Introduction
Gallbladder cancer (GBC) is the most common biliary
tract cancer (BTC) with characteristic thickening of the
gallbladder (GB) wall. Most of the GBCs arise from the epithelial
lining of the GB and the cystic duct (1,2), and
most GBCs are adenocarcinomas (1).
The incidence of GBC is higher in the Indian subcontinent than
Western counterparts (3). In India,
GBC is more common in the northern region when compared with the
southern region (~10-folds) (2).
The only curative option for GBC is surgical
resection, however, most of the patients present with unresectable
disease at an advanced stage owing to the absence of symptoms in
the early stages of the disease (4,5). There
is no standard chemotherapy established for advanced disease.
Palliative chemotherapy with gemcitabine/cisplatin combination or
gemcitabine based chemotherapy regimens are recommended for the
treatment of metastatic GBC by several treatment guidelines
(3,6). Gemcitabine/oxaliplatin (Gemox)
combination has shown more potency when compared with
gemcitabine/cisplatin combination, and the activity and
tolerability of Gemox regimen has been evaluated in cancers,
including advanced GBC (7).
Studies have revealed the expression of vascular
endothelial growth factor (VEGF) receptor in BTCs and GBCs
(8). Addition of an anti-VEGF agent
to the chemotherapy could normalize the tumor vasculature and
reduce the interstitial pressure in tumors leading to overall
improved results. Bevacizumab, an anti-VEGF, is an approved agent
for the treatment of metastatic colorectal cancer and has shown
effectiveness in several other cancers (9). Bevacizumab in addition to gemcitabine
and oxaliplatin chemotherapy (Gemox-B regimen) has shown antitumor
activity with tolerable safety in patients with advanced GBCs
(8). We report here a case of a
patient with GBC adenocarcinoma metastasized to liver and
peritoneum who was treated with biosimilar bevacizumab based
modified Gemox-B regimen.
Case report
A 47-year-old woman presented with complaints of
right shoulder pain, abdominal pain, decreased appetite and
dyspepsia for 6 months. There was no weight loss, fever,
comorbidity or any surgical history. Ultrasonography (USG) in July
2019 revealed polypoidal wall thickening of gallbladder with
maximum thickness in the fundal region and a mild central
intrahepatic biliary radical dilatation suspicious of neoplastic
etiology. Bilateral ovaries were bulky (left > right) and
hypoechoic. There was moderate left sided hydronephrosis, with
upper hydroureter while the distal ureter was obscured by bowel
gas.
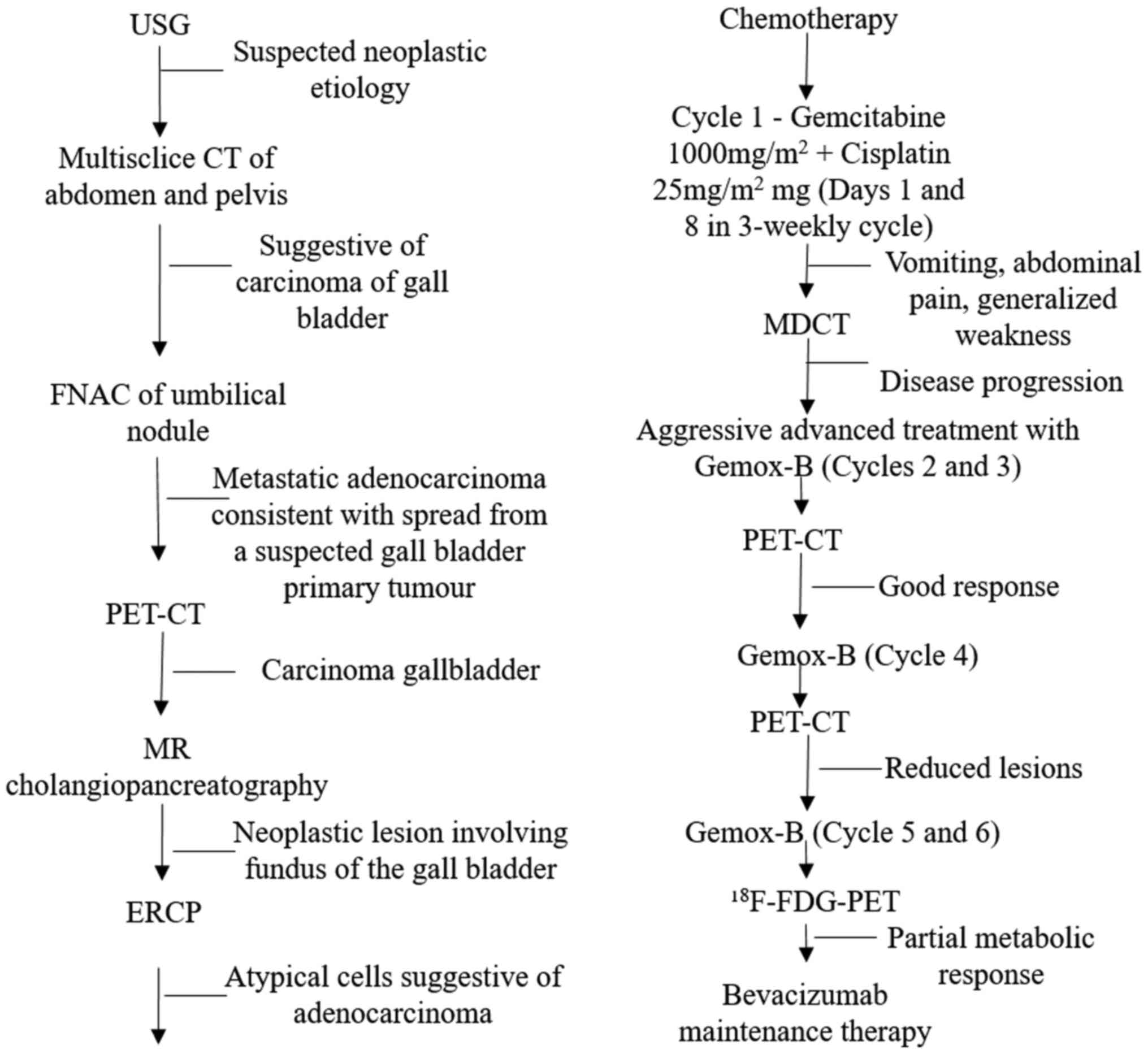
Fig. 1 provides the
details of diagnostic and treatment approaches used in this
patient. The computed tomography (CT) showed irregular
heterogeneously enhanced eccentric wall thickening involving the
gallbladder mainly in the fundal region, loss of fat plane with
segment V of liver and hepatic flexure of colon, focally indistinct
fat plane of the D1 segment of duodenum with pericholecystic fat
stranding and enhanced circumferential wall of the cystic duct
suggestive of GBC. Multiple irregularly shaped hypodense
heterogeneously enhancing soft tissue nodules were seen in the
pericholecystic region and serosal surface of the ascending colon,
and caecum/small soft tissue density nodule in the anterior
abdominal wall in the umbilical region. Also, multiple small
hypodense nodules scattered in both lobes of liver mainly in
segment VIII, IVb and III, were seen. These findings were
suggestive of metastatic disease. Mild ascites was present.
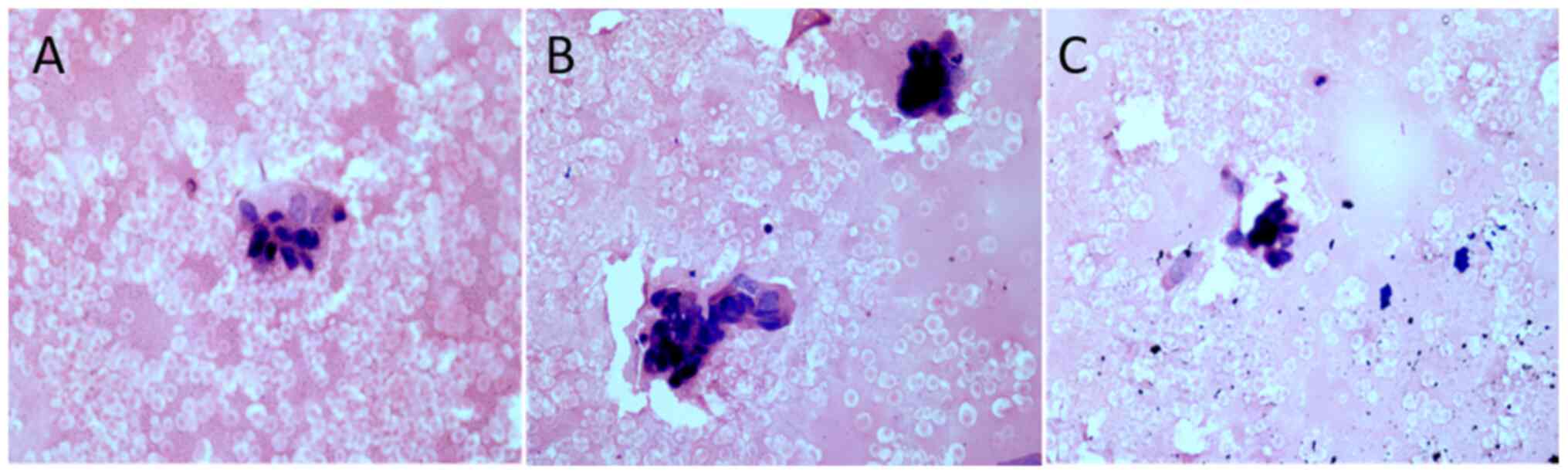
The histological examination revealed few scattered
clusters of medium sized ductal cells exhibiting pleomorphic,
irregular and mildly overlapping dark staining nuclei (Fig. 2).
The baseline positron emission tomography with
2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed
tomography (18F-FDG PET-CT) done on 11-Jul-2019 revealed
asymmetric FDG avid enhancing thickening of wall of the distal body
and fundic region of the GBC (soft tissue mass measuring
3.4x2.8x2.9 cm; standardized uptake value [SUVmax]:
6.08), and confirmed the peritoneal and liver metastasis with
narrowing of the common bile duct (CBD). Bulky bilateral ovaries
(left ovary: 3.8x3.0x2.9; left more than right with avid FDG
uptake) with left sided hydronephrosis and hydroureter were seen
(SUVmax: 5.35).
Her laboratory investigations were abnormal-CA-125:
117.2 U/ml (normal: <46 U/ml), CEA: 5.5 ng/ml (normal: <5
ng/ml), CA19.9: 1433.0 U/ml (normal: <37 U/ml), creatinine: 1.3
mg/dl (normal: 0.84-1.21 mg/dl), total bilirubin: 5.5 mg/dl
(normal: 0.3 mg/dl), alanine transaminase (ALT): 356 U/l (normal:
<40 U/l), aspartate transaminase (AST): 95 U/l (normal: <40
U/l), alkaline phosphatase (ALP): 421 U/l (normal: 20-140 IU/l),
gamma-glutamyl transferase (GGT): 187 U/l (normal: <30 IU/l).
Her total proteins were 7.84 g/dl (normal: 6-8.3 g/dl), albumin
4.02 g/dl (normal: 3.4-5.4 g/dl) and globulin 3.82 g/dl (normal:
2-3.5 g/dl).
Magnetic resonance cholangiopancreatography (MRCP)
performed on 16-Jul-2019 suggested neoplastic lesion involving
fundus of the gallbladder infiltrating adjacent liver parenchyma.
She underwent endoscopic retrograde cholangiopancreatography (ERCP)
with CBD stenting on 18-Jul-2019. Biliary stricture was seen and
self-expandable metallic stent (SEMS; 10x80 mm) was placed for
Bismuth type II biliary stricture with cholangitis.
Her liver function tests gradually improved over one
week The cancer staging was evaluated as
T3N2M1 - Stage IV. The
patient was planned to receive three chemotherapy cycles with
intravenous (IV) gemcitabine 1,000 mg/m2 and cisplatin
25 mg/m2 on Days 1 and 8 every 21 days. She received the
1st dose of cycle 1 chemotherapy on 6-Aug-2019 (Day 1)
and the second dose on 13-Aug-2019 (Day 8).
After 4-5 days of the first chemotherapy dosing, she
complained of vomiting (green colored), abdominal pain and
generalized weakness (all grade 1-2), which improved with
conservative treatment. On multidetector CT (MDCT) performed on
22-Aug-2019, few heterogeneously enhancing lesions were seen in the
liver (largest: 13 mm, Segment IV-a). A large heterogeneously
enhancing centrally necrotic mass lesion in the gastrosplenic
region, encasing the tail of pancreas suggestive of large
metastatic deposits (7.5x4.2x8.7) was a new finding. There was a
mild interval increase in the size of bilateral ovarian lesions.
These aforementioned MDCT findings were suggestive of disease
progression, and hence, the regimen was changed to an aggressive
approach with triple drug combination (modified Gemox with
bevacizumab) of gemcitabine 900 mg/m2, oxaliplatin 80
mg/m2 (Days 1 and 8 in each 3-weekly cycle) and
bevacizumab 7.5 mg/Kg (on Day 1 in each 3-weekly cycle). Her
symptoms gradually improved with the treatment. The modified
Gemox-B regimen was well-tolerated with no significant abnormal
laboratory investigations. In view of clinical response, patient
was treated with four cycles of bevacizumab based chemotherapy.
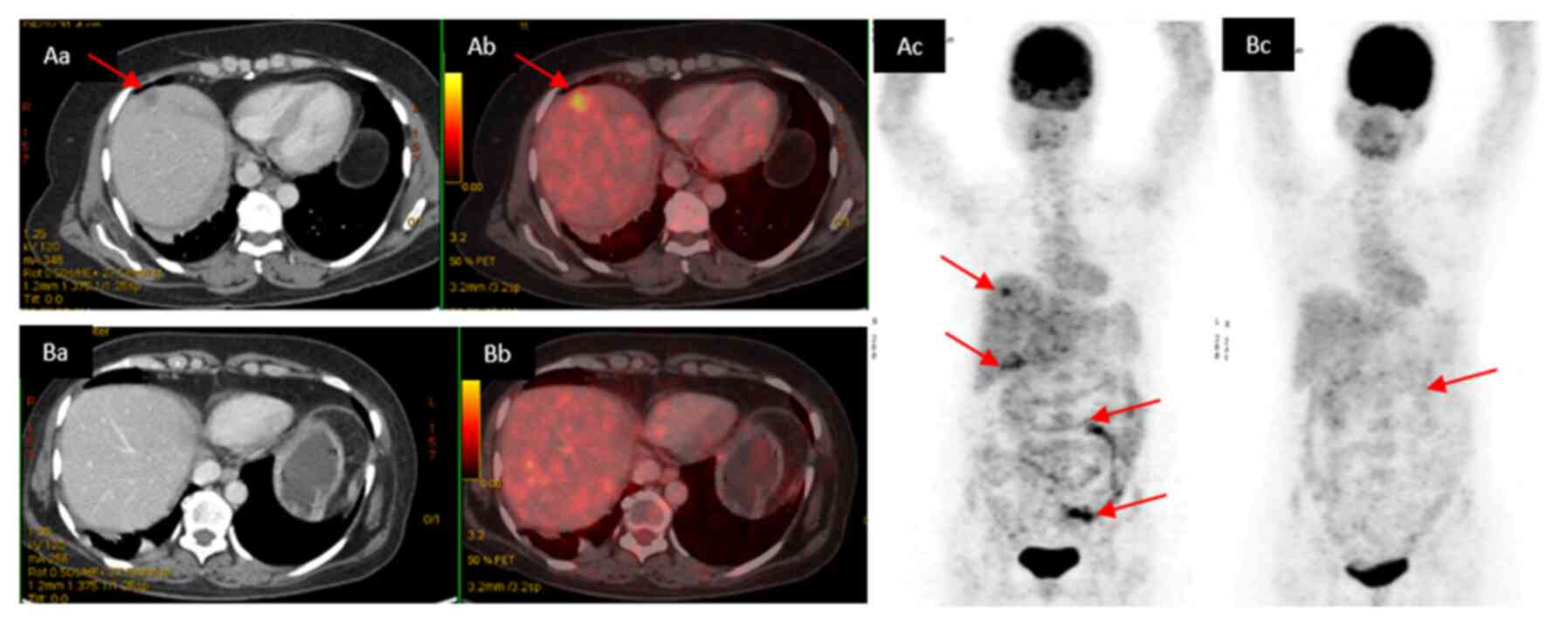
Interim PET-CT showed reduction in lesions all over
except in the peritoneum; liver lesions were resolved. The baseline
(Fig. 3A) and interim PET-CT images
are shown (Fig. 3B).
The wall of gallbladder was irregularly thickened,
which was significantly reduced in size and metabolic activity
(SUVmax was 2.45; largest measuring size: 10 mm). The
previously noted left ovarian lesions showed reduction in size
(3x2.5 cm; SUVmax: 1.88). Subsequently, the patient was
administered fifth and sixth chemotherapy cycles.
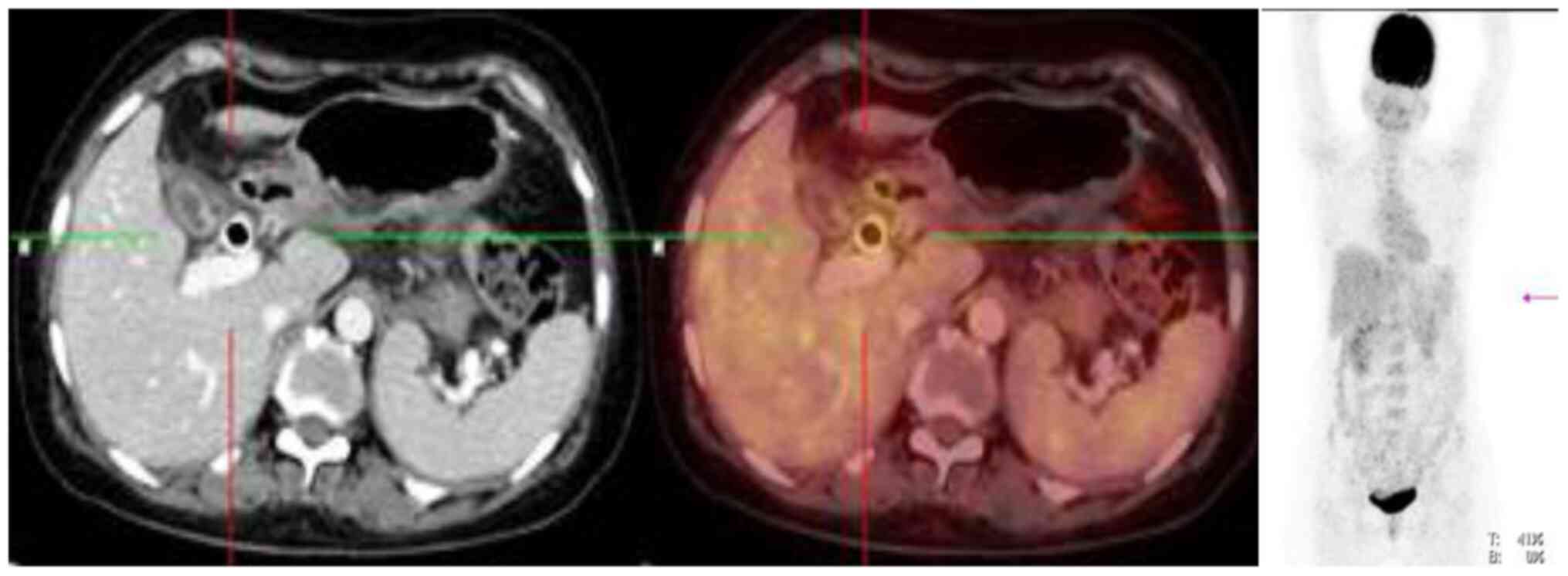
The 18F-FDG PET-CT after six chemotherapy
cycles showed interval reduction in metabolic activity of
ill-defined soft tissue lesions involving fundus of gallbladder.
The SUVmax was 1.77 and measured approximately 1.5x1 cm.
Interval reduction in the size of non-FDG avid serosal deposits in
subhepatic space was seen, and low grade metabolically active
bilateral ovarian lesions were unchanged; left ovarian lesion with
SUVmax 1.44 measuring ~3.1x2.1 cm and right ovarian
lesion with SUVmax 1.72 measuring ~2.1x1.6 cm. There was
interval reduction in the number of non-FDG avid liver lesions. No
other evidence of metabolically active disease was seen (Fig. 4).
Her CA19-9 levels decreased to 70 U/ml. Further
maintenance treatment with bevacizumab (7.5 mg/kg 3-weekly) was
planned in view of the excellent response. The patient tolerated
the maintenance treatment well without any adverse events (AEs).
Unfortunately, she developed clinically progressive disease with
reappearance of symptoms like right sided abdominal pain, nausea
and loss of appetite in June 2020 (progression free interval: ~11
months). PET-CT done in June 2020 suggested persistent primary
gallbladder lesion with mild increase in the number and metabolic
activity of the serosal deposits in the subhepatic space. Mild
increases in the size and metabolic activity were noted in the
bilateral ovarian lesions. Currently, the patient has been started
on palliative chemotherapy with capecitabine.
Discussion
Gallbladder cancer is a very aggressive and
difficult to treat BTC (10).
Studies have reported 6-9 months progression free survival and
9.8-14 months overall survival in advanced unresected GBC patients
(11). Surgery remains the only
curative option but only 10% patients are eligible as majority of
the cases are presented at an advanced disease state (6,12).
Most GBCs do not respond well to chemotherapy with a single agent
(13). For locally advanced or
metastatic unresectable GBC, gemcitabine and cisplatin based
chemotherapy is the recommended treatment option (3,14).
Furthermore, chemotherapy in combination with bevacizumab has shown
promising results in the treatment of BTCs (15). We report here a case of a
47-year-old woman with GBC metastasized to liver and peritoneum who
was treated with a biosimilar bevacizumab based Gemox-B regimen.
The patient underwent ERCP with CBD stenting following which she
received the first chemotherapy cycle with gemcitabine and
cisplatin. The MDCT performed after the first chemotherapy cycle
demonstrated a new finding of a large necrotic mass lesion encasing
the pancreatic tail suggesting large metastatic deposits
(7.5x4.2x8.7). Such a large mass could not be attributed to the
post ERCP pancreatitis, which was confirmed by an independent
radiologist and indicated disease progression. Hence, the patient
was switched to an aggressive bevacizumab based Gemox-B
regimen.
Several factors including advanced age, female
gender and Indian origin are among the established risk factors
(12), and abdominal pain,
discomfort, jaundice, vomiting, abdominal mass and ascites are
common clinical manifestations of GBC (1), which were also seen in our
patient.
Majority of the GBCs arise in the fundus (60%),
infiltrate directly in the liver, and spread to different segments
(IV and V) of liver and peritoneum, and are of adenocarcinoma (98%)
histology (12,16,17),
similar to as seen in our patient. A decrease in pretreatment CA
19-9 levels after chemotherapy are of prognostic relevance in
patients with BTCs (12,18). In our patient, the CA19.9 levels
were decreased from 1433 U/ml at baseline to 70 U/ml post
treatment.
The NCCN guidelines suggest investigation with MDCT
or contrast-enhanced MRI with MRCP and chest CT in case of
suspicious GBC. Similar imaging modalities were carried-out in our
patient. The treatment guidelines suggest gemcitabine- or
fluoropyrimidine-based chemotherapy for the treatment of advanced
unresectable GBCs (3,6). Gemcitabine has shown clinical benefit
rates (complete response+partial response+stable disease) of 15-60%
in GBC cases (12). The combination
of gemcitabine and cisplatin when compared with gemcitabine alone
has resulted in increased response rates (26 vs. 16%) and OS (11.7
vs. 8.1 months) in GBC (19). A
systematic review of clinical studies show that gemcitabine and
oxaliplatin combination (Gemox) regimen has a better toxicity
profile without significant difference in the efficacy compared
with cisplatin/gemcitabine regimen (20). Furthermore, Gemox regimen has
demonstrated a response rate of 26 to 50% and an OS of 11 to 15.4
months in the treatment of advanced gallbladder and BTCs (12).
The combination of molecularly targeted agents with
chemotherapy have also been evaluated in patients with BTCs with
promising efficacy and tolerability (21-23).
Letelier et al, have reported that VEGF-A are expressed in
81% (183/224) of GBC cases (24).
These findings suggest a possible role of anti-VEGF agent for the
treatment of GBCs. Bevacizumab, an anti-VEGF, has been successfully
combined with chemotherapy for several cancers including GBC
(8). The Gemox-B regimen has
reported promising results (response rate: 40%, median OS: 12.7
months) in the treatment of GBCs in a phase II study (8). Innovator biologic bevacizumab based
Gemox-B regimen has been used previously in the GBCs. In our
patient, a biosimilar bevacizumab (Bevatas of Intas Pharmaceuticals
Limited, India) based modified Gemox-B regimen was used, which
demonstrated a partial response as per RECIST 1.1 and a partial
metabolic response (PMR). Intas' biosimilar bevacizumab was
approved in India in 2017, with an intent to provide a
cost-effective (up to 40%) alternative formulation of innovator
bevacizumab to the Indian patients (25).
The FDG combined with PET scan is an early,
sensitive, pharmacodynamic marker of the tumoricidal effect of
chemotherapeutic agents. In our patient, 18F-FDG-PET
analysis showed a mean decrease in SUVmax of ~70%
(SUVmax-baseline: 6.08; post-treatment 1.77; difference:
4.31) suggesting a PMR as per EORTC criteria (26). Previous studies have correlated that
a significantly larger decrease in SUVmax was observed
in patients with longer PFS (>6 months) and overall survival
(OS, >12 months) (26).
The reported tumor response rate (complete response
rate + partial response rate + stable disease) in the phase II
study with Gemox-B regimen was 69%. However, complete response was
not seen in any of the patients (out of 35) (8). In our patient, a partial response was
observed after Gemox-B chemotherapy. At 6 months follow-up post
bevacizumab based chemotherapy, the patient developed disease
progression showing a progression-free survival of ~11 months,
which is comparatively greater than those reported in the
literature (11). The patient is
still alive and currently receiving palliative chemotherapy with
capecitabine. In our patient, there was no grade III or IV AEs
observed which is in line with the results from the phase II study
where Gemox-B regimen showed a favorable tolerability profile in
patients with BTCs (8).
Overall, biosimilar bevacizumab based modified
Gemox-B regimen resulted in a partial response and a progression
free survival of ~11 months in a patient with advanced gallbladder
cancer. The current case could provide insights for biosimilar
bevacizumab based chemotherapy regimen options for the palliative
treatment of metastatic gallbladder carcinoma and need to be
further evaluated in clinical studies.
Acknowledgements
The authors would like to thank Dr Jaykumar Sejpal
and Dr Mujtaba A. Khan (both Intas Pharmaceuticals Ltd.) for their
medical review of the manuscript. The authors would also like to
thank Mr. Shreekant Sharma (ISMPP CMPP™; Intas
Pharmaceuticals Ltd.) for his support in developing the
concept/medical writing, additional editorial support and follow-up
with the journal. Finally, the authors acknowledge Dr Venugopal
Madhusudhana (ISMPP CMPP™; Intas Pharmaceuticals Ltd.)
for additional editorial assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SVA, MT, AT, SS and SHA participated in study
design, data collection, data interpretation, and writing/review of
the case report. All authors substantially contributed to
interpretation of data, critical revision of manuscript, and
consented to the final version of the case report. SVA and SHA
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
A signed informed consent document was obtained from
the patient for the publication of this case report and associated
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maplanka C: Gallbladder cancer, treatment
failure and relapses: The peritoneum in gallbladder cancer. J
Gastrointest Cancer. 45:245–255. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dutta U, Bush N, Kalsi D, Popli P and
Kapoor VK: Epidemiology of gallbladder cancer in India. Chin Clin
Oncol. 8(33)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Valle JW, Borbath I, Khan SA, Huguet F,
Gruenberger T and Arnold D: ESMO Guidelines Committee. Biliary
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 (Suppl 5):v28–v37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang H, Ling W and Luo Y:
Contrast-enhanced ultrasound findings of gallbladder adenocarcinoma
with sarcomatoid carcinoma accompanied by intrahepatic metastasis:
A case report and literature review. Medicine (Baltimore).
97(e10773)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oh J, Steel M, Conklin C and
Aquino-Parsons C: Metastatic gallbladder adenocarcinoma to the
endometrium: A case report and review of literature. Cureus.
11(e5258)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
National Comprehensive Cancer Network.
Hepatobilliary Cancers (Version 2.2019). (2019). Accessed: Jannuary
31, 2020: https://www.nccn.org/professionals/physician_gls/PDF/hepato-biliary.pdf.
|
|
7
|
André T, Tournigand C, Rosmorduc O,
Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun
L, Houry S, et al: Gemcitabine combined with oxaliplatin (GEMOX) in
advanced biliary tract adenocarcinoma: A GERCOR study. Ann Oncol.
15:1339–1343. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhu AX, Meyerhardt JA, Blaszkowsky LS,
Kambadakone AR, Muzikansky A, Zheng H, Clark JW, Abrams TA, Chan
JA, Enzinger PC, et al: Efficacy and safety of gemcitabine,
oxaliplatin, and bevacizumab in advanced biliary-tract cancers and
correlation of changes in 18-fluorodeoxyglucose PET with clinical
outcome: A phase 2 study. Lancet Oncol. 11:48–54. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Keating GM: Bevacizumab: A review of its
use in advanced cancer. Drugs. 74:1891–1925. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Uji M, Mizuno T, Ebata T, Sugawara G,
Igami T, Uehara K and Nagino M: A case of advanced intrahepatic
cholangiocarcinoma accidentally, but successfully, treated with
capecitabine plus oxaliplatin (CAPOX) therapy combined with
bevacizumab: A case report. Surg Case Rep. 2(63)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sacchetti F, Ardito F, Vecchio FM and
Giuliante F: Exceptional long-term survivor (12 years) with
metastatic gallbladder cancer. Glob Surg 5, 2019.
|
|
12
|
Kanthan R, Senger JL, Ahmed S and Kanthan
SC: Gallbladder cancer in the 21st century. J Oncol.
2015(967472)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Caldow Pilgrim CH, Groeschl RT, Quebbeman
EJ and Gamblin TC: Recent advances in systemic therapies and
radiotherapy for gallbladder cancer. Surg Oncol. 22:61–67.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Athauda A, Fong C, Lau DK, Javle M,
Abou-Alfa GK, Morizane C, Steward K and Chau I: Broadening the
therapeutic horizon of advanced biliary tract cancer through
molecular characterisation. Cancer Treat Rev.
86(101998)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang W, Zhou H, Wang Y, Zhang Z, Cao G,
Song T, Zhang T and Li Q: Systemic treatment of advanced or
recurrent biliary tract cancer. Biosci Trends. 14:328–341.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lim KS, Peters CC, Kow A and Tan CH: The
varying faces of gall bladder carcinoma: Pictorial essay. Acta
Radiol. 53:494–500. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoshimitsu K, Nishihara Y, Okamoto D,
Ushijima Y, Nishie A, Yamaguchi K, Taketomi A and Honda H: Magnetic
resonance differentiation between T2 and T1 gallbladder carcinoma:
Significance of subserosal enhancement on the delayed phase dynamic
study. Magn Reson Imaging. 30:854–859. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Harder J, Kummer O, Olschewski M, Otto F,
Blum HE and Opitz O: Prognostic relevance of carbohydrate antigen
19-9 levels in patients with advanced biliary tract cancer. Cancer
Epidemiol Biomarkers Prev. 16:2097–2100. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fiteni F, Nguyen T, Vernerey D, Paillard
MJ, Kim S, Demarchi M, Fein F, Borg C, Bonnetain F and Pivot X:
Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment
of advanced biliary tract cancer: A systematic review. Cancer Med.
3:1502–1511. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Malka D, Fartoux L, Rousseau V, Trarbach
T, Boucher E, De La Fouchardiere C, Faivre SJ, Viret F, Blanc JF,
Assenat E, et al: Gemcitabine and oxaliplatin (GEMOX) alone or in
combination with cetuximab as first-line treatment for advanced
biliary cancer: Final analysis of a randomized phase II trial
(BINGO). J Clin Oncol. 30(4032)2012.
|
|
22
|
Gruenberger B, Schueller J, Heubrandtner
U, Wrba F, Tamandl D, Kaczirek K, Roka R, Freimann-Pircher S and
Gruenberger T: Cetuximab, gemcitabine, and oxaliplatin in patients
with unresectable advanced or metastatic biliary tract cancer: A
phase 2 study. Lancet Oncol. 11:1142–1148. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Safran H, Miner T, Resnick M, Dipetrillo
T, McNulty B, Evans D, Joseph P, Plette A, Millis R, Sears D, et
al: Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: A
phase I study for advanced pancreaticobiliary cancer. Am J Clin
Oncol. 31:140–144. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Letelier P, Garcia P, Leal P, Ili C,
Buchegger K, Riquelme I, Sandoval A, Tapia O and Roa JC:
Immunohistochemical expression of vascular endothelial growth
factor A in advanced gallbladder carcinoma. Appl Immunohistochem
Mol Morphol. 22:530–536. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Intas Launches Bevatas (bevacizumab) in
India. [cited 09 Nov 2020]. Available from urihttps://www.bigmoleculewatch.com/2017/10/17/intas-launches-bevatas-bevacizumab-india/?highlight=bevatassimplehttps://www.bigmoleculewatch.com/2017/10/17/intas-launches-bevatas-bevacizumab-india/?highlight=bevatas.
|
|
26
|
Young H, Baum R, Cremerius U, Herholz K,
Hoekstra O, Lammertsma AA, Pruim J and Price P: Measurement of
clinical and subclinical tumour response using
[18F]-fluorodeoxyglucose and positron emission
tomography: Review and 1999 EORTC recommendations. European
Organization for Research and Treatment of Cancer (EORTC) PET Study
Group. Eur J Cancer. 35:1773–1782. 1999.PubMed/NCBI View Article : Google Scholar
|