node-negative status and negative lymphovascular infiltration were
favorable prognostic factors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HN contributed to the conception, design, analysis
and integrity of the current study. MK, YT, EN and HT performed the
experiments. MS performed the pathological evaluation. NH and MK
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jikei University School of Medicine, and patient
consent was obtained.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu K, Ding P, Wu Y, Tian W, Pan T and
Zhang S: Global patterns and trends in the breast cancer incidence
and mortality according to sociodemographic indices: An
observational study based on the global burden of diseases. BMJ
Open. 9(e028461)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Malorni L, Shetty PB, De Angelis C,
Hilsenbeck S, Rimawi MF, Elledge R, Osborne CK, De Placido S and
Arpino G: Clinical and biologic features of triple-negative breast
cancers in a large cohort of patients with long-term follow-up.
Breast Cancer Res Treat. 136:795–804. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nogi H, Kobayashi T, Suzuki M, Tabei I,
Kawase K, Toriumi Y, Fukushima H and Uchida K: EGFR as paradoxical
predictor of chemosensitivity and outcome among triple-negative
breast cancer. Oncol Rep. 21:413–417. 2009.PubMed/NCBI
|
|
9
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hahnen E, Lederer B, Hauke J, Loibl S,
Kröber S, Schneeweiss A, Denkert C, Fasching PA, Blohmer JU,
Jackisch C, et al: Germline mutation status, pathological complete
response, and disease-free survival in triple-negative breast
cancer: Secondary analysis of the GeparSixto randomized clinical
trial affiliations expand. JAMA Oncol. 3:1378–1385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bonnefoi H, Litière S, Piccart M,
MacGrogan G, Fumoleau P, Brain E, Petit T, Rouanet P, Jassem J,
Moldovan C, et al: Pathological complete response after neoadjuvant
chemotherapy is an independent predictive factor irrespective of
simplified breast cancer intrinsic subtypes: A landmark and
two-step approach analyses from the EORTC 10994/BIG 1-00 phase III
trial. Ann Oncol. 25:1128–1136. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kuroi K, Toi M, Ohno S, Nakamura S, Iwata
H, Masuda N, Sato N, Tsuda H, Kurosumi M and Akiyama F: Prognostic
significance of subtype and pathologic response in operable breast
cancer; a pooled analysis of prospective neoadjuvant studies of
JBCRG. Breast Cancer. 22:486–495. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–572. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Herschkowitz JI, Simin K, Weigman VJ,
Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S,
Chandrasekharan S, et al: Identification of conserved gene
expression features between murine mammary carcinoma models and
human breast tumors. Genome Biol. 8(R76)2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12(R68)2010.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Ding L, Lu Z, Lu Q and Chen YH: The
claudin family of proteins in human malignancy: A clinical
perspective. Cancer Manag Res. 5:367–375. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Masuda H, Baggerly KA, Wang Y, Zhang Y,
Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD,
Pietenpol JA, Hortobagyi GN, et al: Differential response to
neoadjuvant chemotherapy among 7 triple-negative breast cancer
molecular subtypes. Clin Cancer Res. 19:5533–5540. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11(e0157368)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES,
Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al: Adjuvant
capecitabine for breast cancer after preoperative chemotherapy. N
Engl J Med. 376:2147–2159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Denkert C, Liedtke C, Tutt A and von
Minckwitz G: Molecular alterations in triple-negative breast
cancer-the road to new treatment strategies. Lancet. 389:2430–2442.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Damaskos C, Garmpi A, Nikolettos K,
Vavourakis M, Diamantis E, Patsouras A, Farmaki P, Nonni A,
Dimitroulis D, Mantas D, et al: Triple-negative breast cancer: The
progress of targeted therapies and future tendencies. Anticancer
Res. 39:5285–5296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nedeljković M and Damjanović A: Mechanisms
of chemotherapy resistance in triple-negative breast cancer-how we
can rise to the challenge. Cells. 8(957)2019.PubMed/NCBI View Article : Google Scholar
|