Introduction
Pancreatic neuroendocrine tumor (PNET) arising from
neuroendocrine cells throughout the body of pancreas is rare
cancer. This type of cancer accounts for 1-3% of all pancreatic
cancer and ~85% of the cases are designated as nonfunctional tumors
which do not secrete clinically significant hormones, whereas 15%
of the cases are known as functional PNET which secrete hormones
leading to clinical symptoms (1).
However, the incidence of PNET has been increasing by ~1,000 new
patients every year (2). A total of
3,379 patients received treatment for PNET, and the annual
prevalence of PNET in 2010 is estimated to be 2.69 per 100,000
population, which is ~1.2 times higher than that in 2005 according
to a nationwide epidemiological survey in Japan (3). Overall they have a better prognosis
than common pancreatic cancers, and 5-year survival rate of
localized and resected tumors is ~55% (1). However, 5-year survival rate of
unresectable tumors is only ~15% (1,2). The
National Comprehensive Cancer Network guideline, as well as the
European Neuroendocrine Tumor Society (ENETS) guideline, recommends
the resection of all locoregional PNET cases (4,5).
The newest version of the World Health Organization
classification system established in 2017 are classified as
well-differentiated tumors or poorly differentiated tumors, with
grading based on the mitotic count and/or the Ki-67 labeling index.
PNETs with well-differentiated histology include those of grade 1
(G1) and grade 2 (G2) as well as a new subset of grade 3 (G3).
Poorly differentiated pancreatic neuroendocrine carcinomas are
those with G3 histology called as pancreatic neuroendocrine
carcinoma (PNEC) (1).
Laparoscopic distal pancreatectomy (LDP) has been
covered by insurance since 2012 in Japan. LDP with lymph node
dissection just around pancreas has been one of the treatment
options for insulinoma and nonfunctional PNET of <2 cm in our
hospital because insulinoma are almost benign and nonfunctional
PNET of <2 cm have a small risk of lymph node metastases
(6-13).
LDP with lymph node dissection just around pancreas
appears to be technically simple procedure enough to innovate the
laparoscopic pancreatectomy for young surgeon and institution with
less experience of this procedure.
We, therefore, hypothesized that LDP with lymph node
dissection just around the pancreas would provide a safe and good
short-term outcome for insulinoma and nonfunctional PNET of <2
cm.
Patients and methods
Patient selection
We identified patients from our prospectively
maintained database in Department of Hepato-Biliary-Pancreatic
Surgery at Jikei University Hospital between Oct, 2009 and Jun,
2019. Twenty-six patients with PNET who underwent distal
pancreatectomy were analyzed. We performed a retrospective review
of these patients who were histologically diagnosed with PNET.
Preoperative diagnosis and size determination were performed using
CT, MRI, somatostatin scintigraphy, and endoscopic ultrasound (EUS)
with or without guided fine needle aspiration (FNA), and tumor
function was determined by various hormone tests and SASI tests.
Pathological findings and tumor grade were based on the World
Health Organization Classification 2017 for Pancreatic
Neuroendocrine Neoplasms. The medical records were analyzed
retrospectively for patient age, gender, performance status (PS),
Body mass index (BMI), Tumor size, WHO grade, function, operation
time, estimated intraoperative blood loss, blood transfusion,
residual tumor, lymph node metastasis, complications (such as
hemorrhage, pancreatic fistula, intra-abdominal abscess, and
surgical site infection) as well as postoperative hospitalization
period among the patients in the two groups i.e., those who
underwent laparoscopic surgery and those who underwent open
surgery. The PS was classified as per the classification system by
The American Society of Anesthesiologist (ASA).
Treatment
Patients with insulinoma or nonfunctional PNET of
<2 cm underwent LDP with lymph node dissection just around the
pancreas. Patients who displayed other kinds of PNET or those with
nonfunctional PNET >2 cm underwent open distal pancreatectomy
(ODP) with systematic lymph node dissection. All procedures were
performed by hepato-biliary-pancreatic surgeons of our department.
Later, patients had an imaging study and blood tests performed
every 3 months for 5 years in follow-up clinic.
LDP technique
Under general endotracheal anesthesia, patients were
placed in a supine position. In laparoscopic pancreatectomy, an
umbilical scope trocar and four trocars (two 12 mm and two 5 mm
trocars) were utilized. The surgical field was prepared by placing
the table in a reverse Trendelenburg position, which was slightly
rotated to the bottom right. Then, the greater omentum was opened
toward the craniad of the spleen with a dissection of the left
gastroepiploic and the short gastric vessels through the greater
curvature and toward the caudad of the spleen with a dissection of
the splenicocolic ligament to prevent injuring the transverse
colon. Next, the retroperitoneum was dissected below and lateral of
the spleen. The posterior stomach wall was then fixed to the
abdominal wall to enable viewing of the omental bursa and pancreas.
The superior mesenteric vein was identified from the colic vein,
and the pancreas was tunneled right above the portal vein.
Thereafter, the splenic artery was isolated and ligated first
(artery-first technique), followed by transecting the pancreas
right above the portal vein with a triple-row stapler. The partial
pancreatectomy site, particularly on the left side merely above the
portal vein, was then transected. Subsequently, the retropancreatic
plane on the Toldt's fascia was dissected from the caudad to the
craniad of the pancreas; the lymph nodes were also dissected around
the pancreas and spleen. After the complete resection of the
specimen containing the body and tail of the pancreas and spleen,
it was removed using an endobag through the umbilical wound with or
without prolongation. After the hemostasis and injury were
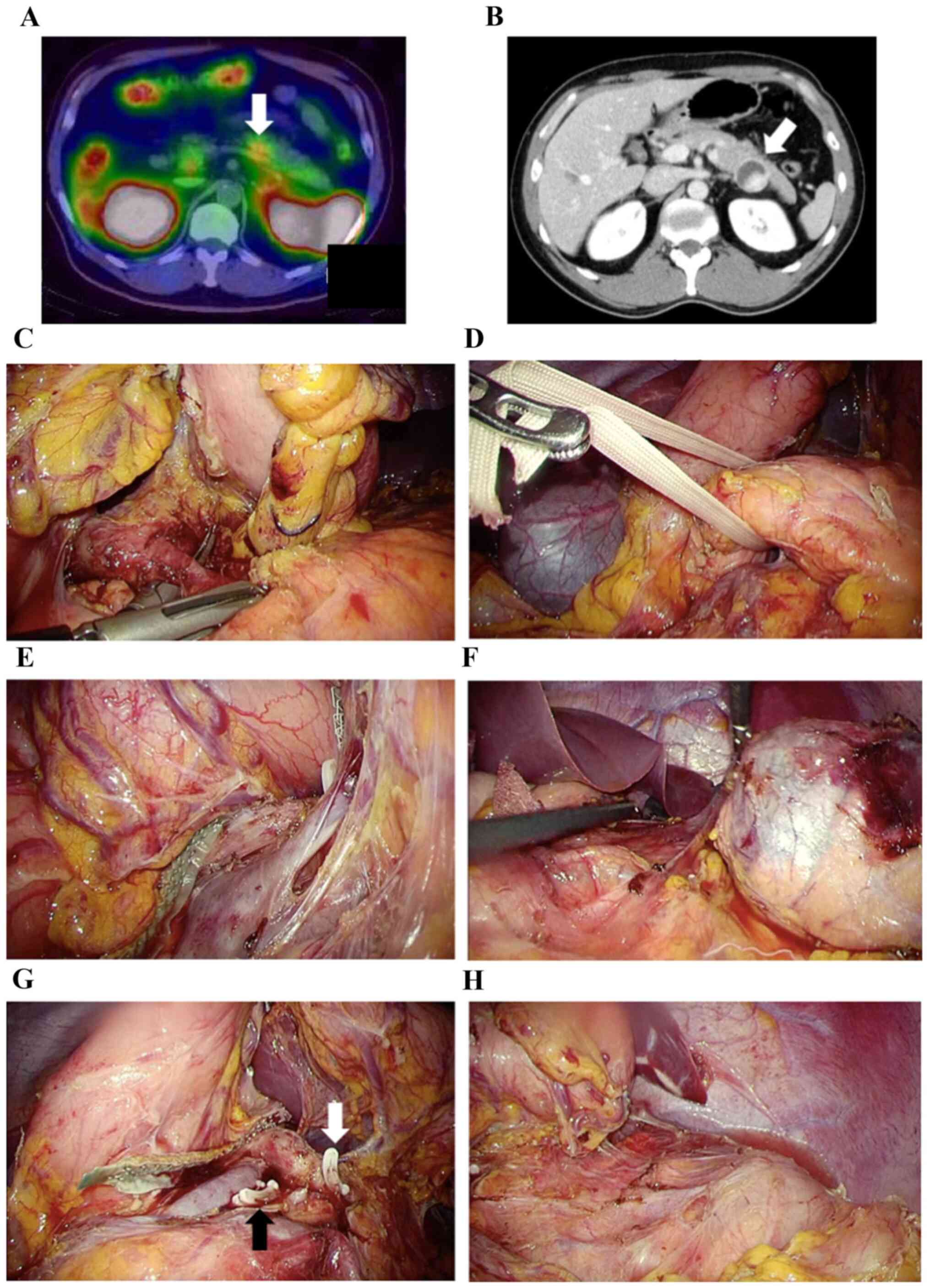
reviewed, a drain was positioned around the pancreas stump. (shown
in Fig. 1).
Statistical analysis
Data is expressed as mean ± standard deviation (SD).
Differences in the continuous data were compared using Student's
t-test. Differences between other characteristics with small sample
sizes were tested using chi-square test or Fisher's exact test.
P-values were considered statistically significant when <0.05.
All statistical tests were performed using the SPSS software
package (v 23.0).
Results
Patient characteristics
Thirteen patients who underwent LDP and 13 patients
who underwent ODP were identified. Table I shows the patient characteristics,
such as their age, gender, and BMI, which were found to be
comparable between the LDP and ODP groups. Patients were primarily
with low PS scores (1; 77%) in LDP group and patients were with low
PS scores (1; 54%) in OP group with no statistical difference.
Tumor diameter in LDP group tended to be smaller than that in the
ODP group as an indication of laparoscopic surgery. According to
WHO grade, eleven patients of G1 and two patients of G2 in LDP
group, and five patients of G1, five patients of G2, and three
patients of pancreatic neuroendocrine carcinoma (PNEC) in ODP group
were classified. Two patients with insulinoma include LDP group and
1 patient with gastrinoma were included in the ODP group. Tumor
function in all patients was diagnosed by preoperative hormonal
tests. They did not exhibit lymph node enlargement in preoperative
diagnostic imaging.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Factor | LDP (n=13) | ODP (n=13) | P-value |
|---|
| Age,
yearsa | 47.9±11.0 | 50.2±15.8 | 0.670 |
| Sex, n
(male:female) | 7:6 | 7:6 | >0.999 |
| ASA score, n
(1:2) | 10:3 | 7:6 | 0.205 |
| BMI,
kg/m2 | 23.1±4.0 | 23.6±4.6 | 0.806 |
| Tumor diameter,
mm | 19.9±9.9 | 35.0±25.0 | 0.060 |
| WHO grade, n
(G1:2:3:NEC)b | 11:2:0:0 | 5:5:0:3 | 0.154 |
| Function, n
(yes:no) | 2:11 | 1:12 | 0.500 |
Surgical factors and postoperative
outcomes
As shown in Table
II, Distal pancreatectomy required 290.2±114.6 and 337.3±130.7
min to be performed in the LDP and ODP groups, respectively, with
no statistical difference. Of note, the estimated blood loss in the
LDP and ODP groups was 122.3±171.8 and 649.2±692.6 ml,
respectively, which was statistically significant. Meanwhile,
requirement for blood transfusion and residual tumor was very
similar among the two groups. Regarding complications, SSI in LDP
group trended to be fewer than that in ODP group, with no
statistical difference, and the overall complications were found to
be similar. Postoperative hospitalization periods were
statistically longer in the ODP group than that in the LDP group.
This was also a feature of the laparoscopic procedure which is
described in Table III.
 | Table IISurgical and pathological factors. |
Table II
Surgical and pathological factors.
| Factor | LDP (n=13) | ODP (n=13) | P-value |
|---|
| Operation time,
mina | 290.2±114.6 | 337.3±130.7 | 0.338 |
| Estimated blood loss,
mla | 122.3±171.8 | 649.2±692.6 | 0.019 |
| Blood transfusion, n
(no:yes) | 13:0 | 12:1 | 0.500 |
| Residual tumor, n
(R0:R1) | 13:0 | 12:1 | 0.500 |
| Lymph node
metastasis, n (no:yes) | 13:0 | 12:1 | 0.500 |
 | Table IIIComplications and postoperative
hospital stay. |
Table III
Complications and postoperative
hospital stay.
| Factor | LDP (n=13) | ODP (n=13) | P-value |
|---|
| Overall complication,
n (%) | 4(31) | 7(54) | 0.234 |
| Hemorrhage, n
(%) | 0 (0) | 0 (0) | - |
| Pancreatic fistula, n
(%) | 1(8) | 4(31) | 0.161 |
| Intraabdominal
abscess, n (%) | 2(15) | 2(15) | >0.999 |
| Surgical site
infection, n (%) | 2(15) | 6(46) | 0.101 |
| Postoperative
hospital stay, daysa | 10.7±7.4 | 20.9±12.7 | 0.022 |
We found that only one patient from the ODP group
had developed metastases of the dissected lymph node, as described
in Table IV. This patient, who had
been diagnosed with gastrinoma, underwent partial pancreatectomy
with systematic lymph node dissection. Pathological findings showed
that it was a 6 mm G1 gastrinoma, and she displayed no recurrence
after surgery for the next 3 years.
 | Table IVMetastatic case and recurrent
cases. |
Table IV
Metastatic case and recurrent
cases.
| Case | Sex | Age, years | Functional or
not | Grade | Tumor size, mm | Procedure (positive
LN/harvest LN) | DFS, months | Site of
recurrence |
|---|
| Metastatic case on
surgical specimen by pathological findings | |
| 1 | Female | 50 | Gastrinoma | G1 | 6 | ODP (1/12) | 30 | No relapse |
| Recurrent
cases | |
| 1 | Male | 43 | Non functional | NEC | 41 | ODP (0/8) | 1 | Liver and lung |
| 2 | Male | 41 | Non functional | NEC | 20 | ODP (0/17) | 21 | Liver |
Further, two patients from the ODP group, developed
recurrence of PNET, are described in Table IV. These were male patients in
their 40s and had been diagnosed with nonfunctional PNET, and they
underwent distal pancreatectomy with lymph node dissection.
Pathological findings discovered PNETs of 41 and 20 mm in the two
patients. Further, a recurrence of liver metastases was detected at
1 and 21 months, respectively, after surgery in the two
patients.
Discussion
Recently, laparoscopic surgery and robotic surgery
are increasingly being performed in some developed countries on
patients with various gastrointestinal and pancreatic NETs.
Although there have been very few reports that study the safety and
long-term prognosis of laparoscopic surgery for NET in the
gastrointestinal tract, conventional laparoscopic surgery that is
performed for esophageal, gastric and colon cancer, and which
requires systemic lymph node dissection, has achieved results
comparable to open surgery. Based on these results, it is now
considered that NET can be safely performed (14-16).
In our analysis, LDP performed for PNET with small tumor size
(<2 mm) was comparable to the results of the ODP; therefore,
partial resection was performed in many cases. Furthermore, in
situations where the surgical technique was similar, LDP tended to
result in significantly less bleeding and shorter operation times
than that with open surgery. However, this was not true when the
tumor diameter was extremely large. The postoperative complications
were not different between the two groups; however, the
postoperative hospitalization period was significantly shorter in
the LDP group. Of note, some of the previous studies on low
malignant tumor also demonstrated that LDP shows perioperative
outcomes and long-term prognosis that is either equal or better
than that in ODP (17-19).
From the results of this study and others, we believe that LDP
appears to be safe and minimally invasive and may become more
common in patients with PNET who do not require systemic
lymphadenectomy or vascular resection.
In this context, it is essential to discussion the
lymph node metastasis and lymph node dissection of PNET. It is
noteworthy that lymph node metastasis is seldom identified because
>90% of insulinomas are benign (20). Therefore, enucleation without lymph
node dissection is recommended if it has no malignant findings and
can be performed safely without damage to the main pancreatic duct
(6-8).
On the other hand, distal pancreatectomy is recommended when it is
in close proximity to the main pancreatic duct. In addition, other
functional NETs have high malignancy and poor prognosis (21-29).
Of note, despite the rate of lymph node metastasis in gastrinoma
being as high as 60% or even higher, improvement of prognosis by
dissection has been reported (30,31).
Even in our data, one patient with gastrinoma which was just only 6
mm G1 tumor underwent partial pancreatectomy and lymph node
dissection with long-term disease-free survival despite lymph node
metastasis being found by pathology. Recently, there are some
reports that it is safe to follow-up small nonfunctional NET with a
tumor diameter of <2 cm (9-13).
On the other hand, lymph node metastasis has been reported even
when the tumor diameter is <1 cm (32-34).
However, this includes the poorly differentiated tumors and
requires careful attention in their interpretation. In our data,
one patient who relapsed had a high grade NEC tumor with a diameter
of 20 mm. Further, lymph node metastasis of G1 PNET of <1 cm has
been reported to be extremely low (32-34).
However, in such cases, the tumor size is either too small or FNA
is too difficult, which results in the PNET grade not being
diagnosed before surgery. Furthermore, PNEC resection results and
postoperative prognostic factors have remained unreported, and the
surgical indication for PNEC is still unclear from the guidelines.
Two cases were found to have recurrence though distal
pancreatectomy with numerous lymph nodes around the pancreas was
performed (Table IV). Therefore,
we could not mention to the lymph node dissection for PNEC.
Hence, laparoscopic pancreatectomy with
lymphadenectomy around the pancreas, which is a minimally invasive
procedure, could be the standard procedure, and an effective
surgical technique for this approach is required for low-malignancy
PNET. In other words, insulinoma and nonfunctional PNET <2 cm
are good indications of laparoscopic pancreatectomy with lymph node
dissection around the pancreas, and introducing laparoscopic
pancreatectomy to an inexperienced institution is a good
opportunity.
Our report has the limitations of being a
retrospective analysis of a single institution and involving a
relatively small sample size. However, the strength of the analysis
is that these are valuable individual findings of rare cancer.
In conclusion, although LDP has not sufficiently
examined and accumulated evidence on long-term treatment results,
it is clearly minimally invasive. In particular, it is considered
to be one of the important treatment options in such a PNET region
in which various range of malignancy for selected patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HShio, YS and TG analyzed and interpreted the
patient data on PNET. HShio and YS wrote the manuscript. HShib, YS,
TG and TI made substantial contributions in data analysis and
interpretation. HShib, TH, JY, KF and SO helped in acquiring the
data for the work. HShio and TI conceived the concept and designed
the study. HShio and YS were responsible for assessing and
confirming the authenticity of all raw data. TI critically revised
the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The Jikei University School of Medicine [approval no.
27-177(8062)], and written informed consent was obtained from each
patient.
Patient consent for publication
Written informed consent was obtained from all
subjects for publication of the present study. A copy of the
written consent is available for review upon requests.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Batukbhai BDO and De Jesus-Acosta A: The
molecular and clinical landscape of pancreatic neuroendocrine
tumors. Pancreas. 48:9–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ries LAG, Young JL, Keel GE, Eisner MP,
Lin YD and Horner M-J (eds): SEER Survival Monograph: Cancer
Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and
Tumor Characteristics. National Cancer Institute, SEER Program, NIH
Pub. No. 07-6215, Bethesda, MD, 2007.
|
|
3
|
Ito T, Igarashi H, Nakamura K, Sasano H,
Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, et
al: Epidemiological trends of pancreatic and gastrointestinal
neuroendocrine tumors in Japan: A nationwide survey analysis. J
Gastroenterol. 50:58–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shah NP: NCCN guidelines updates:
Discontinuing TKI therapy in the treatment of chronic myeloid
leukemia. J Natl Compr Canc Netw. 17:611–613. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zandee WT and de Herder WW: The evolution
of neuroendocrine tumor treatment reflected by ENETS Guidelines.
Neuroendocrinology. 106:357–365. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hirshberg B, Cochran C, Skarulis MC,
Libutti SK, Alexander HR, Wood BJ, Chang R, Kleiner DE and Gorden
P: Malignant insulinoma: Spectrum of unusual clinical features.
Cancer. 104:264–272. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Roldo C, Missiaglia E, Hagan JP, Falconi
M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A and
Croce CM: MicroRNA expression abnormalities in pancreatic endocrine
and acinar tumors are associated with distinctive pathologic
features and clinical behavior. J Clin Oncol. 24:4677–4684.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Klöppel G, Couvelard A, Perren A,
Komminoth P, McNicol AM, Nilsson O, Scarpa A, Scoazec JY,
Wiedenmann B, Papotti M, et al: ENETS consensus guidelines for the
standards of care in neuroendocrine tumors: Towards a standardized
approach to the diagnosis of gastroenteropancreatic neuroendocrine
tumors and their prognostic stratification. Neuroendocrinology.
90:162–166. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Choi JH, Choi YH, Kang J, Paik WH, Lee SH,
Ryu JK and Kim YT: Natural history of small pancreatic lesions
suspected to be nonfunctioning pancreatic neuroendocrine tumors.
Pancreas. 47:1357–1363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rosenberg AM, Friedmann P, Del Rivero J,
Libutti SK and Laird AM: Resection versus expectant management of
small incidentally discovered nonfunctional pancreatic
neuroendocrine tumors. Surgery. 159:302–309. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jung JG, Lee KT, Woo YS, Lee JK, Lee KH,
Jang KT and Rhee JC: Behavior of small, asymptomatic,
nonfunctioning pancreatic neuroendocrine tumors (NF-PNETs).
Medicine (Baltimore). 94(e983)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gaujoux S, Partelli S, Maire F, D'Onofrio
M, Larroque B, Tamburrino D, Sauvanet A, Falconi M and Ruszniewski
P: Observational study of natural history of small sporadic
nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol
Metab. 98:4784–4789. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee LC, Grant CS, Salomao DR, Fletcher JG,
Takahashi N, Fidler JL, Levy MJ and Huebner M: Small,
nonfunctioning, asymptomatic pancreatic neuroendocrine tumors
(PNETs): Role for nonoperative management. Surgery. 152:965–974.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Takatsu Y, Fukunaga Y, Nagasaki T,
Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S and Ueno M: Short-
and long-term outcomes of laparoscopic total mesenteric excision
for neuroendocrine tumors of the rectum. Dis Colon Rectum.
60:284–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Downs-Canner S, Van der Vliet WJ, Thoolen
SJ, Boone BA, Zureikat AH, Hogg ME, Bartlett DL, Callery MP, Kent
TS, Zeh HJ and Moser AJ: Robotic surgery for benign duodenal
tumors. J Gastrointest Surg. 19:306–312. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shiroshita H, Inomata M, Bandoh T, Uchida
H, Akira S, Hashizume M, Yamaguchi S, Eguchi S, Wada N, Takiguchi
S, et al: Endoscopic surgery in Japan: The 13th national survey
(2014-2015) by the Japan Society for Endoscopic Surgery. Asian J
Endosc Surg. 12:7–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang J, Jin J, Chen S, Gu J, Zhu Y, Qin
K, Zhan Q, Cheng D, Chen H, Deng X, et al: Minimally invasive
distal pancreatectomy for PNETs: Laparoscopic or robotic approach?
Oncotarget. 8:33872–33883. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fernández-Cruz L, Molina V, Vallejos R,
Jiménez Chavarria E, Lõpez-Boado MA and Ferrer J: Outcome after
laparoscopic enucleation for non-functional neuroendocrine
pancreatic tumours. HPB (Oxford). 14:171–176. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haugvik SP, Gaujoux S, Røsok B, Gladhaug
IP, Dousset B and Edwin B: Laparoscopic versus open pancreas
resection for neuroendocrine tumours: Need for evaluation of
oncological outcomes. HPB (Oxford). 16(871)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Herder WW: Insulinoma.
Neuroendocrinology. 80 (Suppl 1):S20–S22. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ghaferi AA, Chojnacki KA, Long WD, Cameron
JL and Yeo CJ: Pancreatic VIPomas: Subject review and one
institutional experience. J Gastrointest Surg. 12:382–393.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Patel FB, Khagi S, Daly KP, Lechan RM,
Ummaritchot V and Saif MW: Pancreatic neuroendocrine tumor with
ectopic adrenocorticotropin production: A case report and review of
literature. Anticancer Res. 33:4001–4005. 2013.PubMed/NCBI
|
|
23
|
Garbrecht N, Anlauf M, Schmitt A, Henopp
T, Sipos B, Raffel A, Eisenberger CF, Knoefel WT, Pavel M, Fottner
C, et al: Somatostatin-producing neuroendocrine tumors of the
duodenum and pancreas: Incidence, types, biological behavior,
association with inherited syndromes, and functional activity.
Endocr Relat Cancer. 15:229–241. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wermers RA, Fatourechi V, Wynne AG, Kvols
LK and Lloyd RV: The glucagonoma syndrome: Clinical and pathologic
features in 21 patients. Medicine (Baltimore). 75:53–63.
1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Öberg K: Pancreatic endocrine tumors.
Semin Oncol. 37:594–618. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kuo SC, Gananadha S, Scarlett CJ, Gill A
and Smith RC: Sporadic pancreatic polypeptide secreting tumors
(PPomas) of the pancreas. World J Surg. 32:1815–1822.
2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Soga J: Endocrinocarcinomas (carcinoids
and their variants) of the duodenum. An evaluation of 927 cases. J
Exp Clin Cancer Res. 22:349–363. 2003.PubMed/NCBI
|
|
28
|
Fendrich V, Waldmann J, Bartsch DK and
Langer P: Surgical management of pancreatic endocrine tumors. Nat
Rev Clin Oncol. 6:419–428. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Falconi M, Eriksson B, Kaltsas G, Bartsch
DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G,
Klöppel G, et al: ENETS consensus guidelines update for the
management of patients with functional pancreatic neuroendocrine
tumors and non-functional pancreatic neuroendocrine tumors.
Neuroendocrinology. 103:153–171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Giovinazzo F, Butturini G, Monsellato D,
Malleo G, Marchegiani G and Bassi C: Lymph nodes metastasis and
recurrences justify an aggressive treatment of gastrinoma. Updates
Surg. 65:19–24. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bartsch DK, Waldmann J, Fendrich V,
Boninsegna L, Lopez CL, Partelli S and Falconi M: Impact of
lymphadenectomy on survival after surgery for sporadic gastrinoma.
Br J Surg. 99:1234–1240. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Jutric Z, Grendar J, Hoen HM, Cho SW,
Cassera MA, Newell PH, Hammill CW, Hansen PD and Wolf RF: Regional
metastatic behavior of nonfunctional pancreatic neuroendocrine
tumors: Impact of lymph node positivity on survival. Pancreas.
46:898–903. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Curran T, Pockaj BA, Gray RJ, Halfdanarson
TR and Wasif N: Importance of lymph node involvement in pancreatic
neuroendocrine tumors: Impact on survival and implications for
surgical resection. J Gastrointest Surg. 19:152–160.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gratian L, Pura J, Dinan M, Roman S, Reed
S and Sosa JA: Impact of extent of surgery on survival in patients
with small nonfunctional pancreatic neuroendocrine tumors in the
United States. Ann Surg Oncol. 21:3515–3521. 2014.PubMed/NCBI View Article : Google Scholar
|