Introduction
Breast cancer is the most common site-specific
cancer and the leading cause of cancer-related death in women aged
20 to 39 years (1). The disease is
a current global health problem, and more than 1 million cases are
newly diagnosed each year (2). It
is estimated that 1 in 8 of women in the United States (12.4%) is
affected by invasive breast cancer in their lifetime (3). Moreover, in 2018, approximately 64,000
woman were diagnosed with in situ breast cancer, and
approximately 41 thousand of women in the US died from this disease
(4). However, approximately 50% of
breast cancer incidence globally and 60% of breast cancer mortality
occur in middle- to low-income countries, including Thailand, where
the age-standardized incidence of breast cancer was 28.5 per
100,000 from 2010 to 2012 (2,5).
Currently, strategies for breast cancer diagnosis include imaging
and pathological studies, and clinicopathological aspects such as
tumor size, TNM stage, hormone receptor status, and molecular
subtype are used for therapeutic planning and prognostication
(6).
β2-microglobulin (β2-M) is a low-molecular-weight
protein consisting of a single chain of 100 amino acids that is a
part of the invariant light chain of the HLA antigen molecule
(7,8). It is expressed on the membrane of
almost all nucleated cells and is detectable in all body fluids as
a shedding product of cell membranes (9,10).
Ninety percent of β2-M is eliminated through glomerular filtration
and is almost completely reabsorbed by the proximal convoluting
tubules. At the clinical level, serum and urine β2-M concentrations
are used to monitor glomerular and tubular nephropathies (11,12).
The levels of serum and urine β2-M are also increased in patients
with neoplastic diseases, including multiple myeloma, lymphoma and
leukemia (13-16).
Increased serum β2-M levels reflect increased cellular turnover
rate and disease progression in some hematologic malignancies
(17,18). For example, a β2-M value of less
than 4 µg/ml was found to correlate with better survival in
multiple myeloma (19). Increased
β2-M serum levels in patients with breast cancer has been reported
(20,21). An immunohistochemical (IHC) study
reported that serum β2-microglobulin levels in patients with breast
cancers were significantly higher than those in patients with
benign breast tumors. In addition, the expression levels of β2-M
protein in breast cancer tissue were found to be significantly
different among patients with the 4 molecular subtypes (22,23).
However, the clinical value of serum β2-M as a prognostic marker
and predictor of survival needs further study (24).
The aim of this study was to evaluate the
association between serum β2-M levels and the clinicopathological
characteristics of breast cancer patients, especially the intrinsic
subtypes and clinical stages. In addition, an in vitro study
of the influence of β2-M on the cellular migration of a breast
cancer cell line was conducted.
Materials and methods
Subjects and study protocols
The study design was a prospective cohort. Serum
samples from a total of 200 female patients with histologically
confirmed invasive breast cancer at Songklanagarind Hospital and
adequate pathological data were collected from 2017 to 2019 after
informed consent was obtained. The exclusion criteria included
those with abnormal renal function, those who had previously
received any form of treatment for breast cancer and those with
other cancers. Pathologic stage and cancer subtype were identified
according to the AJCC 8th Edition (25) and St. Gallen International Expert
Consensus 2013(26), respectively.
Metastatic work-up included abdominal ultrasound and chest
computerized tomography. The metastatic status of all patients was
confirmed by radiology and histopathological evidence. Treatment of
breast cancer in our institute followed the Adult Cancer Treatment
Guideline of Thai National Health Security Office (2018) with
individual adjustment according to the functional status. Patients
were appointed every 3 months during the period of active
treatment, then every 6 months, thereafter. The Human Research
Ethics Committee of the Faculty of Medicine, Prince of Songkla
University, approved the study protocol (Reference no.
60-040-10-1).
Serum β2-M
Serum β2-M level was measured once at the time of
breast cancer diagnosis. Blood was collected under aseptic
precautions. Serum was separated and immediately analyzed by
immunological agglutination with latex reaction enhancement assay
using Immunoturbidimetric Assay kit (Roche/Hitachi). The ratio of
reaction is 2:180:80 for sample:buffer:latex suspension. The
concentration of β2-microglobulin was evaluated by measuring the
agglutination reaction at 570 nm compared to the absorbance of
standard β2-microglobulin.
IHC staining
The expression of ER, PR, HER-2 and Ki67 in tumor
tissues was evaluated by IHC staining with the following primary
antibodies: Anti-ER (Thermo Scientific, Inc., clone SP1, 1/250
dilution), anti-PR (Leica Biosystems, 1/2500 dilution), anti-Ki-67
antigen (Dako Glostrup, 1/250 dilution), and anti-HER2 (Ventana).
The staining results were reported by a certified pathologist as
the number of positive cells per 100 cancer cells. In cases with
equivocal HER2 results, the specimens were further studied by HER-2
dual in situ hybridization (HER2-DISH).
HER2-DISH
HER2 and chromosome 17 probes were used for
two-colour chromogenic in situ hybridization of
formalin-fixed paraffin-embedded human breast cancer specimens
following the VENTANA BenchMark XT automated slide staining
protocol. The slides were evaluated under light microscopy for HER2
and chromosome 17 signals in at least 20 nuclei. Calculation of the
HER2/chromosome 17 ratio was performed by dividing the total number
of HER2 signals in the target area by the total number of
chromosome 17 signals in the same area.
Cell migration assay
The cell migration assay was conducted in a 24-well
plate with Transwell chambers (8-µm pore PET membrane; Falcon,
Fisher Scientific). The low migratory cell line, MCF-7, obtained
from American Type Culture Collection (ATCC; Catalog HTB-2), and
routinely cultured on monolayers at ≤80% confluence in RPMI-1640
medium (Gibco) containing 10% FBS at 37˚C, 5% CO2 was
used in this study. After starvation for 24 h, 2x105
cells in 100 µl 1% FBS RPMI-1640 medium with or without recombinant
β2-microglobulin (Abcam) were added to the upper compartment of the
chamber, whereas the lower chamber contained RPMI-1640 with 10%
FBS. After 72 h of incubation at 37˚C, the cells on the upper
surface were removed using a cotton swab. The membranes were fixed
with 70% ethanol for 10 min at room temperature and stained with
0.1% crystal violet for 10 min. The number of migrated cells was
quantified by counting cells in five different fields of view under
a light microscope at a magnification of 200x. The data are
presented as the mean ± SD.
Statistical analysis
The association between β2-M levels and
clinicopathological factors was analyzed by using unpaired
Student's t-test or one-way ANOVA with Tukey's post hoc test. As
age at diagnosis was a major confounder, patients were
subcategorized into 2 groups on the basis of age, those aged 25-55
years and those aged >55 years, for analysis of the association
between metastatic status and β2-M level. For the Transwell
migration study, one-way ANOVA followed by Turkey's post hoc test
was used to determine statistical significance between groups. A
receiver operating characteristic (ROC) curve was plotted using the
sensitivity and specificity of each β2-M cut-off that predicted
metastatic status. Survival analysis used log rank test and
Kaplan-Meier survival plot with cancer related death used as a
censor in overall survival (OS) analysis. Beginning date used
operative date and survival data were as of December 2020. All data
were analyzed with Statistical Package Stata 14.0 (Stata
Corporation). A P-value of less than 0.05 was considered
statistically significant.
Results
Patients and clinical data
A total of 200 patients were included in this study.
The mean age at the time of diagnosis was 54 years (range 25-88
years). The most common histological type was invasive ductal
carcinoma (188 cases; 94%), where the most frequent tumor grade was
grade III (83 cases; 41.5%). The percentage of patients with
positive lymphovascular invasion was 36.5% (73 cases). Lymph node
involvement was most frequently seen in the N0 group (121 cases;
60.5%). T2 was the highest group among the T stages (108 cases;
54%). The percentage of patients with distant metastasis was 4% (8
cases). Regarding tumor molecular subtypes, luminal B was the most
common tumor subtype, followed by luminal A (Table I). The example of HER-2 staining and
HER-2-DISH was showed as Fig.
S1.
 | Table ISerum β2-M levels in patients with
breast cancer according to pathological parameters. |
Table I
Serum β2-M levels in patients with
breast cancer according to pathological parameters.
| Variable | No. (%) | Average β2-M, µg/ml
(range) | P-value |
|---|
| Total cases | 200 (100.0) | 1.83 (0.5-4.2) | |
| Age, years | | | <0.01a |
|
0-55 | 110 (55.0) | 1.64 (0.5-4.0) | |
|
>55 | 90 (45.0) | 2.05 (0.9-4.2) | |
| Tumor side | | | 0.70a |
|
Right | 101 (50.5) | 1.84 (1.0-4.2) | |
|
Left | 99 (49.5) | 1.81 (0.5-4.0) | |
| Histologic type | | | 0.02a |
|
Invasive
ductal carcinoma | 188 (94.0) | 1.80 (0.5-4.2) | |
|
Lobular
carcinoma and others | 12 (6.0) | 2.21 (1.9-2.5) | |
| Tumor grade | | | 0.41b |
|
Grade-1 | 36 (18.0) | 1.79 (1.0-3.8) | |
|
Grade-2 | 81 (40.5) | 1.89 (1.1-4.0) | |
|
Grade-3 | 83 (41.5) | 1.77 (0.5-4.2) | |
| Lymphovascular
invasion | | | 0.54a |
|
No | 127 (63.5) | 1.84 (0.9-4.2) | |
|
Yes | 73 (36.5) | 1.79 (0.5-4.0) | |
| T-stage | | | 0.17b |
|
T1 | 75 (37.5) | 1.79 (1-3.8) | |
|
T2 | 108 (54.0) | 1.81 (0.5-4) | |
|
T3 | 9 (4.5) | 2.26 (1.3-4.2) | |
|
T4 | 8 (4.0) | 1.79 (1.3-2.3) | |
| N-stage | | | 0.72b |
|
N0 | 121 (60.5) | 1.85 (0.9-4.0) | |
|
N1 | 52 (26.0) | 1.83 (0.5-4.2) | |
|
N2 | 18 (9.0) | 1.71 (1.3-2.5) | |
|
N3 | 9 (4.5) | 1.71 (1.2-2.1) | |
| M-stage | | |
<0.01a |
|
M0 | 192 (96.0) | 1.80 (0.5-4.0) | |
|
M1 | 8 (4.0) | 2.40 (1.4-4.2) | |
| Clinical stage | | | 0.15b |
|
Stage 1 | 58 (29.0) | 1.83 (1.0-3.8) | |
|
Stage 2 | 104 (52.0) | 1.81 (0.5-4.0) | |
|
Stage 3 | 30 (15.0) | 1.70 (1.2-3.1) | |
|
Stage 4 | 8 (4.0) | 2.40 (1.4-4.2) | |
| ER status | | | 0.75a |
|
Negative | 42 (21.0) | 1.80 (0.9-4.0) | |
|
Positive | 158 (79.0) | 1.83 (0.5-4.2) | |
| PR status | | | 0.74a |
|
Negative | 68 (34.0) | 1.84 (0.9-4.2) | |
|
Positive | 132 (66.0) | 1.81 (0.5-4.0) | |
| HER2 status | | | 0.71b |
|
Negative | 133 (66.5) | 1.82 (0.5-4.0) | |
|
Equivocal | 22 (11.0) | 1.92 (1.3-3.3) | |
|
Positive | 45 (22.5) | 1.81 (0.9-4.2) | |
| Intrinsic
subtype | | | 0.54b |
|
Luminal
A | 78 (39.0) | 1.77 (0.5-4.0) | |
|
Luminal
B | 88 (44.0) | 1.89 (0.9-4.2) | |
|
HER-2 | 18 (9.0) | 1.72 (0.9-2.6) | |
|
Triple
negative | 24 (12.0) | 1.85 (1.2-4.0) | |
β2-M levels were associated with
metastatic status and survival rate in breast cancer patients
The serum β2-M levels in female breast cancer
patients according to each pathological parameter are shown in
Table I. Statistically significant
differences in β2-M levels were found to be associated with age,
histologic type, and metastatic status. As our data showed that
serum β2-M levels were correlated with age, the level was
re-analyzed with age stratification. The association between β2-M
levels and metastatic status held true only in those aged >55
years (Table II).
 | Table IIComparing serum β2-M levels
stratified by age group. |
Table II
Comparing serum β2-M levels
stratified by age group.
| Age group | β2-M levels in no
metastasis group, µg/ml | β2-M levels in
metastasis group, µg/ml |
P-valuea |
|---|
| 25-55 years (N
106:4) | 1.63 | 1.98 | 0.14 |
| >55 years (N
86:4) | 2.01 | 2.82 | 0.01 |
To evaluate the correlation between serum β2-M
levels and the prediction of metastatic status in breast cancers, a
receiver operating curve (ROC) was constructed, as shown in
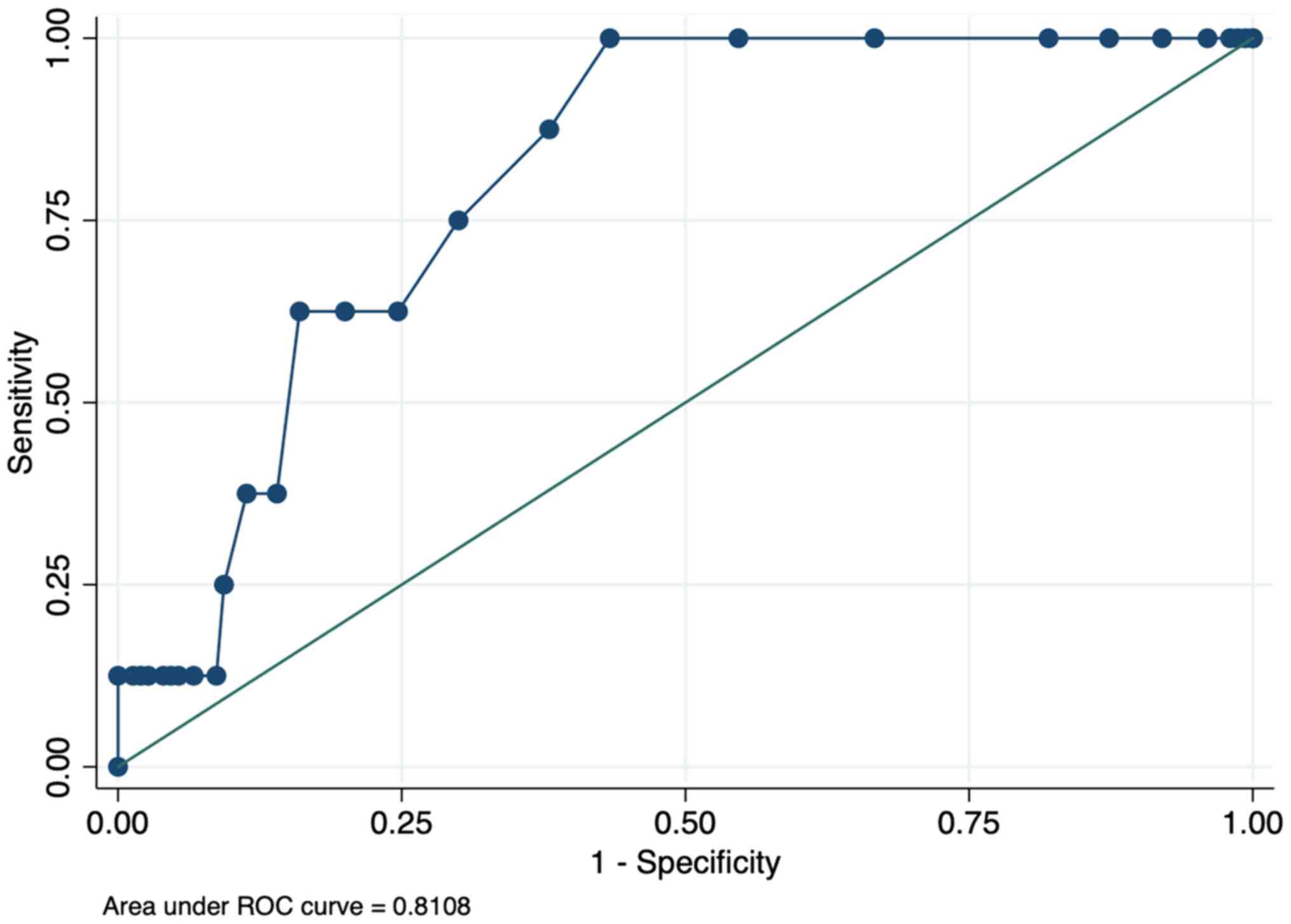
Fig. 1. The area under the curve
(AUC) was 0.78. If a serum β2-M level of 1.9 µg/ml was used as a
cut-off, the sensitivity to diagnose the metastatic status was
87.5%, the specificity was 65.0%, and the diagnostic odds ratio was
2.47. Details of the diagnostic value of β2-M using different
cut-off values are shown in Table
SI. When the ROCs of β2-M performance for diagnosing metastasis
were replotted by age subgroup, the AUC of the group of age 25-to
55-year-old group and the >55-year-old group were 0.75 (logistic
regression P-value 0.22) and 0.82 (P-value 0.03), respectively.
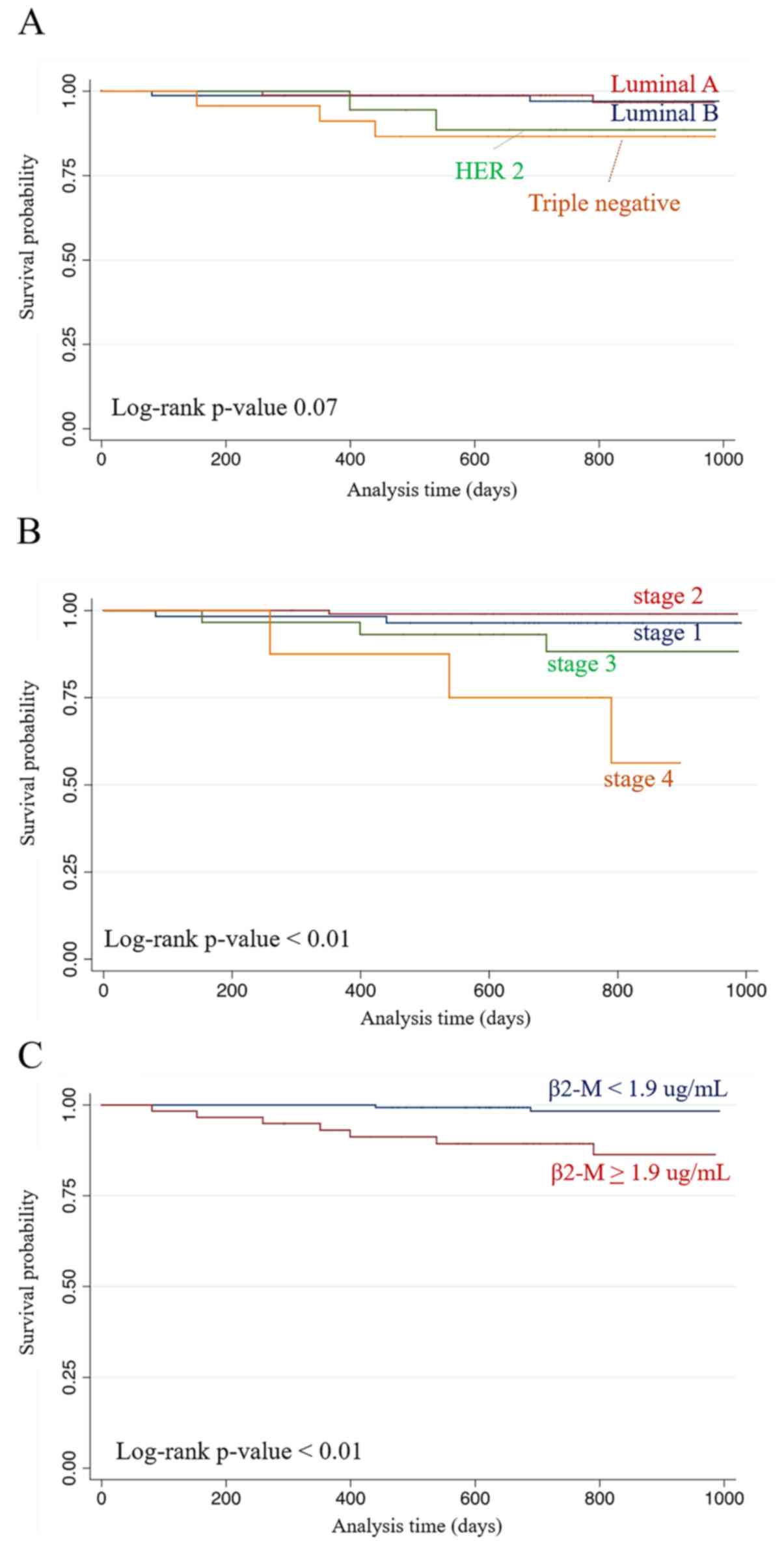
Median follow-up duration was 27.8 months and 24-month OS in all
cases was 95.7%. Two-year overall survival (2-year OS) analysis was
performed by cancer types, stages, and β2-M levels. The results
showed that no significance of difference was found in 2-years OS
when patients were sub-grouped by cancer types (log rank
P-value=0.07) (Fig. 2A). However,
when sub-grouping by stage of cancer, the significance of 2-year OS
was found with the percentage of 96.4, 99.0, 88.2 and 75.0% for
stages I-III and IV, respectively (log rank P-value <0.01)
(Fig. 2B). Two-year OS in cases
with serum β2-M levels lower than 1.9 µg/ml (98.3%) was also
significantly higher than that of high serum β2-M (89.3%, log rank
P-value <0.01) (Fig. 2C).
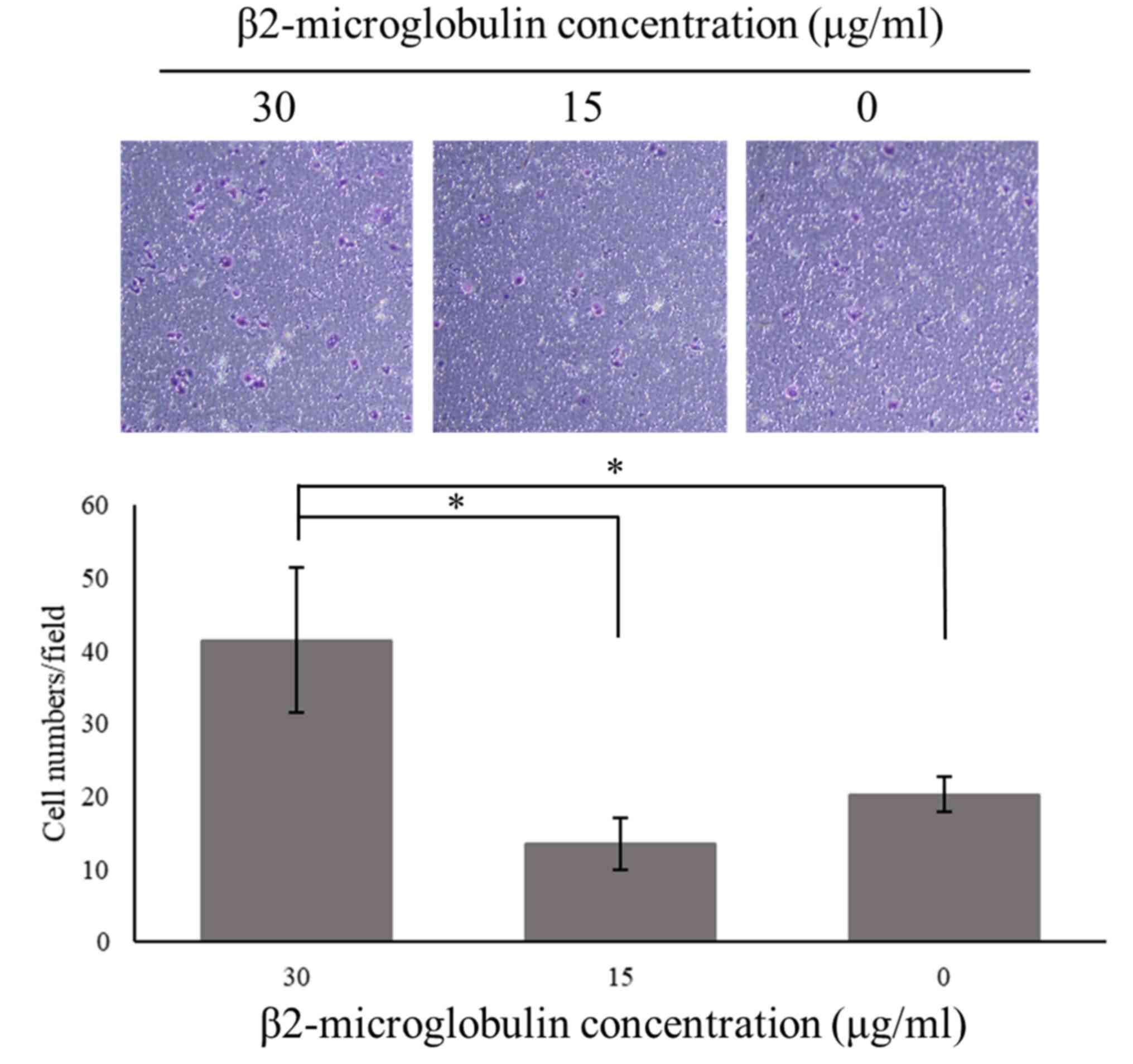
β2-M promoted migration ability of
human breast cancer cell line
To study the influence of β2-M on the metastatic
capability of breast cancer, the low migratory MCF-7 cell line was
subjected to the Transwell cell migration assay. Cells were treated
with human recombinant β2-M at different concentrations and
incubated for 72 h. The results revealed that ectopic treatment
with β2-M stimulated MCF-7 breast cancer cell migration, especially
at 30 µg/ml, and that the number of migrated cells was decreased at
lower concentrations (Fig. 3).
These findings indicated that β2-M had a direct functional effect
on MCF-7 cell movement.
Discussion
Human leukocyte antigen (HLA) consists of a heavy
chain containing the alpha1, 2 and 3 domains and a light chain,
which is a linked form of β2-M. β2-M can be dissociated from HLA
molecules and found as a free form in extracellular fluid and can
also be detected in the blood (27,28).
Generally, the levels of β2-M is directly dependent on the kidney's
function and cell turnover rate; however, some studies have found
correlation between the serum level of this protein and several
cancers (29). The association
between female breast cancer and tissue expression β2-M has been
observed in previous reports (22,23).
Higher serum β2-M in breast cancer patients has been reported since
1977 by Transdale and colleagues (30). The mean serum β2-M in our cases was
lower than that reported by Teasdale. Interlaboratory variation may
explain this disparity. Our main findings of an association between
high serum β2-M levels and metastatic status in breast cancer were
consistent with recent studies which reported higher serum β2-M
levels in metastatic breast cancer patients than in those with
early-stage or locally advanced diseases (31). In addition, our study also provided
the suggested cut-off value of β2-M at 1.9 µg/ml that might predict
metastatic status and also demonstrated poorer survival probability
in patients with high serum β2-M.
Roles of β2-M in the promotion of the epithelial to
mesenchymal transition (EMT) in cancers, which is the key scenario
in cancer metastasis have been reported (32). Overexpression of β2-M in cancers
induces the invasion and migration ability of breast, lung, and
renal cancer cell lines (33). In
addition, β2-M induces bone and soft tissue metastasis in mice, and
it can be used as a therapeutic target (29,33).
This evidence supports our findings and recent studies reporting
high serum levels of β2-M in cancer patients with distant
metastasis (34). In accordance
with our results, when poorly migrating cancer cells were exposed
to high levels of recombinant β2-M, a higher migration ability was
observed. This finding supports the role of β2-M in promoting
cancer cell metastasis. However, the detailed mechanism of β2-M in
cancer metastasis should be further evaluated. Our data suggested
that serum β2-M might be used as a marker for advanced disease at
the time of diagnosis or during post-treatment follow-up.
Another consideration is that our data showed that
serum β2-M levels significantly varied with age, which may be
related to the fact that β2-M excretion decreases with a reduction
in glomerular filtration rate in the elderly. This same
physiological process may explain the significant association
between the serum β2-M level and metastasis in the group of
patients aged >55 years but not in the younger patients. Intact
renal function and rapid clearance of β2-M from the circulation
results in less exposure of cancer cells to this
migration-promoting substance. The limitations of our study were
its cross-sectional design; in which no chronological data on
alteration of β2-M level can be analyzed. However, our data was
consistent with a previous study in that patients with metastatic
breast cancer and high β2-M significantly had poorer survival
(35).
In conclusion, serum β2-M levels were significantly
higher in women with metastatic breast cancer than in those with
cancer of less advanced stages. In addition, the high level was
correlated with poorer survival outcome. Serum β2-M may be a
non-invasive marker of metastatic status in breast cancer, and the
cut-off level of 1.9-2.0 µg/ml might be used for metastatic
prediction with a greater than 85% sensitivity.
Supplementary Material
Immunohistochemical and DISH analysis
of HER-2 in breast cancer. (A) Immunohistochemical score 0. No
staining on tumor cell membrane. (B) Immunohistochemical score 1.
Faintly perceptible staining on >10% tumor cell membrane. (C)
Immunohistochemical score 2. Moderate staining on >10% tumor
cell membrane. (D) Immunohistochemical score 3. Strong staining on
>10% tumor cell membrane. (E) Case with HER-2 amplification
using DISH analysis. Pink color indicates the reference probe of
Chr. 17 centromere, while brown color is the target probe for
HER-2. Scale bars, 200 μm. DISH, dual color in situ
hybridization.
Sensitivity, specificity and positive
likelihood ratio of each β2-microglobulin cut-off value in
predicting metastatic breast cancer.
Acknowledgements
The authors would like to thank Mr. David Patterson
of the International Affairs Office, Faculty of Medicine, Prince of
Songkla University (Hat Yai, Songkhla, Thailand) for manuscript
proofreading and language editing.
Funding
This research was supported and funded by the Faculty of
Medicine, Prince of Songkla University (grant no. REC 60-040-010-1)
with postdoctoral fellowship program of the Faculty of Medicine,
Prince of Songkla University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM and SSan conceived the study. SJ, SM, SSam, KK,
JS and SSan developed the methodology. SSan provide software. SM
and SSan validated the data. SJ, SSan, JS and SM performed formal
analysis. SJ, KK and JS performed investigations. SSam and KK
provided resources. SJ, SSan and SM curated data. SJ and JS wrote
the original draft. SSan and JS reviewed and edited the manuscript.
SSan and SM visualized data. SM supervised the study. SM was the
project administrator. SM acquired funding. The authenticity of all
the raw data was confirmed by SSan and SM. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The board of The Human Research Ethics Committee of
Faculty of Medicine, Prince of Songkla University approved the
study protocol (reference no. 60-040-10-1; Hat Yai, Songkhla,
Thailand). Written informed consent was obtained at the time of
original sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fidler MM, Gupta S, Soerjomataram I,
Ferlay J, Steliarova-Foucher E and Bray F: Cancer incidence and
mortality among young adults aged 20-39 years worldwide in 2012: A
population-based study. Lancet Oncol. 18:1579–1589. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Forman D, Bray F, Brewster DH, Gombe MC,
Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R and Ferlay
J (eds): Cancer incidence in five continents. Vol. 10 (electronic
version). IARC Scientific Publications No. 164. International
Agency for Research on Cancer, Lyon, 2013.
|
|
3
|
Parada H Jr, Sun X, Tse CK, Olshan AF and
Troester MA: Lifestyle patterns and survival following breast
cancer in the carolina breast cancer study. Epidemiology. 30:83–92.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alkabban FM and Ferguson T: Breast cancer.
In: StatPearls. StatPearls Publishing, Treasure Island (FL),
2020.
|
|
5
|
Imsamran W, Chaiwerawattana A, Wiangnon S,
Pongnikorn D, Suwanrungrung K, Sangrajrang S and Buasom R: Cancer
in Thailand. Vol. 8, 2010-2012. New Thammada Press (Thailand) Co.,
Ltd., 2015.
|
|
6
|
Park S, Koo JS, Kim MS, Park HS, Lee JS,
Lee JS, Kim SI and Park BW: Characteristics and outcomes according
to molecular subtypes of breast cancer as classified by a panel of
four biomarkers using immunohistochemistry. Breast. 21:50–57.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Grey HM, Kubo RT, Colon SM, Poulik MD,
Cresswell P, Springer T, Turner M and Strominger JL: The small
subunit of HL-A antigens is beta 2-microglobulin. J Exp Med.
138:1608–1612. 1973.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cresswell P, Springer T, Strominger JL,
Turner MJ, Grey HM and Kubo RT: Immunological identity of the small
subunit of HL-A antigens and beta2-microglobulin and its turnover
on the cell membrane. Proc Natl Acad Sci USA. 71:2123–2127.
1974.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Karlsson FA, Groth T, Sege K, Wibell L and
Peterson PA: Turnover in humans of beta 2-microglobulin: The
constant chain of HLA-antigens. Eur J Clin Invest. 10:293–300.
1980.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Karlsson FA, Wibell L and Evrin PE: Beta
2-microglobulin in clinical medicine. Scand J Clin Lab Invest
Suppl. 154:27–37. 1980.PubMed/NCBI
|
|
11
|
Hofstra JM, Deegens JK, Willems HL and
Wetzels JF: Beta-2-microglobulin is superior to
N-acetyl-beta-glucosaminidase in predicting prognosis in idiopathic
membranous nephropathy. Nephrol Dial Transplant. 23:2546–2551.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nozue T, Michishita I and Mizuguchi I:
Predictive value of serum cystatin C, β2-microglobulin, and urinary
liver-type fatty acid-binding protein on the development of
contrast-induced nephropathy. Cardiovasc Interv Ther. 25:85–90.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Diem H, Fateh-Moghadam A and Lamerz R:
Prognostic factors in multiple myeloma: Role of beta
2-microglobulin and thymidine kinase. Clin Investig. 71:918–923.
1993.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Musarurwa C and Matarira HT:
Beta-2-microglobulin in multiple myeloma. Cent Afr J Med. 50:19–20.
2004.PubMed/NCBI
|
|
15
|
Vassilakopoulos TP, Nadali G, Angelopoulou
MK, Dimopoulou MN, Siakantaris MP, Kontopidou FN, Karkantaris C,
Kokoris SI, Dimitriadou EM, Calpadaki C, et al:
Beta(2)-microglobulin in Hodgkin's lymphoma: Prognostic
significance in patients treated with ABVD or equivalent regimens.
J BUON. 10:59–69. 2005.PubMed/NCBI
|
|
16
|
Berrebi A, Bassous L, Haran M, Shtalrid M
and Shvidel L: The significance of elevated beta 2-microglobulin
(b2-m) in chronic lymphocytic leukemia (CLL): Evidence of in vitro
secretion following activation of CLL cells. Leuk Res.
34:e248–e249. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vassilakopoulos TP, Nadali G, Angelopoulou
MK, Siakantaris MP, Dimopoulou MN, Kontopidou FN, Karkantaris C,
Kokoris SI, Kyrtsonis MC, Tsaftaridis P, et al: The prognostic
significance of beta(2)-microglobulin in patients with Hodgkin's
lymphoma. Haematologica. 87:701–708. 2002.PubMed/NCBI
|
|
18
|
Tsimberidou AM, Kantarjian HM, Wen S,
O'Brien S, Cortes J, Wierda WG, Koller C, Pierce S, Brandt M,
Freireich EJ, et al: The prognostic significance of serum beta2
microglobulin levels in acute myeloid leukemia and prognostic
scores predicting survival: Analysis of 1,180 patients. Clin Cancer
Res. 14:721–730. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Durie BG, Stock-Novack D, Salmon SE,
Finley P, Beckord J, Crowley J and Coltman CA: Prognostic value of
pretreatment serum beta 2 microglobulin in myeloma: A Southwest
oncology group study. Blood. 75:823–830. 1990.PubMed/NCBI
|
|
20
|
Papaioannou D, Geggie P and Klassen J:
Study of serum beta-2 microglobulin levels in breast cancer
patients. Clin Chim Acta. 99:37–41. 1979.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Klein B, Levin I, Kfir B, Mishaeli M,
Shapira J and Klein T: The significance of soluble interleukin-2,
soluble interleukin-2 receptors, soluble ICAM-1 and beta
2-microglobulin in breast cancer patients. Tumour Biol. 16:290–296.
1995.PubMed/NCBI
|
|
22
|
Li K, Du H, Lian X, Yang S, Chai D, Wang
C, Yang R and Chen X: Characterization of β2-microglobulin
expression in different types of breast cancer. BMC Cancer.
14(750)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chai D, Li K, Du H, Yang S, Yang R, Xu Y
and Lian X: β2-microglobulin has a different regulatory molecular
mechanism between ER+ and ER- breast cancer
with HER2. BMC Cancer. 19(223)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tsuda H, Hirohashi S, Higuchi K and
Shimosato Y: Beta-2-microglobulin expression in relation to
amplification of oncogenes and prognosis in breast carcinoma.
Histopathology. 16:500–502. 1990.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou B, Xu L, Ye J, Xin L, Duan X and Liu
Y: The prognostic value of the 8th edition of the American joint
committee on cancer (AJCC) staging system in HER2-enriched subtype
breast cancer, a retrospective analysis. Anticancer Res.
37:4615–4621. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khaled H, Gamal H, Lotayef M, Knauer M and
Thürliman B: The St. Gallen international expert consensus
conference on the primary therapy of early breast cancer 2017:
Egyptian view. Breast Cancer Res Treat. 172:545–550.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wibell L: The serum level and urinary
excretion of beta2-microglobulin in health and renal disease.
Pathol Biol (Paris). 26:295–301. 1978.PubMed/NCBI
|
|
28
|
Karlsson FA, Dahlberg PA, Venge P and
Roxin LE: Serum myoglobin in thyroid disease. Acta Endocrinol
(Copenh). 94:184–187. 1980.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shi C, Zhu Y, Su Y, Chung LW and Cheng T:
Beta2-microglobulin: Emerging as a promising cancer therapeutic
target. Drug Discov Today. 14:25–30. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Teasdale C, Mander AM, Fifield R, Keyser
JW, Newcombe RG and Hughes LE: Serum beta2-microglobulin in
controls and cancer patients. Clin Chim Acta. 78:135–143.
1977.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Petekkaya I, Aksoy S, Roach EC, Okoh AK,
Gecmez G, Gezgen G, Isler DC, Dogan E, Babacan T, Sarici F, et al:
Impact of inflammatory markers on the prognosis of patients with
operable breast cancer. J BUON. 19:673–680. 2014.PubMed/NCBI
|
|
32
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Josson S, Nomura T, Lin JT, Huang WC, Wu
D, Zhau HE, Zayzafoon M, Weizmann MN, Gururajan M and Chung LW:
β2-Microglobulin induces epithelial to mesenchymal transition and
confers cancer lethality and bone metastasis in human cancer cells.
Cancer Res. 71:2600–2610. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen CH, Su CY, Chien CY, Huang CC, Chuang
HC, Fang FM, Huang HY, Chen CM and Chiou SJ: Overexpression of
beta2-microglobulin is associated with poor survival in patients
with oral cavity squamous cell carcinoma and contributes to oral
cancer cell migration and invasion. Br J Cancer. 99:1453–1461.
2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Petekkaya I, Unlu O, Roach EC, Gecmez G,
Okoh AK, Babacan T, Sarici F, Keskin O, Arslan C, Petekkaya E, et
al: Prognostic role of inflammatory biomarkers in metastatic breast
cancer. J BUON. 22:614–622. 2017.PubMed/NCBI
|