Introduction
Gastric cancer (GC) ranks as the second-most prevalent cancer among men and the fifth-most prevalent cancer among women when considering all types of cancer reported in China (1). The presence of human epidermal growth factor receptor 2 (HER2) overexpression accounts for 15-20% of gastric adenocarcinomas. The incorporation of trastuzumab, a monoclonal antibody targeting HER2, into chemotherapy has led to a significant increase in the median overall survival (mOS) as compared with that attributed to chemotherapy alone (2). In particular, alpha-fetoprotein (AFP)-secreting GC (AFP-GC) is a relatively rare, aggressive malignancy with a prevalence of 1.3-15% among all GC cases worldwide. Currently, no standard therapy is available for AFP-GC, which consequently results in extremely poor prognoses for patients afflicted with this condition. Similarly, no standard of care exists for treating patients with advanced GC characterized by AFP- and HER2-positivity.
Apatinib, produced by the Hengrui Medicine Company of China, is a small-molecular tyrosine kinase inhibitor administered orally that functions as an antiangiogenesis agent via targeting vascular endothelial growth factor receptor-2 (VEGFR-2). At present, the Chinese Society of Clinical Oncology (CSCO) has recommended apatinib be used as a third-line treatment for advanced GC (3). So far, only one study explored the use of apatinib for GC with AFP-secretion (4). Meanwhile, no investigation has assessed apatinib treatment in AFP-secretive and HER2-positive metastatic GC patients. We herein report that apatinib monotherapy was used to effectively treat a patient with chemotherapy-refractory advanced GC with AFP- and HER2-positivity.
Case report
Patient presentation
An 86-year-old male patient was referred to The Affiliated Hospital of Jiujiang University (Jiujiang, China) with upper abdominal distention for almost two weeks on November 28, 2019. Contrast-enhanced abdominal computed tomography (CT) revealed a prominent thickening of the gastric wall (4.0x2.5 cm2) near the incisura angularis (Fig. 1). Upper abdominal magnetic resonance imaging (MRI) demonstrated a nodule measuring 1.8x1.6 cm2 in the S6 segment of the liver with the characteristics of a ‘low in, low out’ enhancement mode in the arterial, portal, equilibrium, and delay phases (Fig. 1). Painless gastroscopy revealed an ulcer lesion sized 4.0x2.5 cm2 in the anterior wall of the stomach near the incisura angularis (Fig. 2), while the pathology from the gastric biopsy indicated poorly-moderately differentiated adenocarcinoma of stomach (Fig. 3). The immunohistochemistry (IHC) score of HER2 was equivocal (2+) and was confirmed by detecting HER2 amplification by fluorescence in-situ hybridization (FISH) (Fig. 3). The serum AFP level was extremely high (4,235.0 ng/ml; normal range: 0-25 µg/l) at the time of initial diagnosis; however, IHC suggested that AFP was negative in the gastric biopsy. Then, tumor cells were further found with positive expression of embryonic stem cell markers, i.e., glypican-3 (GPC3) and Sal-like protein 4 (SALL4) by IHC staining (Fig. 3).
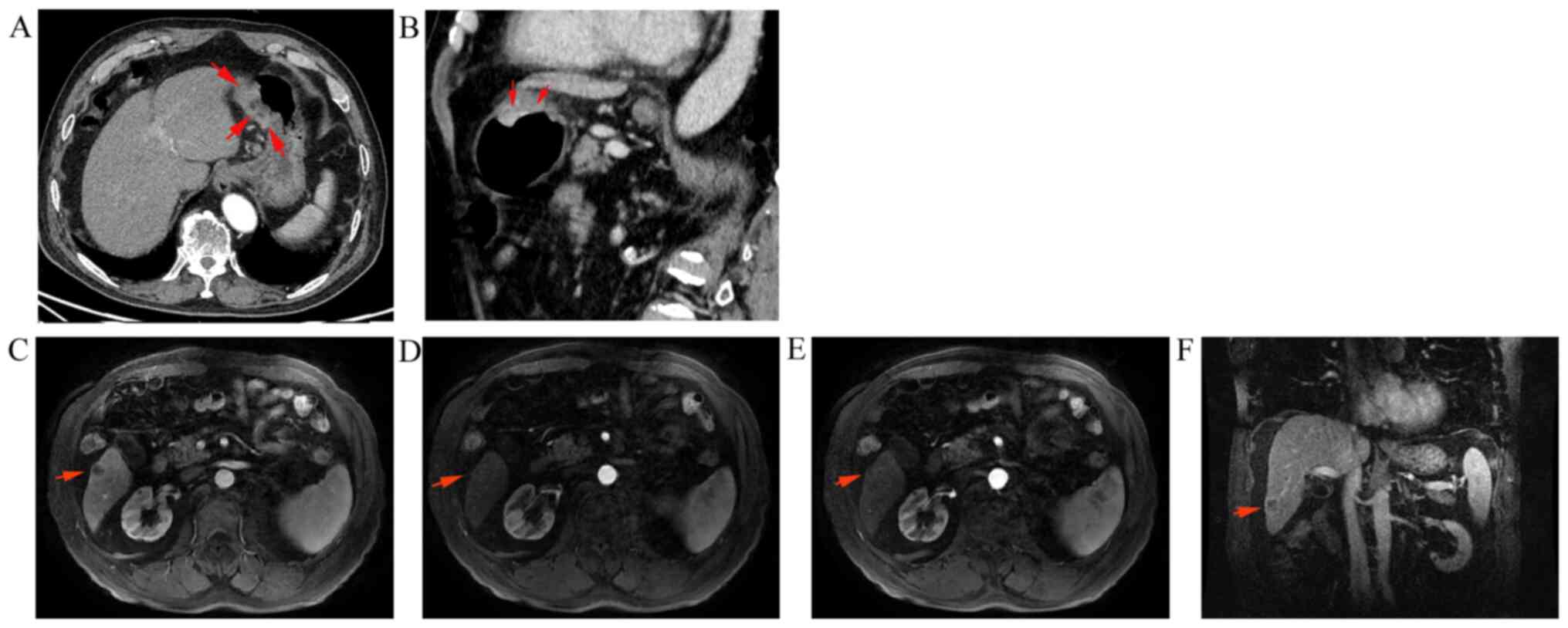 |
Figure 1
Abdominal CT and MRI findings at the first medical examination. (A) Gastric lesions (4.0x2.5 cm2) on the CT cross-section. (B) Gastric lesions (4.0x2.5 cm2) on the CT coronal section. The arrows indicate the gastric lesions in (A and B) (C) Liver metastasis (1.8x1.6 cm2) on the MRI transaxial section (arterial phase). (D) Liver metastasis on the MRI transaxial section (portal phase). (E) Liver metastasis on the MRI transaxial section (equilibrium phase). (F) Liver metastasis on the MRI coronal section (delay phase). The arrows indicate the liver metastasis in (C-E).
|
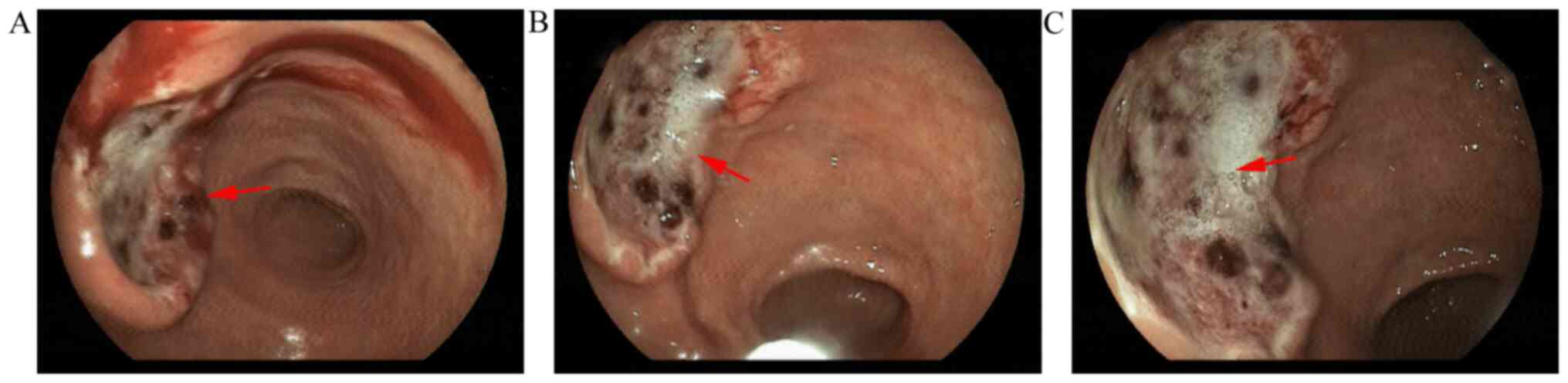 |
Figure 2
Gastroscopy features of the gastric cancer lesion. An ulcer lesion measuring 4.0x2.5 cm2 was present in the anterior wall of the stomach incisura angularis. (A) Hemorrhage image of gastric cancer lesion after histopathologic examination. (B) Image of gastric cancer lesion during biopsy. (C) Static image of gastric cancer lesion before histopathologic examination. The arrows indicate the gastric cancer lesion.
|
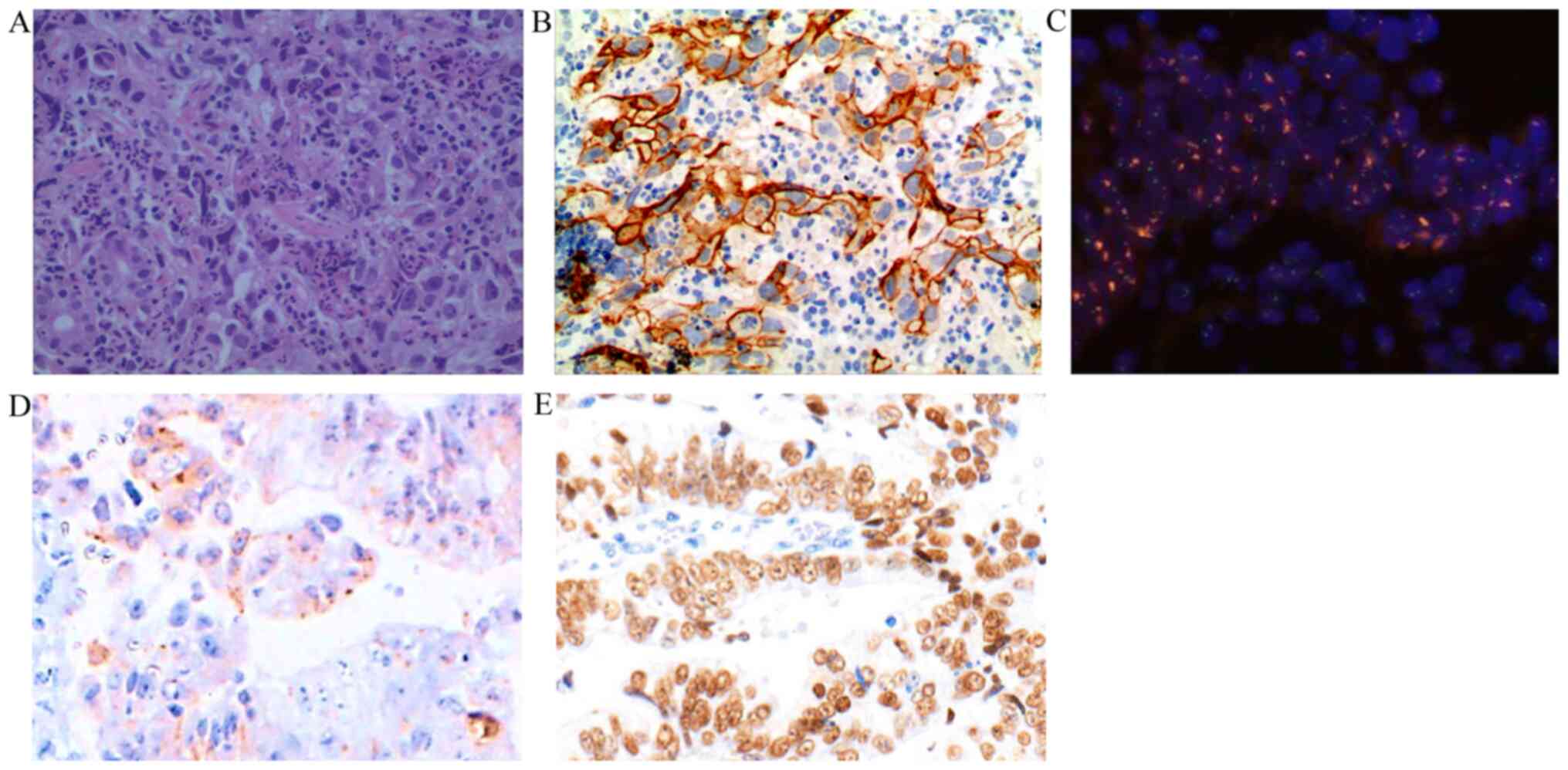 |
Figure 3
Pathological image. (A) Hematoxylin and eosin staining. Magnification, x400. The major histological type was adenocarcinoma. (B) Immunohistochemical staining for HER2 in the adenocarcinoma. Magnification, x400. Immunohistochemistry grading was 2+. (C) FISH analysis. Magnification, x400. FISH detection revealed the amplification of HER2/neu (clusters of red signals). (D) Immunohistochemical staining for glypican-3. Magnification, x400. (E) Immunohistochemical staining for Sal-like protein 4. Magnification, x400. HER2, epidermal growth factor receptor 2; FISH, fluorescence in situ hybridization.`
|
First-line therapy
According to the above findings, the patient was diagnosed with AFP-secretive and HER2-positive advanced GC with liver metastasis. Subsequently, systemic chemotherapy was initiated with the regimen of S-1 (tegafur/gimeracil/oteracil) (80 mg/day for two weeks) and oxaliplatin (100 mg/m2 on day 1 every three weeks), with trastuzumab given at a dose of 8 mg/kg every three weeks on the first day of the first cycle, followed by 6 mg/kg every three weeks, as the first-line treatment. After two cycles, the patient's symptom of upper abdominal distention had not improved significantly. Furthermore, his serum AFP level had markedly increased to 6,856.0 ng/ml. Therefore, the clinical response was determined to be disease progression according to the RECIST 1.1 guidelines.
Second-line therapy
Due to the adverse event of grade IV myelosuppression during the first attempt at chemotherapy, we moved on to trying second-line therapy with docetaxel (60.0 mg/m2 on day 1 every three weeks) in combination with trastuzumab (6 mg/kg on day 1 every three weeks) for two cycles. At this stage, the patient complained of upper abdominal pain, and his AFP level increased further to 8,675.0 ng/ml. Re-examination CT imaging of the upper abdomen found that the gastric lesion and metastatic liver lesion had increased in size to 7x5 cm2 and 2.5x2.1 cm2, respectively (Fig. 4). Unfortunately, the patient still showed myelosuppression of grade IV and recovered through the use of granulocyte colony-stimulating factor. Disease progression was confirmed as the clinical outcome.
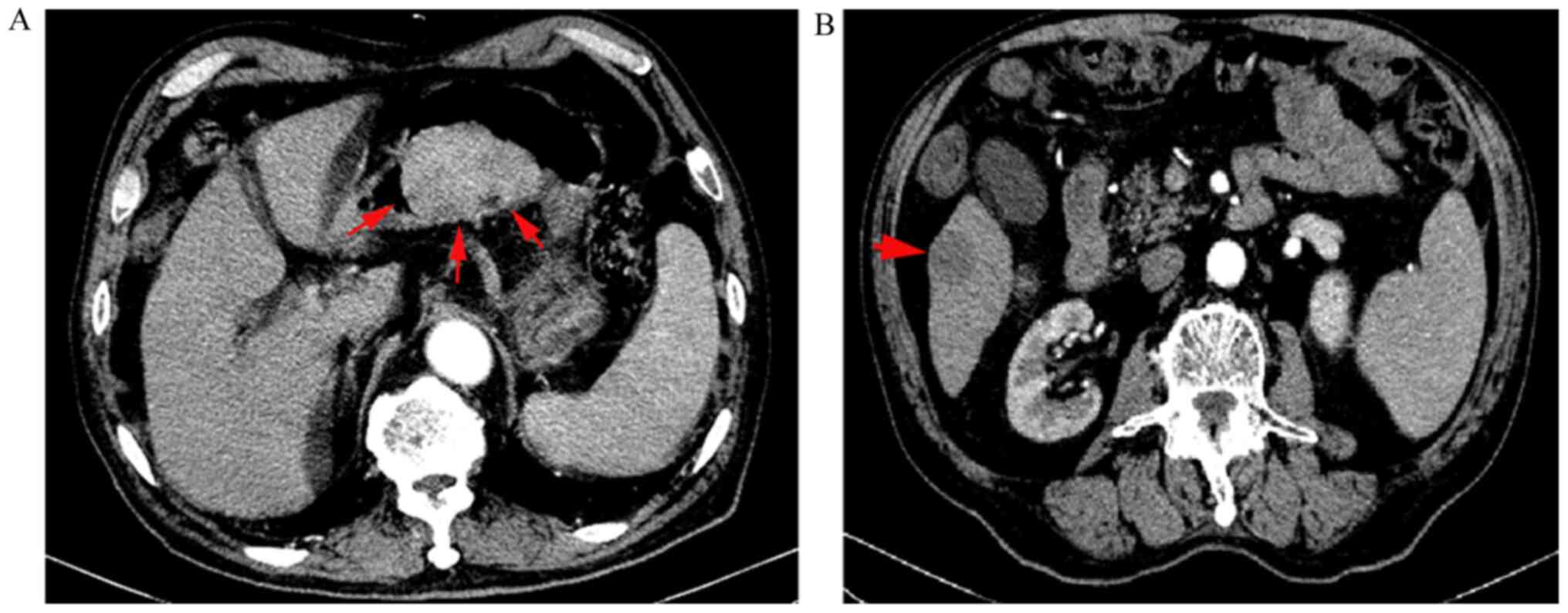 |
Figure 4
Abdominal CT findings after four cycles of chemotherapy. (A) Gastric lesion increased to 7x5 cm2. The arrows indicate the gastric lesion. (B) Liver lesion increased to 2.5x2.1 cm2. The arrow indicates the liver lesion.
|
Third-line therapy
The observation of disease progression with both first- and second-line chemotherapy plus trastuzumab suggested that the patient was refractory to these treatments. At this point, we chose apatinib as third-line therapy based on the 2019 CSCO guideline for GC. Due to the patient's advanced age, the dose of apatinib was adjusted from the standard dose of 850 mg/d down to 500 mg/d (every four weeks/cycle).
General status
After two cycles of apatinib treatment, the patient reported the disappearance of upper abdominal pain and an improvement in his appetite. Also, we found that his Eastern Cooperative Oncology Group score had increased from the initial 60 points at diagnosis to 90 points.
Serological marker and imaging finding
Two cycles of apatinib therapy later, the serum AFP level exhibited a sharp decrease from the previous level of 8,675.0 ng/ml to 620 ng/ml. Additionally, upper abdominal CT imaging revealed that the gastric lesion and the liver lesion had decreased in size to 2.3x1.9 cm2 (by 67%) and 1.9x1.7 cm2 (by 24%), respectively (Fig. 5).
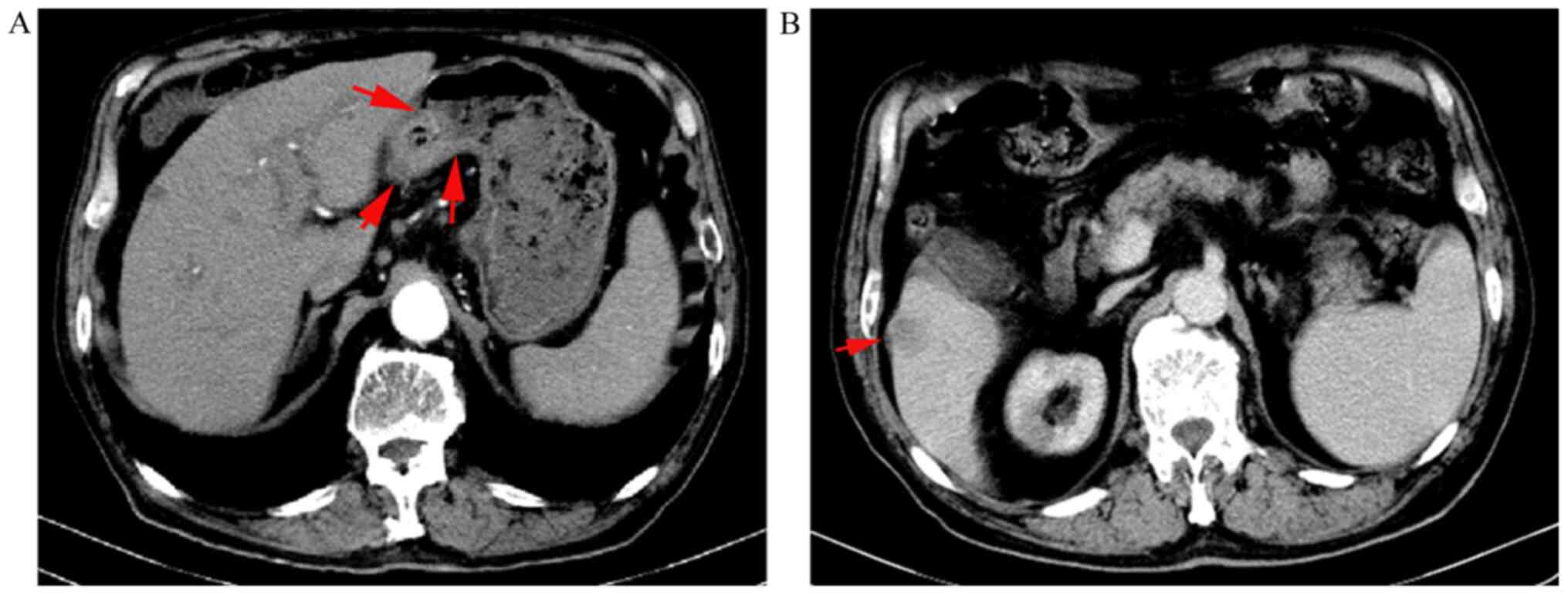 |
Figure 5
Abdominal CT findings after two cycles of apatinib. (A) Gastric lesion decreased to 2.3x1.9 cm2. The arrows indicate the gastric lesion. (B) Liver lesion decreased to 1.9x1.7 cm2. The arrow indicates the liver lesion.
|
Adverse events
Fortunately, the patient only displayed mild hand-foot syndrome and elevated blood pressure, which was ascribed to the reduced dose of apatinib administered. At the same time, the antihypertensive agent of amlodipine was given to deal with hypertension.
Clinical efficacy
According to the CT imaging findings, the gastric lesion and liver metastatic lesion had decreased in size by 67 and 24%, respectively, as compared with prior to receiving apatinib treatment (Fig. 5). The clinical outcome was designated as partial remission per the RECIST 1.1 guidelines. The patient has continued to receive apatinib therapy and undergo follow-up evaluation.
Discussion
The notable feature of AFP-GC is a low rate of surgical success and a high rate of liver and lymph node metastasis, which collectively contribute to a very poor prognosis. Moreover, the majority of patients with AFP-GC presenting at initial diagnosis with an advanced stage of the disease. Due to the rarity of AFP-GC, there is no available standard of care for patients with this condition. The mOS of the patients with AFP-GC is significantly shorter than that of those without AFP-GC (12.6 vs. 22.1 months; P<0.001) (5).
As an inhibitor of HER2, trastuzumab is well-known as a therapeutic agent for HER2-positive breast cancer. Similarly, trastuzumab's incorporation into chemotherapy as first-line therapy significantly improved the clinical outcome of HER2-positive GC patients. Several clinical studies have reported that the median progression-free survival and mOS lengths of patients with HER2-positive GC ranged from 7.1 to 9.2 months and 13.8 to 19.5 months with first-line treatment, respectively (6-9). However, the role of trastuzumab as a second-line therapy remains unclear (10).
In this case, IHC and FISH analysis confirmed the HER2/neu-positivity pathologically. Despite the negative IHC staining result for AFP, the serum level of AFP was markedly increased. Notably, sensitive markers of embryonic stem cells, i.e., GPC3 and SALL4, were confirmed to be positive pathologically, which strongly suggested that this patient was diagnosed with AFP-producing GC rather than primary liver cancer. Therefore, the final diagnosis of our patient was advanced AFP-secreting and HER2-positive GC with liver metastasis. Both first- and second-line chemotherapy plus trastuzumab failed to control the disease progression, indicating that the patient was resistant to conventional chemotherapy combined with trastuzumab. This result strongly suggested that the status of AFP-secretion enhanced the drug resistance of HER2-positive GC. In this context, it is imperative to identify novel therapeutic strategies that work against advanced GC characterized by AFP-secretion and HER2-positivity.
Tumor neovascularization plays a crucial role in tumorigenesis and tumor progression. Molecular biological studies have reported that the frequency of vascular endothelial growth factor (VEGF)-C expression was significantly greater in AFP-GC patients than in patients without AFP-secretion; however, no significant difference in VEGF expression was recorded between these two groups (11). As a lymphangiogenic factor, VEGF-C is responsible for the generation of excess lymph vessels and the distant metastasis of cancer cells, which could constitute an explanation for the biological behavior of AFG-producing GC, i.e., the high tendency for liver and lymph node metastasis (12,13). As one of the receptors of VEGF-C, VEGF receptor 2 has been considered to be a novel predictor of survival in gastric cancer (14). Of coincidence, apatinib is a very small-molecule tyrosine kinase inhibitor that targets VEGF receptor 2, which has been approved as a third-line agent for advanced GC by CSCO (3). As such, we employed apatinib in the present case found to be resistant to first- and second-line chemotherapy. Encouragingly, apatinib monotherapy displayed incredible efficacy in this advanced AFP-producing and HER2-positive GC case. Also, the patient's serum AFP level was sharply reduced following treatment with apatinib, which furthermore confirmed the good clinical outcome of this therapeutic option.
Based on the case outcome described above, we speculated that AFP-secretion could contribute to the chemoresistance of HER2-positive GC. Apatinib may be a promising anticancer agent against advanced AFP-producing and HER2-positive GC. Whether it is appropriate to use apatinib as first-line therapy to treat this unique type of GC deserves further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XD designed and drafted the manuscript. JD revised the manuscript. XD and JD acquired the raw data and figures as the patient's medical intern and primary medical oncologist, respectively. XD and JD confirmed the authenticity of all the raw data. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The requirement for patient consent for participation was waived by the ethics committee because the patient received the standard third-line treatment of apatinib.
Patient consent for publication
The requirement for patient consent for publication was waived by the ethics committee because the patient received the standard third-line treatment of apatinib.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Feng RM, Zong YN, Cao SM and Xu RH: Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 39(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 376:687–697. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, et al: Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: Results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 31:3219–3225. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhu XR, Zhu ML, Wang Q, Xue WJ, Wang YW, Wang RF, Chen SY and Zheng LZ: A case report of targeted therapy with apatinib in a patient with advanced gastric cancer and high serum level of alpha-fetoprotein. Medicine (Baltimore). 95(e4610)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bozkaya Y, Demirci NS, Kurtipek A, Erdem GU, Ozdemir NY and Zengin N: Clinicopathological and prognostic characteristics in patients with AFP-secreting gastric carcinoma. Mol Clin Oncol. 7:267–274. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, Wang J, Xu N, Cheng Y, Bai Y, et al: Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): A multicenter, phase II trial. BMC Cancer. 16(68)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Soularue E, Cohen R, Tournigand C, Zaanan A, Louvet C, Bachet JB, Hentic O, Samalin E, Chibaudel B, de Gramont A and André T: for GERCOR. Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: A retrospective study. Bull Cancer. 102:324–331. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rivera F, Romero C, Jimenez-Fonseca P, Izquierdo-Manuel M, Salud A, Martinez E, Jorge M, Arrazubi V, Mendez JC, Garcia-Alfonso P, et al: Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol. 83:1175–1181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takahari D, Chin K, Ishizuka N, Takashima A, Minashi K, Kadowaki S, Nishina T, Nakajima TE, Amagai K, Machida N, et al: Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naive, HER2-positive advanced gastric cancer. Gastric Cancer. 22:1238–1246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Palle J, Rochand A, Pernot S, Gallois C, Taieb J and Zaanan A: Human Epidermal Growth Factor Receptor 2 (HER2) in advanced gastric cancer: Current knowledge and future perspectives. Drugs. 80:401–415. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kamei S, Kono K, Amemiya H, Takahashi A, Sugai H, Ichihara F, Fujii H and Matsumoto Y: Evaluation of VEGF and VEGF-C expression in gastric cancer cells producing alpha-fetoprotein. J Gastroenterol. 38:540–547. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, et al: The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 9:209–223. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pan Z, Lu X, Zhao J, Gao Q and Wang J: VEGF-C is positively associated with lymphangiogenesis and lymphatic metastasis in rectal cancer. Int J Clin Exp Pathol. 11:1777–1783. 2018.PubMed/NCBI
|
|
14
|
Li T, Yu J, Luo X, Ren W, Zhang Y and Cao B: VEGFR-2 as a novel predictor of survival in gastric cancer: A systematic review and meta-analysis. Pathol Res Pract. 214:560–564. 2018.PubMed/NCBI View Article : Google Scholar
|



















