Introduction
There have been recent advances in the diagnostic
and therapeutic techniques for gastric cancer (GC), but GC remains
as the third leading cause of cancer mortality worldwide (1). Although adjuvant treatment has
prolonged the survival of GC patients, the overall survival (OS)
after surgery for GC remains poor (2,3).
Trophoblast cell-surface antigen 2 (TROP2) encoded
by tumor-associated calcium signal transducer 2
(TACSTD2) gene is a transmembrane glycoprotein expressed in
epithelial cells (4). TROP2 was
identified in human trophoblast and choriocarcinoma cell lines
(5). TROP2 was reported to bind to
claudin1, claudin7, cyclin D1, protein kinase C (PKC),
phosphatidylinositol 4,5-bisphosphate (PIP2), and insulin-like
growth factor 1 (IGF1) (5-7).
It is suspected that by its binding to these proteins, TROP2 might
affect the tight junctions of epithelial cells (8), tumor proliferation (9), podosome formation, Raf and NF-κB
activation (5,8), and IGF1 receptor (IGF1R) suppression
(10).
The expression of TROP2 was described in several
studies as being associated with the invasion and metastasis of
cancer cells, resulting in poor prognoses of GC, pancreatic cancer,
oral cancer, colon cancer, and ovarian carcinoma (11-16).
However, TROP2 was reported to have a tumor-suppressive function in
cervical cancer, lung adenocarcinoma, and head and neck squamous
cell cancer (10,17,18).
The functions of TROP2 are thus a matter of controversy.
Phosphorylation of proteins on cell membrane is important in
considering intracellular signals, but there are few reports on
phospho-TROP2 (pTROP2). In addition, there is no report about the
clinicopathologic significance of pTROP2. We conducted the present
study to clarify the clinicopathologic significance of TROP2 and
pTROP2 in GC. This study is the first to reveal the correlation
between the GC patients' clinicopathologic features and the TROP2
and pTROP2 expression in their tumors.
Materials and methods
Patients
A total of 704 patients who were histologically
confirmed to have primary GC and underwent a resection of gastric
tumor and regional lymph nodes at Osaka City University Hospital
between 1997 and 2006 were enrolled in this study. None of patients
had undergone preoperative radiation and/or chemotherapy. The
pathologic diagnoses and classifications were made according to the
UICC TNM classification of malignant tumors. This study was
approved by Osaka City University ethics committee (reference
number, 924). Informed consent was obtained in writing from all
patients.
Immunohistochemistry of TROP2 and
pTROP2
The expression of TROP2 and pTROP2 were evaluated by
immunohistochemistry. The immunohistochemical determination of them
were examined as the manufacturer's instructions. Briefly, Slides
were deparaffinized and rehydrated with xylene and graded alcohol
series and activated by heating. Endogenous peroxidase was blocked
and then sections were incubated with an anti-mouse antibody for
TROP2 (1:250, sc-376746, Santa Cruz Biotechnology) or anti-rabbit
antibody for pTROP2 (1:200, obtained from Kyoto Sangyo University).
Anti-rabbit antibody for pTROP2 was produced by the following
method. Keyhole limpet hemocyanin (KLH)-conjugated peptides (Trop-2
cytoplasmic domain) with phosphorylated Ser-322 were emulsified
with Freund's complete (first time) and incomplete (from second
time) adjuvant and injected subcutaneously five times into a
12-week-old female New Zealand white rabbit (19). After the fifth immunization, blood
was taken, and an IgG fraction was prepared from the serum by
protein A-Sepharose column chromatography. The sections were
incubated with biotinylated second antibody. They were treated with
streptavidin-peroxidase reagent and counterstained with Mayer's
hematoxylin. TROP2 expression was evaluated by intensity of
staining and percentage of stained tumor cells. Intensity was given
scores 0-3 (0, no; 1, weak; 2, moderate; 3, strong) and the
percentage of stained tumor cells in all tumor cells was given
scores 0-3 (0=0%, 1=1%-30%, 2=31%-70%, 3=71%-100%). The two scores
were multiplied to obtain the final score of 0-9. TROP2 positive
was defined as the score was ≥3. pTROP2 expression was evaluated by
intensity of staining of tumor cells and was given scores 0-3 like
TROP2. pTROP2-positive was defined as the intensity score was
≥1.
Statistical analysis
Statistical analysis was performed by R for Windows
OS (version 3. 5. 2). The association between TROP2 or pTROP2
expression and clinicopathological variables were assessed by the
chi-square test. Survival was measured from the date of surgery. OS
was analyzed by Kaplan-Meier method and compared by log-rank test.
The Cox proportional hazards model was used for univariate analysis
and multivariate analysis. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Immunostaining findings of TROP2 and
pTROP2
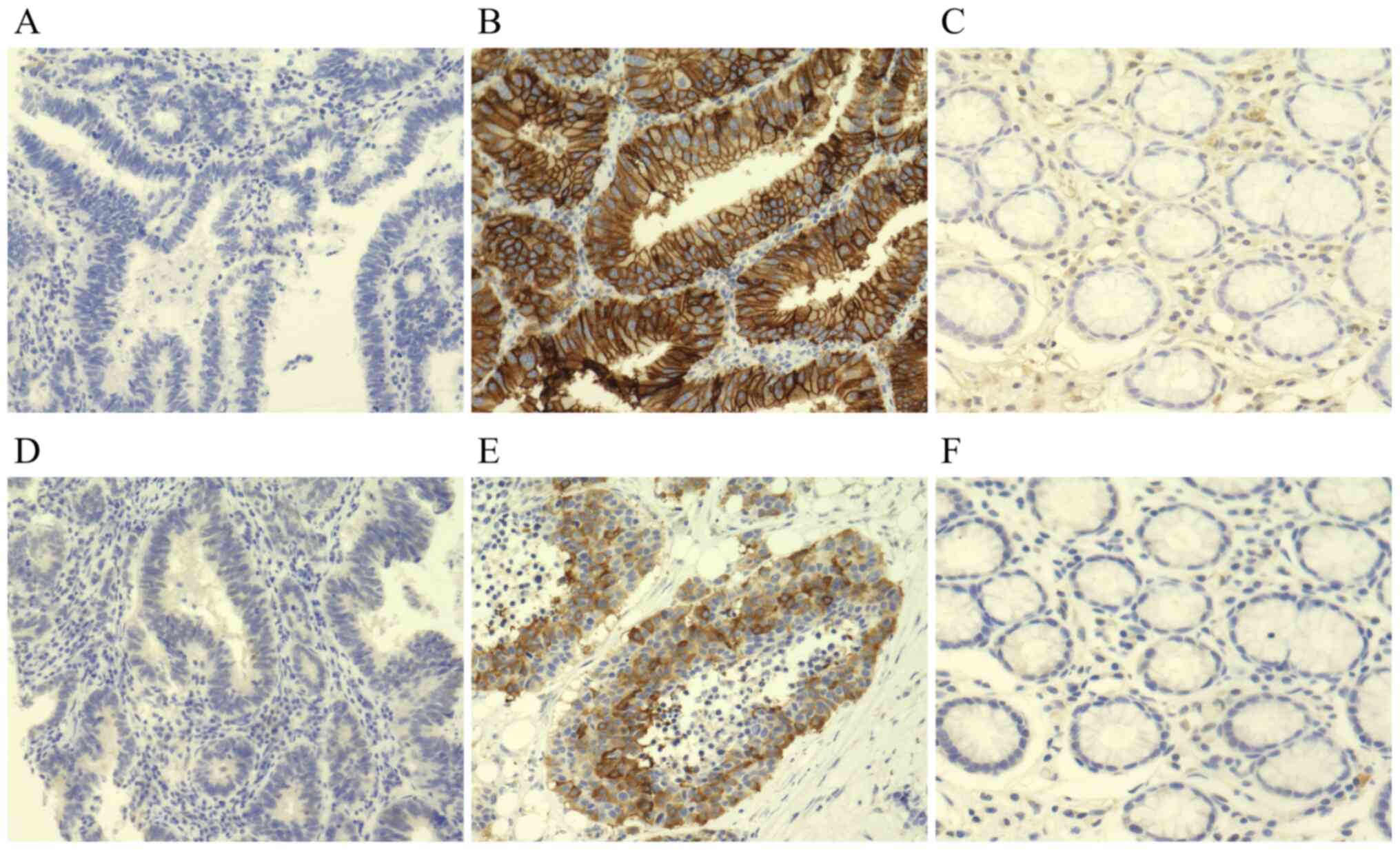
Fig. 1 provides
representative immunostaining patterns of TROP2 and pTROP2. TROP2
and pTROP2 were stained at the cytoplasm and the cell membrane of
cancer cells. TROP2 was expressed mainly at the cell membrane,
whereas pTROP2 was expressed mainly at the cell cytoplasm. Of the
total of 704 cases, 330 (46.9%) were TROP2-positive and 306 (43.5%)
were pTROP2-positive. TROP2 expression was found on stromal cells
in normal gastric tissue but not on mucosa. In contrast, neither
stromal cells nor mucosa did not express pTROP2 in normal gastric
tissue (Fig. 1C and F).
Expression levels of TROP2 and pTROP2
and their correlations with clinicopathological features
The clinicopathological features of all 704 patients
based on the TROP2 and pTROP2 expression in their cancer cells are
summarized in Table I. Compared to
TROP2 negativity of cancer cells, TROP2 positivity of cancer cells
was significantly associated with age >60 years (P<0.01),
male gender (P<0.01), differentiated type (P<0.01), tumor
depth (T3/T4) (P<0.01), lymph node metastasis (P<0.01),
lymphatic invasion (P<0.01), venous invasion (P<0.01), pTROP2
overexpression (P<0.01). The overexpression of pTROP2 was
significantly correlated with differentiated type (P<0.01),
tumor depth (T1/T2) (P<0.01), no lymph node metastasis
(P<0.01), and no lymphatic invasion (P<0.01).
 | Table IAssociation between the levels of
TROP2 and pTROP2 in tumour cells and clinicopathologic features in
704 patients with gastric cancer. |
Table I
Association between the levels of
TROP2 and pTROP2 in tumour cells and clinicopathologic features in
704 patients with gastric cancer.
| | TROP2 | | pTROP2 | |
|---|
| Clinicopathologic
features | Negative, n (%;
n=374) | Positive, n (%;
n=330) | P-value | Negative, n (%;
n=398) | Positive, n (%;
n=306) | P-value |
|---|
| Age, years | | | | | | |
|
<60 | 134 (63.51) | 77 (36.49) | 0.0003 | 120 (56.87) | 91 (43.13) | 0.9058 |
|
≥60 | 240 (48.68) | 253 (51.32) | | 278 (56.39) | 215 (43.61) | |
| Sex | | | | | | |
|
Female | 183 (59.22) | 126 (40.78) | 0.0041 | 176 (56.96) | 133 (43.04) | 0.8410 |
|
Male | 191 (48.35) | 204 (51.65) | | 222 (56.20) | 173 (43.80) | |
| Microscopic type | | | | | | |
|
Differentiated | 133 (41.82) | 185 (58.18) | <0.0001 | 147 (46.23) | 171 (53.77) | <0.0001 |
|
Undifferentiated | 241 (62.44) | 145 (37.56) | | 251 (65.03) | 135 (34.97) | |
| Tumor depth | | | | | | |
|
T1, T2 | 207 (60.00) | 138 (40.00) | 0.0003 | 173 (50.14) | 172 (49.86) | 0.0008 |
|
T3, T4 | 167 (46.52) | 192 (53.48) | | 225 (62.67) | 134 (37.33) | |
| Lymph node
metastasis | | | | | | |
|
Negative | 225 (63.03) | 132 (36.97) | <0.0001 | 184 (51.54) | 173 (48.46) | 0.0078 |
|
Positive | 146 (42.57) | 197 (57.43) | | 211 (61.52) | 132 (38.48) | |
| Lymphatic
invasion | | | | | | |
|
Negative | 181 (65.58) | 95 (34.42) | <0.0001 | 140 (50.72) | 136 (49.28) | 0.0113 |
|
Positive | 193 (45.20) | 234 (54.80) | | 258 (60.42) | 169 (39.58) | |
| Venous
invasion | | | | | | |
|
Negative | 330 (58.51) | 234 (41.49) | <0.0001 | 316 (56.03) | 248 (43.97) | 0.5869 |
|
Positive | 44 (31.43) | 96 (68.57) | | 82 (58.57) | 58 (41.43) | |
| Distant
metastasis | | | | | | |
|
Negative | 358 (53.19) | 315 (46.81) | 0.9881 | 380 (56.46) | 293 (43.54) | 0.7022 |
|
Positive | 16 (53.33) | 14 (46.67) | | 18 (60.00) | 12 (40.00) | |
| pTROP2 | | | | | | |
|
Negative | 243 (61.06) | 155 (38.94) | <0.0001 | | | |
|
Positive | 131 (42.81) | 175 (57.19) | | | | |
Survival
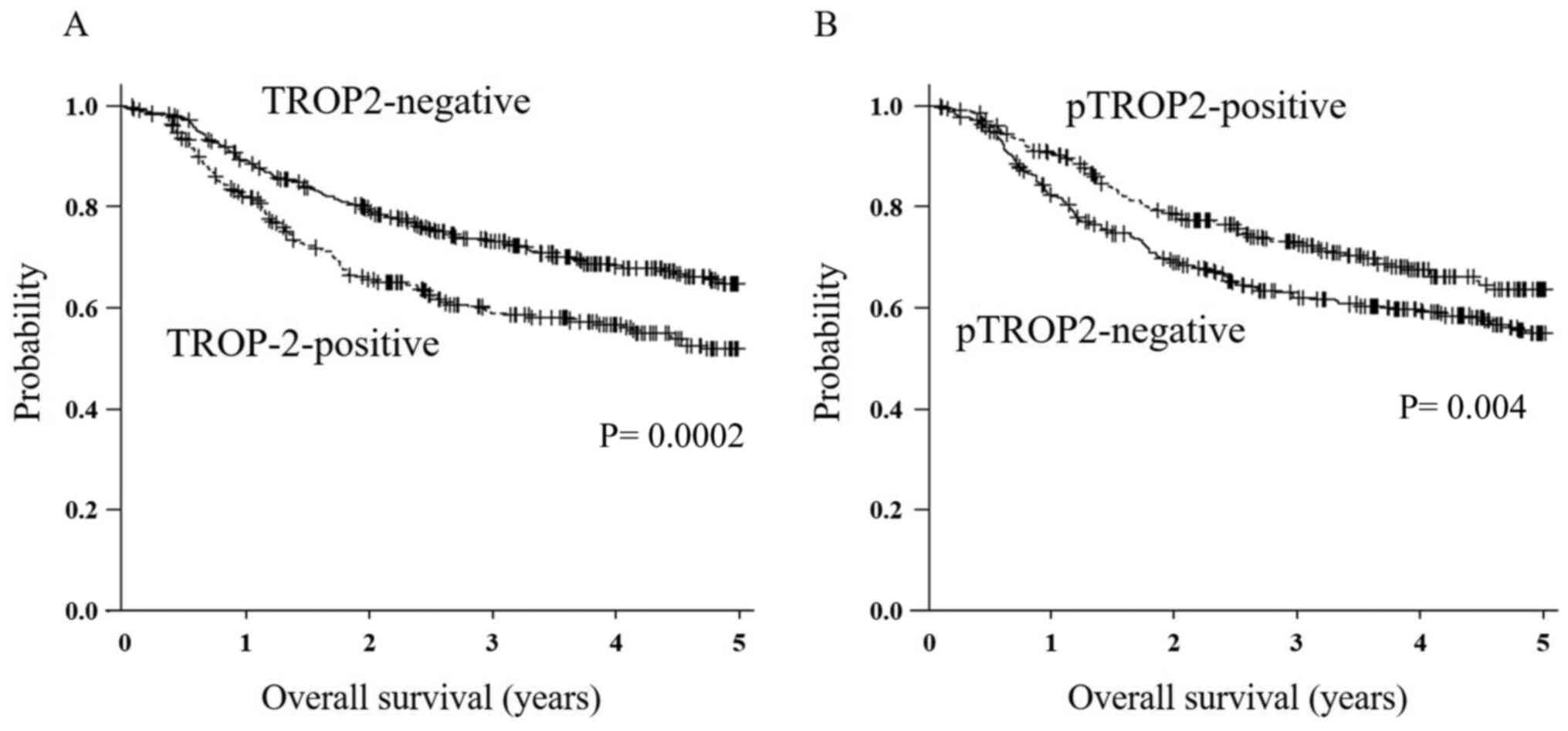
The 5-year OS rate of the 330 patients in the
TROP2-positive group was significantly poorer compared to that of
the TROP2-negative group (P<0.01, Fig. 2A). The 5-year OS rate of the
patients in the pTROP2-positive group was significantly better
compared to that of the pTROP2-negative patients (P<0.01,
Fig. 2B). Our analysis by each
tumor stage revealed that there was no significant difference in OS
between the TROP2-positive and TROP2-negative cases at each tumor
stage. The OS of the pTROP2-positive cases was not significantly
different from that of the pTROP2-negative cases at each stage. OS
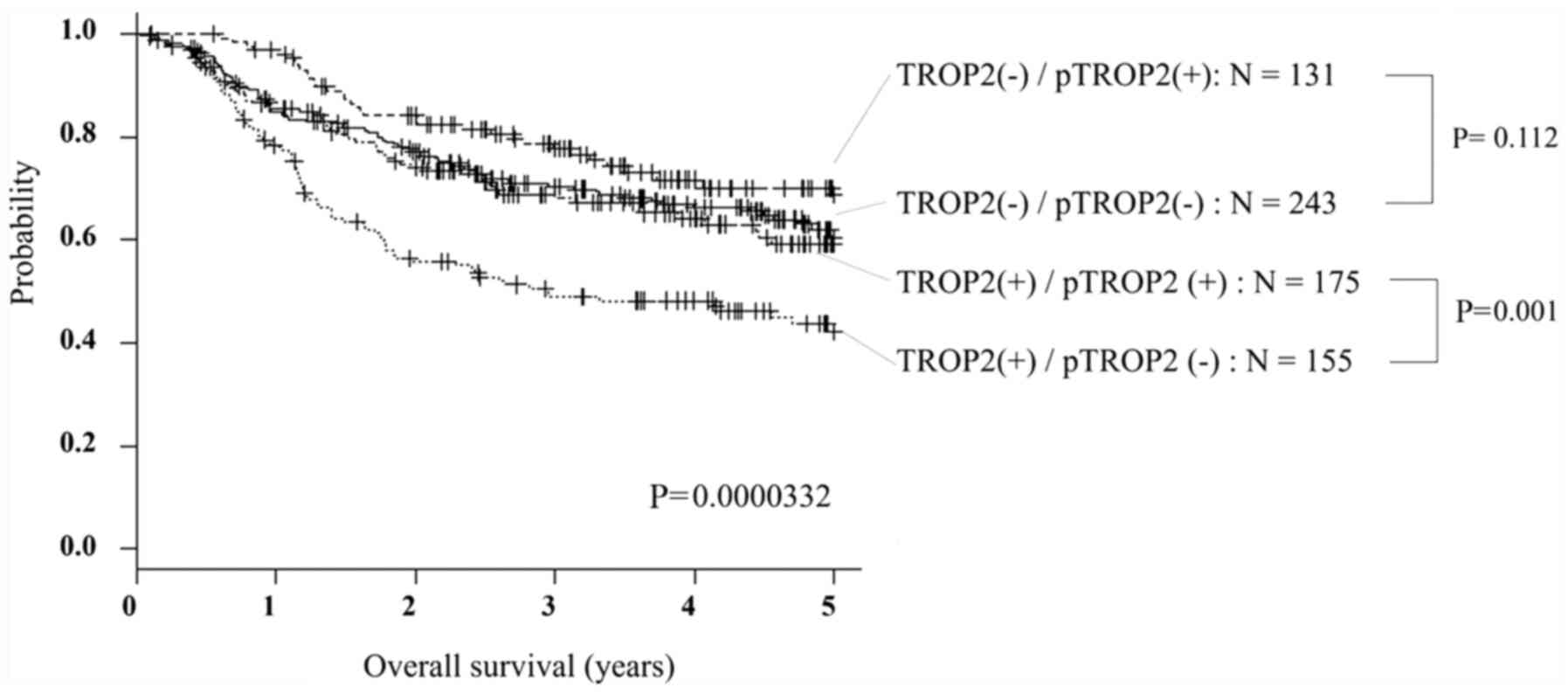
of the 4 groups divided by the expression of TROP2 and pTROP2 was
analyzed by Kaplan-Meier method and compared by log-rank test in
Fig. 3. A subgroup analysis of OS
significantly showed that patients with TROP2 (-)/pTROP2 (+) had a
good prognosis, but patients with TROP2 (+)/pTROP2 (-) had a poor
prognosis (P<0.01). Comparing by TROP2 expression, pTROP2
expression did not affect the prognosis in the case of TROP2
negative (P=0.112), but patients with pTROP2 overexpression had
significantly better prognosis in the case of TROP2-positive
(P<0.01).
Univariate and multivariate
analyses
The results of the univariate and multivariate
analyses for OS are given in Table
II. The univariate analysis showed that poor OS was
significantly correlated with undifferentiated type (P<0.01),
depth of tumor (T3 and T4) (P<0.01), lymph node metastasis
(P<0.01), distant metastasis (P<0.01), venous invasion
(P<0.01), lymphatic invasion (P<0.01), TROP2 overexpression
(P<0.01), and pTROP2 overexpression (P<0.01). There was no
significant difference in age and sex in univariate analysis. Since
univariate analysis showed a correlation between TROP2 and pTROP2
and they could be confounding factors, multivariate analysis was
performed using either TROP2 or pTROP2 and the significant factors
of univariate analysis. The multivariate analysis including TROP2
revealed that undifferentiated type (P<0.05), depth of tumor
(P<0.01), lymph node metastasis (P<0.01), and distant
metastasis (P<0.01) were significantly correlated with poorer
OS. TROP2 and lymphatic invasion were not significantly associated
with OS. The multivariate analysis including pTROP2 revealed that
depth of tumor (P<0.01), lymph node metastasis (P<0.01),
distant metastasis (P<0.01), and lymphatic invasion (P<0.05)
were significantly associated with poorer OS. pTROP2 and
microscopic type were not significantly associated with OS. In both
multivariate analyses, TROP2 and pTROP2 were not significantly
associated with poorer OS.
 | Table IIUnivariate and multivariate Cox
multiple regression analysis with respect to overall survival after
surgery in patients with gastric carcinoma. |
Table II
Univariate and multivariate Cox
multiple regression analysis with respect to overall survival after
surgery in patients with gastric carcinoma.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI)a | P-value | Hazard ratio (95%
CI)b | P-value |
|---|
| TROP2 (positive vs.
negative) | 1.562
(1.221-1.998) | 0.0004 | 1.249
(0.959-1.628) | 0.0994 | | |
| pTROP2 (positive
vs. negative) | 0.701
(0.543-0.905) | 0.0063 | | | 0.911
(0.701-1.185) | 0.4866 |
| Age (≥60 vs. <60
years) | 1.201
(0.915-1.578) | 0.1874 | | | | |
| Sex (male vs.
female) | 1.184
(0.920-1.524) | 0.1907 | | | | |
| Microscopic type
(undifferentiated type vs. differentiated type) | 1.931
(1.487-2.507) | <0.0001 | 1.415
(1.068-1.875) | 0.0156 | 1.289
(0.982-1.691) | 0.0669 |
| Tumor depth
(T3&4 vs. T1&2) | 8.262
(5.866-11.640) | <0.0001 | 3.802
(2.555-5.657) | <0.0001 | 3.762
(2.528-5.598) | <0.0001 |
| Lymph node
metastasis (positive vs. negative) | 6.022
(4.434-8.180) | <0.0001 | 2.382
(1.675-3.386) | <0.0001 | 2.463
(1.739-3.489) | <0.0001 |
| Distant metastasis
(positive vs. negative) | 5.418
(3.620-8.110) | <0.0001 | 2.543
(1.690-3.825) | <0.0001 | 2.522
(1.677-3.792) | <0.0001 |
| Lymphatic invasion
(positive vs. negative) | 5.596
(3.929-7.971) | <0.0001 | 1.477
(0.977-2.232) | 0.0646 | 1.536
(1.020-2.312) | 0.0399 |
Discussion
Our present analyses demonstrated that TROP2
overexpression was significantly associated with tumor depth, lymph
node metastasis, and vessel invasion in GC. Stoyanova et al
(20) reported that the
intracellular domain of TROP2 might stimulate cyclin D1 and c-myc.
TROP2 overexpression might be correlated with the progression of GC
via up-regulations of cyclin D1 and c-myc. In the present patient
series, the OS of the GC patients with TROP2 overexpression was
poor. The univariate analysis indicated that the patients' OS was
significantly correlated with TROP2, whereas the multivariate
analysis demonstrated that TROP2 overexpression was not correlated
with OS. These findings might indicate that TROP2's signal is
associated with the progression of GC cells, and that TROP2 could
be one of the predictive markers for poor survival of GC
patients.
We observed herein that TROP2 overexpression was
associated with the intestinal type of GC. Mühlmann et al
(12) also reported that TROP2 was
correlated with the histological intestinal type of GC. It has been
reported that adhesion molecules such as claudins and cadherins
might play an important role in the histology of cancer cells
(21,22). TROP2's signal up-regulates the tight
junctions (which are associated with histologically intestinal type
of GC), suggesting that TROP2 might be involved in the histological
formation of GC.
In contrast, our analyses revealed that pTROP2
overexpression was associated with tumor depth (T1 or T2), no lymph
node metastasis, and no lymphatic invasion, resulting in a good
prognosis. The overexpression of pTROP2 might have
tumor-suppressive functions with clinical significance that differs
from that of TROP2. Fig. 3 suggests
that although the prognosis is poor when TROP2 is overexpressed,
phosphorylation of TROP2 has a function of reducing cancer
malignancy. Sin et al (17)
reported that TROP2 suppressed IGF1R and ALK signaling as a
tumor-suppressing function. The mechanism of this suppression is
that IGF1 and midkine bind to TROP2 and inhibit the signals of
IGF1R and ALK, which play critical roles in cell growth,
differentiation, transformation, and metastasis (23-25).
Mori et al (19) demonstrated that in colon cancer
cells, PKCα and PKCδ were involved in TROP2 phosphorylation, and
TROP2 phosphorylation changed the localization of claudin7 and
promoted cell motility. TROP2 phosphorylation may have a
suppressive effect on GC, and pTROP2 may inhibit the IGF1R and ALK
signal pathway. The clinicopathologic significance of pTROP2 might
differ among cancer types. Taken together, the above-described
findings and our present results suggest that the phosphorylation
of TROP2 may restrain tumor progression in GC.
Currently, several clinical trials using the
therapeutic agents against TROP2, DS-1062 and IMMU-132, are ongoing
in lung cancer (DS-1062, NCT 03401385), urothelial cancer
(IMMU-132, NCT 03547973), and triple negative breast cancer
(IMMU-132, NCT 04230109). It was reported that IMMU-132 had
efficacy in a heavily pretreated population of patients with
metastatic triple-negative breast cancer (26). Phase III study of IMMU-132 in
patients with metastatic triple negative breast cancer is in
progress (NCT 02574455). Our data suggest that a clinical trial
using these agents might be useful for GC patients with TROP2
expression. In this study, we analyzed the clinicopathological
features of TROP2 and pTROP2, and conclude the data as clinical
significance in patients with GC. We would like to clarify the
effect of TROP2 inhibitors on the proliferation of GC cell lines
in vivo and in vitro in future.
In conclusion, TROP2 might be associated with the
tumor progression of GC cells, resulting in poor prognoses of
patients with GC. pTROP2 might be associated with a
tumor-suppressing function.
Acknowledgements
The authors would like to thank Ms. Akiko Tsuda
(Department of Molecular Oncology and Therapeutics, Osaka City
University Graduate School of Medicine, Osaka 545-8585, Japan) for
technical support of immunohistochemistry. The abstract was
presented at the Annual Meeting of the American Association for
Cancer Research June 22-24 Virtual Meeting II, Sessions Available
Online, 2020 and published as abstract no. 6465 in Cancer Research
80 (Supplement): 2020.
Funding
The present study was supported by Grant-in-Aid for Scientific
Research (B) (grant no. 18H02883).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK and MYa conceived the present study. MYo, TTa,
TTo, HT, KM and MO treated the patients and collected clinical data
of the patients who underwent gastrectomy. HN prepared the
antibody. SK performed the experiments and analyzed them. SK and
MYa confirm the authenticity of all the raw data. SK, MYa, YY, TS,
AS, SN, ST and KK evaluated and interpreted the data. SK and MYa
verified the analytical methods. MYa supervised the findings of
this work. All authors discussed the results and contributed to the
final manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Osaka City
University Ethics Committee (reference no. 924). Informed consent
was obtained in writing from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fornaro L, Vasile E, Aprile G, Goetze TO,
Vivaldi C, Falcone A and Al-Batran SE: Locally advanced
gastro-oesophageal cancer: Recent therapeutic advances and research
directions. Cancer Treat Rev. 69:90–100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gambardella V and Cervantes A: Precision
medicine in the adjuvant treatment of gastric cancer. Lancet Oncol.
19:583–584. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wanger TM, Dewitt S, Collins A, Maitland
NJ, Poghosyan Z and Knauper V: Differential regulation of TROP2
release by PKC isoforms through vesicles and ADAM17. Cell Signal.
27:1325–1335. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cubas R, Li M, Chen C and Yao Q: Trop2: A
possible therapeutic target for late stage epithelial carcinomas.
Biochim Biophys Acta. 1796:309–314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vidmar T, Pavsic M and Lenarcic B:
Biochemical and preliminary X-ray characterization of the
tumor-associated calcium signal transducer 2 (Trop2) ectodomain.
Protein Expr Purif. 91:69–76. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang H, Groth J, Sossey-Alaoui K,
Hawthorn L, Beall S and Geradts J: Aberrant expression of novel and
previously described cell membrane markers in human breast cancer
cell lines and tumors. Clin Cancer Res. 11:4357–4364.
2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shvartsur A and Bonavida B: Trop2 and its
overexpression in cancers: Regulation and clinical/therapeutic
implications. Genes Cancer. 6:84–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guerra E, Trerotola M, Dell' Arciprete R,
Bonasera V, Palombo B, El-Sewedy T, Ciccimarra T, Crescenzi C,
Lorenzini F, Rossi C, et al: A bicistronic CYCLIN D1-TROP2 mRNA
chimera demonstrates a novel oncogenic mechanism in human cancer.
Cancer Res. 68:8113–8121. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang
YL, Jou YS, Hong TM and Yang PC: TROP2 is epigenetically
inactivated and modulates IGF-1R signalling in lung adenocarcinoma.
EMBO Mol Med. 4:472–485. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao W, Zhu H, Zhang S, Yong H, Wang W,
Zhou Y, Wang B, Wen J, Qiu Z, Ding G, et al: Trop2 is overexpressed
in gastric cancer and predicts poor prognosis. Oncotarget.
7:6136–6145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mühlmann G, Spizzo G, Gostner J, Zitt M,
Maier H, Moser P, Gastl G, Zitt M, Müller HM, Margreiter R, et al:
TROP2 expression as prognostic marker for gastric carcinoma. J Clin
Pathol. 62:152–158. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fong D, Moser P, Krammel C, Gostner JM,
Margreiter R, Mitterer M, Gastl G and Spizzo G: High expression of
TROP2 correlates with poor prognosis in pancreatic cancer. Br J
Cancer. 99:1290–1295. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fong D, Spizzo G, Gostner JM, Gastl G,
Moser P, Krammel C, Gerhard S, Rasse M and Laimer K: TROP2: A novel
prognostic marker in squamous cell carcinoma of the oral cavity.
Mod Pathol. 21:186–191. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW,
Yun JP, Zhang MF and Wan DS: Elevated expressions of MMP7, TROP2,
and survivin are associated with survival, disease recurrence, and
liver metastasis of colon cancer. Int J Colorectal Dis. 24:875–884.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bignotti E, Todeschini P, Calza S,
Falchetti M, Ravanini M, Tassi RA, Ravaggi A, Bandiera E, Romani C,
Zanotti L, et al: Trop-2 overexpression as an independent marker
for poor overall survival in ovarian carcinoma patients. Eur J
Cancer. 46:944–953. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sin STK, Li Y, Liu M, Ma S and Guan XY:
TROP-2 exhibits tumor suppressive functions in cervical cancer by
dual inhibition of IGF-1R and ALK signaling. Gynecol Oncol.
152:185–193. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang K, Jones L, Lim S, Maher CA, Adkins
D, Lewis J, Kimple RJ, Fertig EJ, Chung CH, Van Tine BA, et al:
Loss of Trop2 causes ErbB3 activation through a
neuregulin-1-dependent mechanism in the mesenchymal subtype of
HNSCC. Oncotarget. 5:9281–9294. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mori Y, Akita K, Ojima K, Iwamoto S,
Yamashita T, Morii E and Nakada H: Trophoblast cell surface antigen
2 (Trop-2) phosphorylation by protein kinase C α/δ (PKC α/δ)
enhances cell motility. J Biol Chem. 294:11513–11524.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stoyanova T, Goldstein AS, Cai H, Drake
JM, Huang J and Witte ON: Regulated proteolysis of Trop2 drives
epithelial hyperplasia and stem cell self-renewal via β-catenin
signaling. Genes Dev. 26:2271–2285. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Soini Y, Tommola S, Helin H and
Martikainen P: Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of
claudin expression associates with the diffuse subtype. Virchows
Arch. 448:52–58. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stanculescu D, Margaritescu C, Stepan A
and Mitrut AO: E-cadherin in gastric carcinomas related to
histological prognostic parameters. Rom J Morphol Embryol. 52
(Suppl 3):S1107–S1112. 2011.PubMed/NCBI
|
|
23
|
Larsson O, Girnita A and Girnita L: Role
of insulin-like growth factor 1 receptor signalling in cancer. Br J
Cancer. 92:2097–2101. 2005.PubMed/NCBI
|
|
24
|
Rosenzweig SA and Atreya HS: Defining the
pathway to insulin-like growth factor system targeting in cancer.
Biochem Pharmacol. 80:1115–1124. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chiarle R, Voena C, Ambrogio C, Piva R and
Inghirami G: The anaplastic lymphoma kinase in the pathogenesis of
cancer. Nat Rev Cancer. 8:11–23. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Bardia A, Mayer IA, Vahdat LT, Tolaney SM,
Isakoff SJ, Diamond JR, O'Shaughnessy J, Moroose RL, Santin AD,
Abramson VG, et al: Sacituzumab govitecan-hziy in refractory
metastatic triple-negative breast cancer. N Engl J Med.
380:741–751. 2019.PubMed/NCBI View Article : Google Scholar
|