Introduction
Adverse events are a major medical problem in
hospitalized patients (1,2). In particular, adverse events are
frequently observed in patients receiving cancer chemotherapy
because of the narrow therapeutic range of anticancer drugs, which
are associated with a high incidence of toxicity (3,4).
Adverse events often become severe, resulting in dose delays and
reductions, therapy discontinuation, or decreased quality of life,
all of which negatively influence the therapeutic effect (5-7).
Therefore, it is necessary to consider dose adjustment or
withdrawal of anticancer drugs depending on bone marrow function,
renal function, liver function and the state of occurrence of
adverse events in previous treatment in many cancer chemotherapy
regimens (8). Additionally, the
prevention and timely remedy of adverse events in patients is of
the importance. However, many chemotherapy regimens are highly
complex, including prophylactic supportive care, and require
frequent dosage adjustments, which can lead to prescription errors
(9,10). Therefore, medical care related to
the administration of cancer chemotherapy should be performed by a
team of medical staff comprising physicians, nurses, clinical
pharmacists, nutritionists and other medical staff (11,12).
Clinical pharmacists play a critical role in
pharmaceutical practices in cancer chemotherapy, including
reviewing cancer chemotherapy regimens, dose adjustments for organ
dysfunction, weight, age, monitoring for drug-drug interactions,
verifying prescription orders containing anticancer drugs,
monitoring efficacy and adverse events, preventing or alleviating
adverse events, implementing palliative care and providing drug
information to medical staff (13,14).
Even though clinical pharmacists spend a considerable amount of
time ensuring patient safety during the preparation and dispensing
of chemotherapy, only a few studies have evaluated their efforts
related to management in patients receiving cancer chemotherapy
(15,16).
The purpose of this retrospective study was to
evaluate a single pharmacist's interventions on the rate of adverse
events in hospitalized patients with thoracic cancer receiving
cancer chemotherapy.
Materials and methods
Study design and setting
We conducted a single-center, retrospective study at
the 614-bed, tertiary care Gifu University Hospital. We enrolled
hospitalized patients with thoracic cancer who received cancer
chemotherapy in the respiratory medicine ward between April 2013
and May 2014. One of two clinical pharmacists in charge, including
one oncology pharmacy specialist, was based in the respiratory
medicine ward and implemented pharmaceutical care for the patients.
Adverse events were monitored in cooperation with physicians,
nurses and clinical pharmacists and were recorded in each patient's
electronic medical chart. Appropriate drug management for adverse
events was performed by the physician based on his/her own
judgement or on the pharmacist's recommendation, and judgment of
whether or not the intervention improved an adverse event was
continued until the end of the treatment.
Ethics statement
The present study was conducted according to the
guidelines for human studies of the ethics committee of Gifu
University Graduate School of Medicine and the Government of Japan,
and was approved by the university's institutional review board
(approval no. 28-348). In view of the retrospective nature of the
study, informed consent from the subjects was not mandated.
Cancer chemotherapy
In our hospital, all thoracic cancer chemotherapy is
administered based on a treatment protocol agreed upon by
physicians and clinical pharmacists in advance. This protocol sets
the criteria for starting treatment, dose reduction or drug
withdrawal accompanying incidence of adverse events and dose
adjustments for renal and hepatic function based mainly on evidence
from original reports, and prophylactic supportive care, including
antiemetic medication; hydration management for cisplatin; folic
acid supplements and vitamin B12 injections for
pemetrexed; glucocorticoid and histamine H1 for
paclitaxel hypersensitivity; and hydration while on having renal
dysfunction. Standard antiemetic medication was administered based
on the Japanese Society of Clinical Oncology guidelines (17). Additionally, patients who developed
chemotherapy-induced nausea and vomiting were prophylactically
administered aprepitant, olanzapine, lorazepam or prochlorperazine
in addition to standard prophylactic antiemetic therapy at the next
scheduled treatment.
Standardized chemotherapy order forms containing the
dosage of anticancer drug and dose calculation (mg/m2),
start date and time, day of therapy, solution diluent and volume,
infusion rate (drips), route (intravenous push or infusion),
duration of infusion, frequency of anticancer drug administration,
total number of scheduled doses, and prophylactic supportive care
restricted to injections were entered into the electronic medical
chart for each regimen.
Duties of clinical pharmacists
Clinical pharmacists performed the following duties:
Interviewed all patients on admission and documented medications
brought to the hospital and patients' medication history, daily
review of laboratory data, verified prescriptions, monitored and
managed adverse events, provided drug information to medical staff,
and patient education.
Clinical pharmacists provided physicians with timely
information and advice on adverse events; drug interactions; and
appropriate dosages, dose intervals, and routes of administration.
All interventions performed by pharmacists were recorded, including
whether or not their recommendations were accepted by the
physicians.
Assessment and intervention of adverse
events
In the present study, adverse events were defined as
harm due to medications (adverse drug event), radiation therapy, or
events that occurred during the course of the disease, excluding
those due to medical errors, system errors and equipment failure.
The severity of adverse events was graded according to the Common
Terminology Criteria for Adverse Events (National Cancer Institute,
MD, USA) version 4.0. Pharmacotherapy for adverse events was based
on the National Comprehensive Cancer Network guidelines for
supportive care and other clinical practice guidelines for each
adverse event.
Interventions for adverse events were carried out in
patients showing grade ≥2 non-hematological or grade ≥3
hematological adverse events, and the effect of intervention was
evaluated before and after intervention. The judgment whether or
not the intervention improved the adverse events was conducted
until the end of the treatment.
Data analysis
The following patient data were recorded in
specially prepared Microsoft Excel 2010 (Microsoft Corp.)
spreadsheets: Patient age and sex; date of admission and discharge;
diagnosis; purpose of hospitalization; list of private medications;
clinical pharmacists' prescription proposals; and adverse events,
their grade, and outcome of intervention. The duration of hospital
stay was documented in Kaplan-Meier plots and the mean hospital
stay was statistically compared using the Mantel-Cox log rank test.
Data were analyzed using SPSS version 11 (SPSS Inc.) and GraphPad
Prism version 6.0 (GraphPad Software). Comparison of the incidence
of adverse events before and after intervention was statistically
analyzed using McNemar's test for paired non-parametric variables.
P-values of <0.05 was considered statistically significant.
Results
Patient demographics
A total of 484 (337 men and 147 women) patients who
were hospitalized for the purpose of cancer chemotherapy were
enrolled in the present study. Patient demographics are shown in
Table I. The median age of patients
was 66.0 years (5-95th, 56.0-76.0 years), and the mean duration of
hospital stay was 12.3 days (5-95th, 4.0-24.0 days). The most
common cancer type was non-small cell lung cancer (n=355, 73.3%),
followed by small cell lung cancer (n=110, 22.7%), malignant
mesothelioma (n=10, 2.1%) and thymoma/thymic carcinoma (n=9, 1.9%).
The most common cancer chemotherapy regimen was carboplatin (AUC 5)
+ pemetrexed (500 mg/m2) ± bevacizumab (15
mg/m2) (n=88, 18.2%), followed by carboplatin (AUC 5) +
paclitaxel (200 mg/m2) ± bevacizumab (15
mg/m2) (n=85, 17.5%), docetaxel (60 mg/m2)
(n=67, 13.8%), amurubicin (40 mg/m2) (n=41, 8.5%),
carboplatin (AUC 5) + etoposide (100 mg/m2) (n=33,
6.8%), and pemetrexed (500 mg/m2) + bevacizumab (15
mg/m2) (n=30, 6.2%).
 | Table IPatient demographics. |
Table I
Patient demographics.
| Characteristic | Number |
|---|
| Number of patients
(male/female), n | 484 (337/147) |
| Mean age (5-95th
percentiles), years | 66.0 (56.0-76.0) |
| Mean length of
hospital stay (5-95th percentiles), days | 12.3 (4.0-24.0) |
| Type of cancer,
number of patients (%) | |
|
Non-small
cell lung cancer | 355 (73.3) |
|
Small cell
lung cancer | 110 (22.7) |
|
Malignant
meothelioma | 10 (2.1) |
|
Thymoma/thymic
carcinoma | 9 (1.9) |
| Chemotherapy
regimens, number of patients (%) | |
|
Carboplatin
(AUC 5) + pemetrexed (500 mg/m2) ± bevacizumab (15
mg/kg) | 88 (18.2) |
|
Carboplatin
(AUC 5) + paclitaxel (200 mg/m2) ± bevacizumab (15
mg/kg) | 85 (17.5) |
|
Docetaxel
(60 mg/m2) | 67 (13.8) |
|
Amurubicin
(40 mg/m2) | 41 (8.5) |
|
Carboplatin
(AUC 5) + etoposide (100 mg/m2) | 33 (6.8) |
|
Pemetrexed
(500 mg/m2) + bevacizumab (15 mg/kg) | 30 (6.2) |
|
Tyrosine
kinase inhibitors against epidermal growth factor receptor | 23 (4.8) |
|
Nogitecan
(1.0 mg/m2) | 15 (3.1) |
|
Irinotecan
(100 mg/m2) | 14 (2.9) |
|
Cisplatin
(80 mg/m2) + vinorelbine (25 mg/m2) | 12 (2.8) |
|
Vinorelbine
(25 mg/m2) | 9 (1.8) |
|
Carboplatin
(AUC 5) + tegafur/gimeracil/oteracil | 7 (1.4) |
|
Cisplatin
(80 mg/m2) + pemetrexed (500 mg/m2) ±
bevacizumab (15 mg/kg) | 7 (1.4) |
|
Gemcitabine
(1,000 mg/m2) | 7 (1.4) |
|
Cisplatin
(80 mg/m2) + docetaxel (60 mg/m2) | 4 (0.8) |
|
Carboplatin
(AUC 5) + gemcitabine (1,000 mg/m2) | 4 (0.8) |
|
Tegafur/gimeracil/oteracil | 4 (0.8) |
|
Other | 34 (7.0) |
Interventions implemented by the
clinical pharmacist before administration of chemotherapy
Before administration of chemotherapy, the clinical
pharmacist implemented a total of 152 interventions in 101 of the
484 (20.8%) patients. The types of interventions implemented by the
clinical pharmacist before administration of chemotherapy are shown
in Table II. The most frequent
type of intervention was drug addition (n=82, 53.9%), among which
76 interventions (50.0%) were related to addition of supportive
care, followed by dose adjustment (n=27, 17.8%), selection (n=15,
9.9%), discontinuation (n=12, 7.9%), examination addition (n=6,
3.9%) and other (n=10, 6.6%).
 | Table IIDrug-specific interventions by
clinical pharmacist before administration of chemotherapy. |
Table II
Drug-specific interventions by
clinical pharmacist before administration of chemotherapy.
| Category of
intervention (n) | Contents of
intervention (n) |
|---|
| Drug addition
(82) | Antiemetic drugs
(26), vitamin B12 or folic acid on pemetrexed chemotherapy (18),
zoledronic acid or precipitated calcium
carbonate/cholecalciferol/magnesium carbonate for high blood
calcium due to cancer (14), hydration on cisplatin chemotherapy or
renal dysfunction (12), other drugs (12) |
| Drug dose
adjustment (27) | Anticancer drugs
(22), other drugs (5) |
| Drug selection
(15) | Antiemetic drugs
(7), anticancer drugs (8) |
| Drug
discontinuation (12) | Antiemetic drugs
(7), anticancer drug (2), other drugs (3) |
| Examination
addition (6) | Urinary protein
examination on bevacizumab chemotherapy (3), other (3) |
| Other (10) | Drug-drug
interactions (5), change of solution used to dissolve anticancer
drugs (2), other (3) |
Incidence of adverse events
As shown in Table
III, there were a total of 365 adverse events, among which 29
(7.9%), 227 (62.2%), 89 (24.4%), and 20 (5.5%) were grade 1, 2, 3
and 4, respectively. A total of 203 (41.9%) patients had adverse
events, including 12 (2.5%) with grade 1, 106 (21.9%) with grade 2,
69 (14.3%) with grade 3, 16 (3.3%) with grade 4, and 191 (39.4%)
with grade ≥2 events.
 | Table IIIIncidence of adverse events according
to severity. |
Table III
Incidence of adverse events according
to severity.
| Grade | Number | Patients, n
(%) |
|---|
| Grade 1 | 29 | 12 (2.5) |
| Grade 2 | 227 | 106 (21.9) |
| Grade 3 | 89 | 69 (14.3) |
| Grade 4 | 20 | 16 (3.3) |
| All grades | 365 | 203 (41.9) |
| Grade ≥2 | 336 | 191 (39.4) |
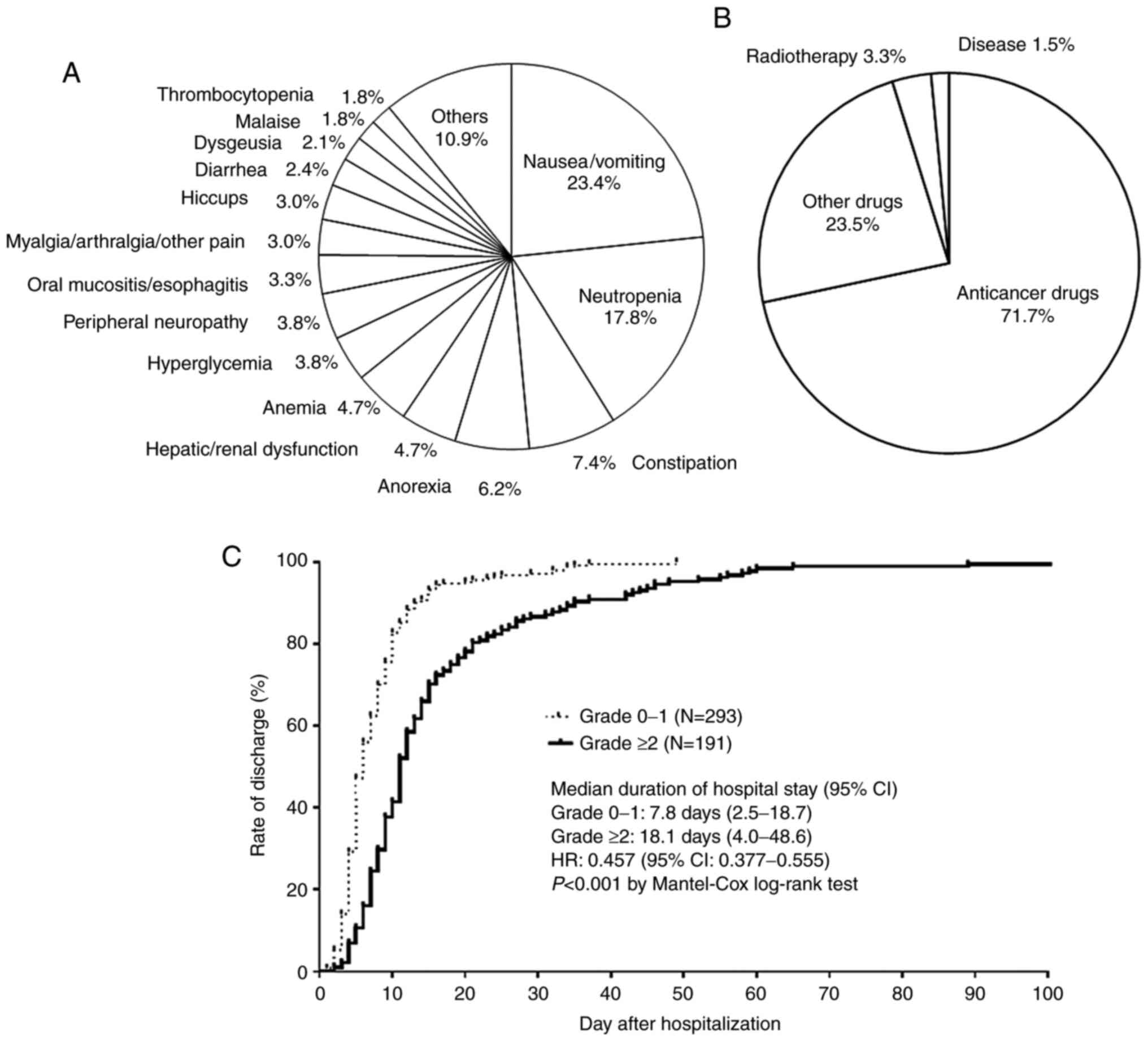
In contrast, the most common adverse event was
nausea/vomiting (23.4%), followed by neutropenia (17.8%),
constipation (7.4%), anorexia (6.2%), hepatic dysfunction/renal
dysfunction (4.7%), anemia (4.7%), hyperglycemia (3.8%), peripheral
neuropathy (3.8%), oral mucositis/esophagitis (3.3%),
myalgia/arthralgia/other pain (3.0%), hiccups (3.0%), diarrhea
(2.4%), dysgeusia (2.1%), malaise (1.8%) and thrombocytopenia
(1.8%) (Fig. 1A). The most common
cause of adverse events was anticancer drugs (71.7%), followed by
other drugs (23.5%), radiotherapy (3.3%) and disease (1.5%)
(Fig. 1B).
As shown in Fig. 1C,
the mean duration of hospitalization was significantly longer among
patients with grade ≥2 events than those with grade 0 and 1 events
[7.8 days, range 2.5-18.7 days vs. 18.1 days, range 4.0-48.6 days;
hazards ratio (HR) 0.457, 95% confidence interval (CI) 0.377-0.555;
P<0.001].
Recommendations by the clinical
pharmacist for treatment of adverse events
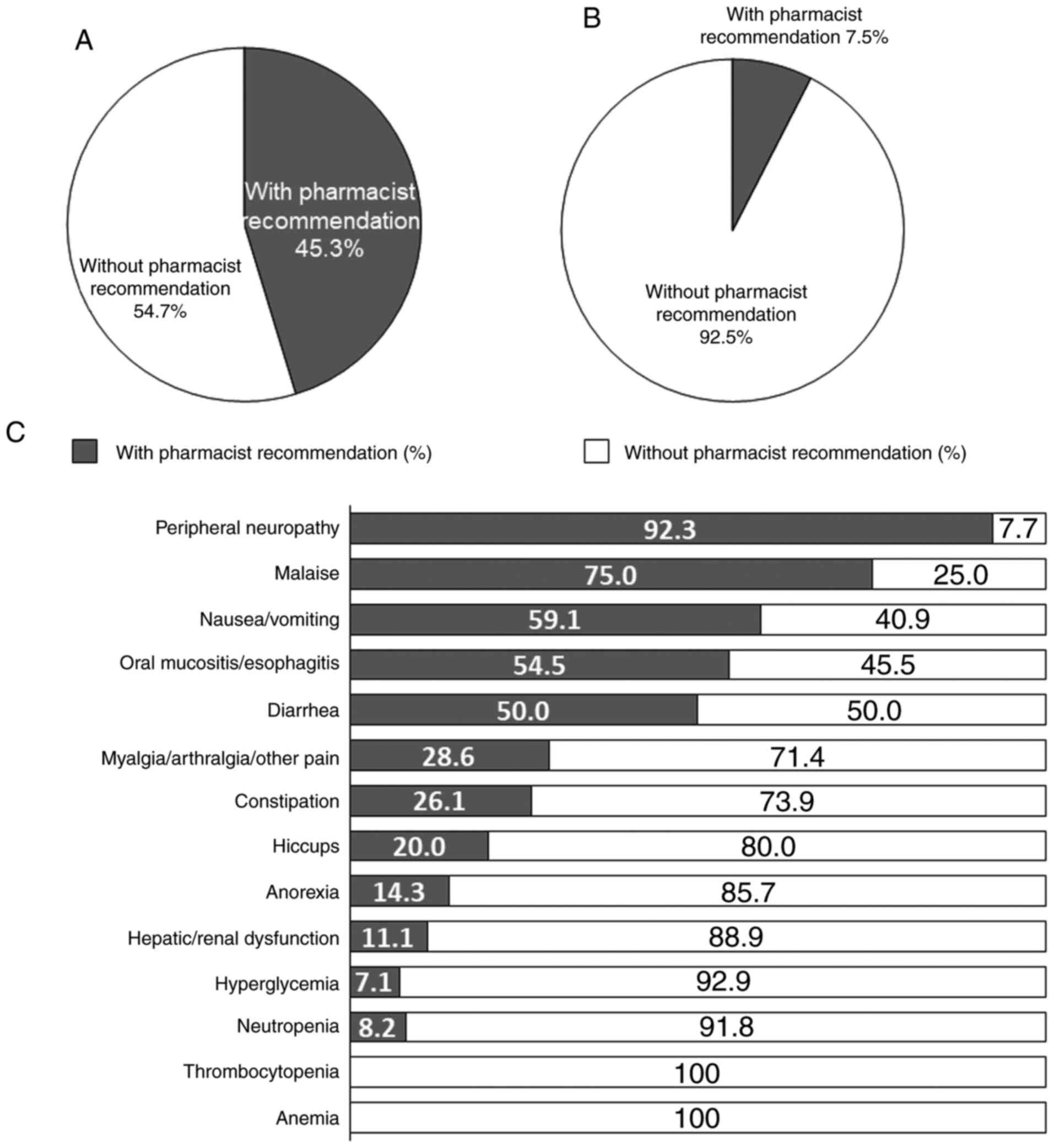
There were 224 medical interventions and 73 follow
ups for grade ≥2 non-hematological or grade ≥3 hematological
adverse events. Seventy-seven of 170 (45.3%) medical interventions
for grade ≥2 non-hematological adverse events were implemented
based on the pharmacist's recommendations (Fig. 2A). In contrast, 4 of 54 (7.5%)
medical interventions for grade ≥3 hematological adverse events
were based on the pharmacist's recommendations (Fig. 2B).
The rates of pharmacist's recommendations in medical
intervention for each of non-hematological adverse events of grade
≥2 or hematological adverse events of grade ≥3 observed was as
follows: Peripheral neuropathy (92.3%), followed by malaise
(75.0%), nausea/vomiting (59.1%), oral mucositis/esophagitis
(54.5%), diarrhea (50.0%), myalgia/arthralgia/other pain (28.6%),
constipation (26.1%), hiccups (20.0%), anorexia (14.3%),
hepatic/renal dysfunction (11.1%), neutropenia (8.2%) and
hyperglycemia (7.1%) (Fig. 2C).
Effect of medical interventions for
adverse events
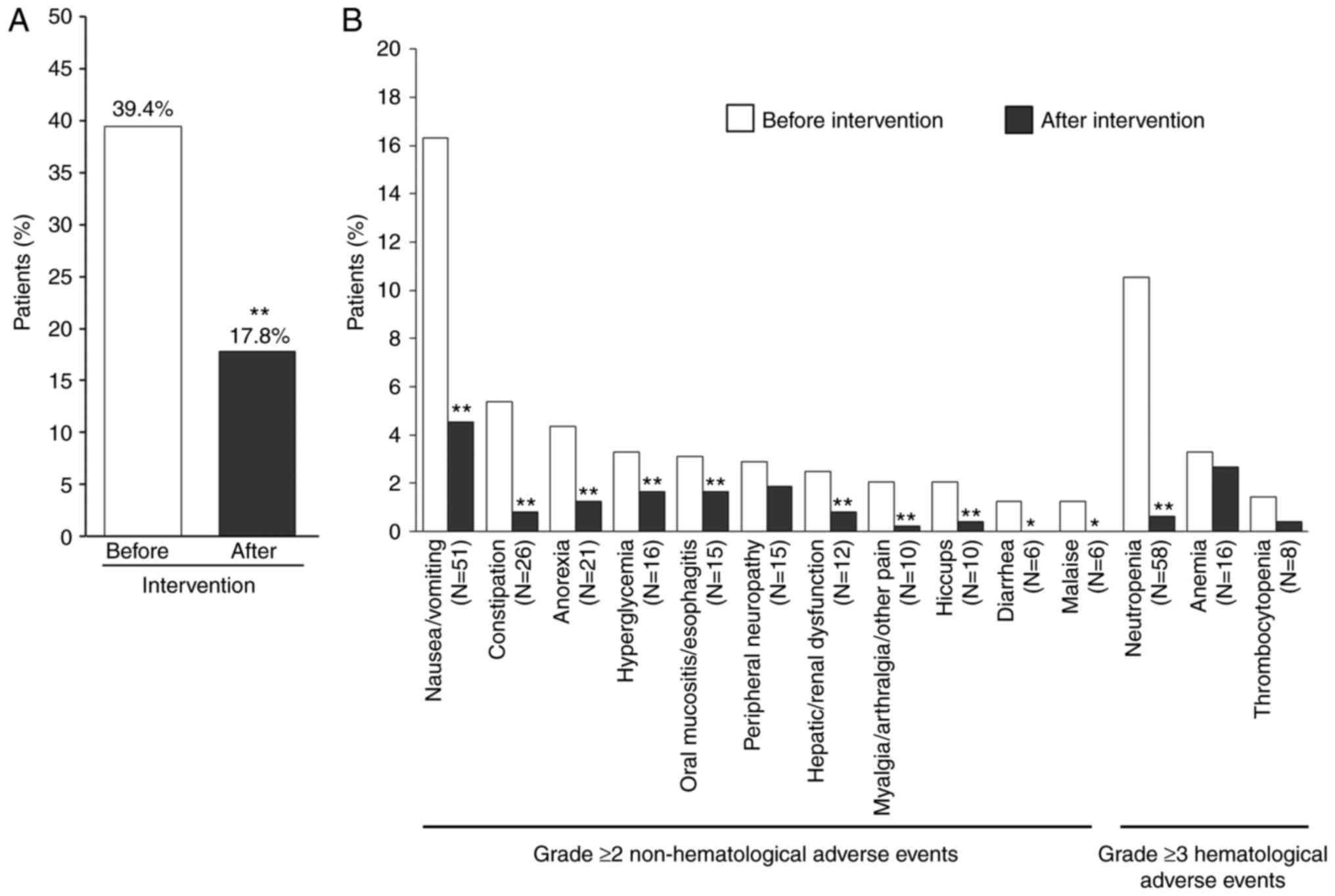
As shown in Fig. 3A,
the incidence of grade ≥2 non-hematological or grade ≥3
hematological adverse events was significantly reduced after
intervention (39.4 vs. 17.8%, P<0.01). Moreover, as shown in
Fig. 3B, the incidence rates of
following non-hematological adverse events of grade ≥2 or
hematological adverse events of grade ≥3 observed were
significantly reduced after implementation of medical interventions
listed in Table IV:
Nausea/vomiting (16.3 vs. 4.5%, P<0.01), constipation (5.4 vs.
0.8%, P<0.01), anorexia (4.3 vs. 1.2%, P<0.01), hyperglycemia
(3.3 vs. 1.7%, P<0.01), oral mucositis/esophagitis (3.1 vs.
1.7%, P<0.05), hepatic or renal dysfunction (2.5 vs. 0.8%,
P<0.01), myalgia/arthralgia/other pain (2.1 vs. 0.2%,
P<0.01), hiccups (2.1 vs. 0.4%, P<0.01), diarrhea (1.2 vs.
0%, P<0.05), malaise (1.2 vs. 0%, P<0.05) and neutropenia
(10.5 vs. 0.6%, P<0.01).
 | Table IVContents of medical interventions for
grade ≥2 adverse events with highest incidence. |
Table IV
Contents of medical interventions for
grade ≥2 adverse events with highest incidence.
| Adverse event
(n) | Intervention
(n) |
|---|
| Nausea/vomiting
(66) | Olanzapine (34),
prochlorperazine (18), metoclopramide (3), granisetron (3), other
(8) |
| Constipation
(33) | Magnesium oxide
(11), sennoside (8), bisacodyl (8), picosulfate (4),
glycerine(2) |
| Anorexia (21) | Follow up (16),
prochlorperazine (3), dexametasone (2) |
| Hepatic or renal
dysfunction (13) | Hepatic
dysfunction: Discontinuation of the suspected drug (3),
glycyrrhizinate (3), renal dysfunction: Hydration (7) |
| Hyperglycemia
(14) | Insulin (14) |
| Peripheral
neuropathy (13) | Pregabalin (11),
duloxetine (2) |
| Oral
mucositis/esophagitis (8) | Steroids (4),
polaprezinc (3), acetaminophen (1) |
|
Myalgia/arthralgia/other pain (9) | Loxoprofen (4),
shakuyaku-kanzo-to (1), follow up (4) |
| Hiccups (11) | Clonazepam (5),
chlorpromazine (3), prochlorperazine (2), shakuyaku-kanzo-to
(1) |
| Diarrhea (6) | Loperamide (2),
trimebutine (2), hange-shashin-to (2) |
| Malaise (3) | Steroids (3) |
| Neutropenia
(55) | G-CSF (55) |
| Anemia (1) | Blood transfusion
(1) |
| Thrombocytopenia
(1) | Blood transfusion
(1) |
Discussion
Here, we report a clinical pharmacist's
interventions for the prevention and treatment of adverse events in
hospitalized patients with thoracic cancer receiving cancer
chemotherapy. Before administration of cancer chemotherapy, the
clinical pharmacist performed a total of 152 interventions for
23.6% of eligible patients, including drug addition (53.9%), dose
adjustment (17.8%), selection (9.9%), discontinuation (7.9%),
examination addition (3.9%), and drug-drug interactions (3.3%).
Therefore, half of the interventions involved addition of a drug
for the prevention of adverse events.
Several studies have reported the efficacy of
clinical pharmacists using physician-approved protocols in
pharmaceutical care (18-20).
In the present study, interventions were implemented by a single
clinical pharmacist before administration of cancer chemotherapy
based on a treatment protocol agreed upon by physicians and
clinical pharmacists in advance. This protocol set the criteria for
starting treatment, dose reduction, drug withdrawal based mainly on
evidence from original reports, and prophylactic supportive care.
As a result, all patients received the appropriate dosage of
anticancer drug and prophylactic supportive therapy based on the
treatment protocol agreed upon by physicians and clinical
pharmacists in advance.
Despite implementation of the appropriate dosage of
anticancer drug and prophylactic supportive therapy with above
mention, 39.4% (191/484) of patients suffered grade ≥2
non-hematological or grade ≥3 hematological adverse events, and the
most common adverse events was nausea/vomiting, followed by
neutropenia, constipation, anorexia, hepatic dysfunction/renal
dysfunction, anemia, hyperglycemia, peripheral neuropathy, oral
mucositis/esophagitis, myalgia/arthralgia/other pain, hiccups,
diarrhea, dysgeusia, malaise and thrombocytopenia. Previous studies
have reported a high incidence of adverse events in patients who
received cancer chemotherapy (21,22).
Borghaei et al reported that 41% (1,624/3,967) of elderly
patients with advanced non-small cell lung cancer on second-line
therapy experienced one or more severe adverse events, of which
hypertension, anemia, and pneumonia were most common (21). In another report, the incidence of
chemotherapy-related adverse events in 1,682 patients with
metastatic breast cancer was 54%, and the most common adverse
events categories were hematological, musculoskeletal/pain related,
gastrointestinal and infection/pyrexia (22).
Our previous findings indicated that implementation
of medical intervention significantly lowered the incidence of a
variety of adverse events in inpatients with head and neck cancer,
including microbial infection, oral mucositis, odynophagia,
neutropenia, insomnia, and constipation (23). Consistent with this, the present
study showed that implementation of medical interventions
significantly reduced the incidence of a variety of adverse events.
Among these, 45.3% of medical interventions for non-hematological
adverse events were implemented based on the pharmacist's
recommendations. Although only 7.5% of interventions for
hematological adverse events were based on the clinical
pharmacist's recommendations, the clinical pharmacist monitored
absolute neutrophil counts and platelet and hemoglobin levels to
assure blood parameters were within acceptable limits for the next
cycle of chemotherapy. The pharmacist's recommendations for medical
interventions primarily contributed to improving peripheral
neuropathy, followed by malaise, diarrhea, oral
mucositis/esophagitis, nausea/vomiting, constipation, hiccups and
myalgia/arthralgia/other pain. Additionally, the medical
interventions significant improved these adverse events, except for
peripheral neuropathy. At present, there are no sufficient
treatment options available for chemotherapy-induced peripheral
neuropathy because the exact pathophysiology is unclear (24).
Several lines of evidence indicate that the
incidence of moderate-to-severe adverse events is associated with
prolonged hospital stay (25-27).
In the present study, the mean time to discharge was markedly
different between patients with grade 0 and 1 events and those with
grade ≥2 events. Therefore, prevention or relief of adverse events
may lead to reduced hospitalization periods.
There are several limitations in the present study.
First, this was a non-randomized single-centre retrospective study.
Second, the sample size was small, limiting our ability to detect
statistically significant differences in the data. Third, the
patient population was limited to inpatients with thoracic cancer
receiving cancer chemotherapy. Therefore, a large-scale,
multi-institutional prospective study is required to confirm our
present findings.
In conclusion, we found that a clinical pharmacist
performed interventions in 23.6% of eligible patients before
administration of cancer chemotherapy. Although all patients
received the appropriate dosage of anticancer drug and prophylactic
supportive therapy based on the agreed treatment protocol, 39.4% of
patients suffered grade ≥2 non-hematological or grade ≥3
hematological adverse events. Implementation of medical
interventions significantly reduced the incidence of adverse
events, among which 45.3 and 7.5% of medical interventions for
non-hematological and hematological adverse events, respectively,
were implemented based on the pharmacist's recommendations. One
pharmacist in the respiratory medicine ward therefore made a marked
contribution to preventing and relieving adverse events in
hospitalized patients with thoracic cancer receiving cancer
chemotherapy.
Acknowledgements
The authors would like to thank Ms. Maya
Tatsumi-Yamada (Gifu University Hospital, Gifu, Japan) for the
assistance in the study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HI, CH, AS and YO conceived and designed the study.
HI, CH, NF, JE, FI, KY and DK were involved with the acquisition of
data. HI, AS and YO analyzed and interpreted the data. HI, CH, JE
and YO assessed the authenticity of all the raw data. HI, CH, NF,
JE, FI, KY, DK, YO and AS drafted the manuscript and revised it
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines for human studies of the ethics committee of Gifu
University Graduate School of Medicine and the Government of Japan,
and was approved by the university's institutional review board
(approval no. 28-348). In view of the retrospective nature of the
study, informed consent from the subjects was not mandated.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vincent C, Neale G and Woloshynowych M:
Adverse events in British hospitals: Preliminary retrospective
record review. BMJ. 322:517–519. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baker GR, Norton PG, Flintoft V, Blais R,
Brown A, Cox J, Etchells E, Ghali WA, Hébert P, Majumdar SR, et al:
The Canadian adverse events study: The incidence of adverse events
among hospital patients in Canada. CMAJ. 170:1678–1686.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hurvitz S, Guerin A, Brammer M, Guardino
E, Zhou ZY, Latremouille Viau D, Wu EQ and Lalla D: Investigation
of adverse-event-related costs for patients with metastatic breast
cancer in a real world setting. Oncologist. 19:901–908.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wong W, Yim YM, Kim A, Cloutier M,
Gauthier-Loiselle M, Gagnon-Sanschagrin P and Guerin A: Assessment
of costs associated with adverse events in patients with cancer.
PLoS One. 13(e0196007)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rashid N, Koh HA, Baca HC, Li Z, Malecha
S, Abidoye O and Masaquel A: Clinical impact of
chemotherapy-related adverse events in patients with metastatic
breast cancer in an integrated health care system. J Manag Care
Spec Pharm. 21:863–871. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Caggiano V, Weiss RV, Rickert TS and
Linde-Zwirble WT: Incidence, cost, and mortality of neutropenia
hospitalization associated with chemotherapy. Cancer.
103:1916–1924. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shune SE, Karnell LH, Karnell MP, Van
Daele DJ and Funk GF: Association between severity of dysphagia and
survival in patients with head and neck cancer. Head Neck.
34:776–784. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mathijssen RH, Sparreboom A and Verweij J:
Determining the optimal dose in the development of anticancer
agents. Nat Rev Clin Oncol. 11:272–281. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ranchon F, Salles G, Späth HM, Schwiertz
V, Vantard N, Parat S, Broussais F, You B, Tartas S, Souquet PJ, et
al: Chemotherapeutic errors in hospitalised cancer patients:
Attributable damage and extra costs. BMC Cancer.
11(478)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Phillips J, Beam S, Brinker A, Holquist C,
Honig P, Lee LY and Pamer C: Retrospective analysis of mortalities
associated with medication errors. Am J Health Syst Pharm.
58:1835–1841. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chewning B and Wiederholt JB: Concordance
in cancer medication management. Patient Educ Couns. 50:75–78.
2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Strasser F, Sweeney C, Willey J,
Benisch-Tolley S, Palmer JL and Bruera E: Impact of a half-day
multidisciplinary symptom control and palliative care outpatient
clinic in a comprehensive cancer center on recommendations, symptom
intensity, and patient satisfaction: A retrospective descriptive
study. J Pain Symptom Manage. 27:481–491. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Iihara H, Ishihara M, Matsuura K,
Kurahashi S, Takahashi T, Kawaguchi Y, Yoshida K and Itoh Y:
Pharmacists contribute to the improved efficiency of medical
practices in the outpatient cancer chemotherapy clinic. J Eval Clin
Pract. 18:753–760. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shah S, Dowell J and Greene S: Evaluation
of clinical pharmacy services in a hematology/oncology outpatient
setting. Ann Pharmacother. 40:1527–1533. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Han JM, Ah YM, Suh SY, Jung SH, Hahn HJ,
Im SA and Lee JY: Clinical and economic impact of pharmacists'
intervention in a large volume chemotherapy preparation unit. Int J
Clin Pharm. 38:1124–1132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah S, Dowell J and Greene S: Evaluation
of clinical pharmacy services in a hematology/oncology outpatient
setting. Ann Pharmacother. 40:1527–1533. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Suzuki H, Suzuki S, Kamata H, Sugama Y,
Demachi K, Ikegawa K, Igarashi T and Yamaguchi M: Impact of
pharmacy collaborating services in an outpatient clinic on
improving adverse drug reactions in outpatient cancer chemotherapy.
J Oncol Pharm Pract. 25:1558–1563. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Martin JK Jr and Norwood MB: Pharmacist
management of antiemetic therapy under protocol in an oncology
clinic. Am J Hosp Pharm. 45:1322–1328. 1988.PubMed/NCBI
|
|
19
|
Horne AL and Dapolite LA: Protocol for
pharmacist management of antineoplastic drug-induced adverse
effects in outpatients. Am J Health Syst Pharm. 54:680–683.
1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gums TH, Uribe L, Vander Weg MW, James P,
Coffey C and Carter BL: Pharmacist intervention for blood pressure
control: Medication intensification and adherence. J Am Soc
Hypertens. 9:569–578. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Borghaei H, Yim YM, Guerin A, Pivneva I,
Shi S, Gandhi M and Ionescu-Ittu R: Severe adverse events impact
overall survival and costs in elderly patients with advanced
non-small cell lung cancer on second-line therapy. Lung Cancer.
119:112–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rashid N, Koh HA, Baca HC, Lin KJ, Malecha
SE and Masaquel A: Economic burden related to chemotherapy-related
adverse events in patients with metastatic breast cancer in an
integrated health care system. Breast Cancer. 8:173–181.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Suzuki A, Kobayashi R, Okayasu S, Kuze B,
Aoki M, Mizuta K and Itoh Y: Pharmacotherapy for adverse events
reduces the length of hospital stay in patients admitted to
otolaryngology ward: A single arm intervention study. PLoS One.
9(e115879)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hou S, Huh B, Kim HK, Kim KH and Abdi S:
Treatment of chemotherapy-induced peripheral neuropathy: Systematic
review and recommendations. Pain Physician. 21:571–592.
2018.PubMed/NCBI
|
|
25
|
Classen DC, Pestotnik SL, Evans RS, Lloyd
JF and Burke JP: Adverse drug events in hospitalized patients.
Excess length of stay, extra costs, and attributable mortality.
JAMA. 277:301–306. 1997.PubMed/NCBI
|
|
26
|
Bates DW, Spell N, Cullen DJ, Burdick E,
Laird N, Petersen LA, Small SD, Sweitzer BJ and Leape LL: The costs
of adverse drug events in hospitalized patients. JAMA. 277:307–311.
1997.PubMed/NCBI
|
|
27
|
Nishida S, Hayashi Y, Suzuki A, Kobayashi
R, Inuzuka T and Itoh Y: Relationship between number of drugs and
duration of hospital stay in older patients with neuromuscular
diseases. Geriatr Gerontol Int. 18:1018–1024. 2018.PubMed/NCBI View Article : Google Scholar
|