Introduction
The human epidermal growth factor receptor 2 (HER2)
oncogene encodes a transmembrane protein (17q12-21.32) with
tyrosine kinase activity, which acts as a growth factor (1). HER2 has been detected with variable
expression in a wide variety of malignant tumors and has been found
to be an adverse prognostic marker in breast (20%) and ovarian
(33%) adenocarcinomas (2,3). Overexpression of the HER2 protein and
amplification of the HER2 gene, or both, occurs in approximately
25% of breast cancers and is associated with aggressive behavior
(4).
Although unequivocal data on HER2 overexpression are
not available for prostate cancer (PCa), evidence suggests that it
may be crucial for disease progression and aggressiveness (5). A recent study supporting these
findings was a comprehensive immunohistochemical (IHC) evaluation
of 2,525 samples, which revealed positive associations between HER2
staining, PCa aggressiveness, and recurrence (6). In addition, high levels of HER2 have
been correlated with tumor growth in LAPC-4 androgen-independent
PCa cells (7). Furthermore,
HER2-dependent signaling may support the development of
castration-resistant PCa (CRPC) through androgen ligand-independent
mechanisms (8).
However, there are no available data on the
influence of castration on HER2-dependent signaling in patients
with castration-sensitive PCa. Investigating the expression of HER2
in patients undergoing hormonal therapy during distinct periods
could also increase our understanding of the mechanisms associated
with the development of castration resistance.
The metastatic potential of PCa may not be fully
understood during the initial risk assessment (9). The tumor's multicentric origin,
associated with genetic mutations, may explain treatment pitfalls
(10,11).
Our research had two objectives: To correlate HER2
expression with the histologic features of PCa subjected to radical
prostatectomy (RP) and to evaluate the role of testosterone
suppression in HER2 expression.
Materials and methods
Patients
RP specimens were obtained from patients who were
consecutively treated at two different institutions from 1998 to
2011 (Santa Casa of São Paulo Hospital and Centro Universitário
FMABC Hospital). The local ethics committee approved the study
(84427718.0.0000.0082 and 06937412.0.1001.0082).
Formalin-fixed paraffin-embedded (FFPE) tissue
blocks of tumor samples were identified and divided into two
groups. group 1 included specimens from individuals who underwent
RP without prior neoadjuvant androgen deprivation therapy (ADT)
(n=42). group 2 (PCa with ADT) included specimens from individuals
who underwent RP after receiving neoadjuvant cyproterone acetate
during distinct periods (200 mg daily for 1-24 months) (n=150;
cohort derived from a previous study) (12).
The patients in group 2 were those who were included
in a study performed in 2014, which proposed hormonal therapy with
neoadjuvant cyproterone before RP. The material was preserved in a
paraffin block using the tissue microarray (TMA) technique for
future studies. We chose to use this cohort because neoadjuvant
cyproterone is not used today. Furthermore, it is not ethical to
suppress testosterone for long periods of time in men who would
undergo radical treatment.
All hematoxylin and eosin (H&E)-stained
histological sections from the RP specimens were reviewed. An index
tumor (highlighted on the slides) was defined as the focus with the
highest Gleason pattern or the largest tumor (in case of a single
pattern). Other prognostic factors evaluated were perineural
invasion, extra-prostatic disease, T stage, serum prostate-specific
antigen (PSA), angiolymphatic invasion, and surgical margins.
Immunohistochemistry
In group 1, four to ten tissue sections (4 µm thick)
were collected from the index tumors and mounted on glass slides.
In group 2, two to four tissue sections (6 µm thick) were mounted
on glass slides from the TMA block, as previously described
(12). Histological sections from
breast carcinoma cases were used as reference patterns for the
positive reactions. Non-neoplastic breast and prostatic tissues
(from an internal sample) were used for negative reactions.
Anti-HER2 antibody A0458, a polyclonal rabbit
anti-human c-erbB-2 oncoprotein antibody (Dako GmbH, Jena,
Germany), was used (incubated at 1:600) for staining in tissue
samples with distinct loss of basal cells (proven PCa). The
sections without any previous confirmation of PCa were not tested.
Antigen recovery was performed according to the HercepTest™ manual
(Dako) (13). The diluted epitope
recovery solution (1:10) was preheated in a tank at 85˚C and
sections were dewaxed at room temperature and immersed in a
preheated epitope recovery solution. They were heated to 97˚C and
incubated for 40±1 min at 97˚C. They were then left in the tank
until they reached a temperature of 85˚C. They were then removed
from the tank and left on the table with the lid closed for
subsequent cooling. After 10 min, the tissue sections were washed
with diluted Dako wash buffer and soaked in this buffer for 5-20
min after epitope recovery and before staining.
All tissue sections were reviewed by two
board-certified genitourinary pathologists (LHSS and MGC). All
features were scored according to the Food and Drug Administration
(FDA) and HercepTest™ manual interpretation (Dako) (13), which comprised intensity,
percentage, and characteristics of the stain (from 0 to 3+), and
then the calculation of a final expression score. The
immunohistochemical expression of HER2 was correlated with
prognostic factors. The Gleason score was reclassified according to
the International Society of Urological Pathology standards for
(14). T staging was assessed using
the clinical tumor node metastasis (TNM) classification standard
(15).
Statistics
The data were analyzed using STATA 14.0 (StataCorp
LP). Frequency tables were selected for descriptive analyses.
Chi-square and Fisher's exact tests were used to assess the
frequency of responses between the groups. For continuous
variables, we used the Mann-Whitney test. In addition, logistic
regression and ordinal logistic regression were applied to
investigate the effect of covariates on the expression of the
HercepTest™. Statistical significance was set at P<0.05.
Results
Technical issues
A total of 192 men were included in this study.
After analysis, 42 patients remained in group 1 and 104 in group 2.
Due to unequivocal cancer tissue in the corresponding TMA section
(remaining 104 samples), 46 samples were excluded from group 2. The
proportion of non-interpretable samples for HER2
immunohistochemistry was 23.9%.
Immunohistochemistry
The demographic characteristics of the patients are
shown in Table I. The mean age was
66 years (interquartile range, 61-80 years). The mean PSA level in
the study was 11.78±12.4 ng/ml (±SD). group 2 presented higher PSA
levels compared to group 1 (7.54±2.70 ng/ml vs. 13.49±14.27 ng/ml;
P=0.0021).
 | Table IBaseline characteristics and outcomes
for all patients. |
Table I
Baseline characteristics and outcomes
for all patients.
| Variable | Total N (%) | group 1 N (%) | group 2 N (%) | P-value |
|---|
| Perineural
invasion | | | | 0.004 |
|
Yes | 63 (43.15) | 26 (61.90) | 37 (35.58) | |
|
No | 83 (56.85) | 16 (38.10) | 67 (64.42) | |
| HER2 expression | | | | <0.001 |
|
2+/3+ | 14 (9.59) | 14 (33.33) | 0 (0.00) | |
|
0/1+ | 132 (90.41) | 28 (66.67) | 104 (100.00) | |
| ISUP | | | | 0.001 |
|
1-2 | 105 (71.92) | 22 (52.38) | 83 (79.81) | |
|
3-5 | 41 (28.08) | 20 (47.62) | 21 (20.19) | |
| Angiolymphatic
invasion | | | | 0.001 |
|
Yes | 30 (20.55) | 16 (38.10) | 14 (13.46) | |
|
No | 116 (79.45) | 26 (61.90) | 90 (86.54) | |
| Surgical
margins | | | | <0.001 |
|
Yes | 28 (19.18) | 18 (42.86) | 10 (9.62) | |
|
No | 118 (80.82) | 24 (57.14) | 94 (90.38) | |
| T stage | | | | <0.001 |
|
T1-T2a | 28 (19.18) | 4 (9.52) | 24 (23.08) | |
|
T2b | 35 (23.97) | 3 (7.14) | 32 (30.77) | |
|
≥T2c | 83 (56.85) | 35 (83.33) | 48 (46.15) | |
| Diabetes | | | | 0.105 |
|
Yes | 7 (4.79) | 4 (9.52) | 3 (2.88) | |
|
No | 139 (95.21) | 38 (90.48) | 101 (97.12) | |
| Hypertension | | | | 0.001 |
|
Yes | 39 (26.71) | 19 (45.24) | 20 (19.23) | |
|
No | 107 (73.29) | 23 (54.76) | 84 (80.77) | |
| Smoking | | | | 0.413 |
|
Yes | 16 (10.96) | 6 (14.29) | 10 (9.62) | |
|
No | 130 (89.04) | 36 (85.71) | 94 (90.38) | |
| Ethnicity | | | | <0.001 |
|
White | 99 (67.81) | 19 (45.24) | 80 (76.92) | |
|
Black | 6 (6.16) | 4 (9.52) | 5 (4.81) | |
|
Mixed ethnic
ancestries | 38 (26.03) | 19 (45.24) | 19 (18.27) | |
| Age
(years)a | 64.88±6.75
(66.00) | 64.48±7.42
(64.50) | 65.04±6.48
(66.00) | 0.729 |
| PSA
(ng/ml)a | 11.78±12.41
(8.55) | 7.54±2.70
(7.15) | 13.49±14.27
(9.70) | 0.002 |
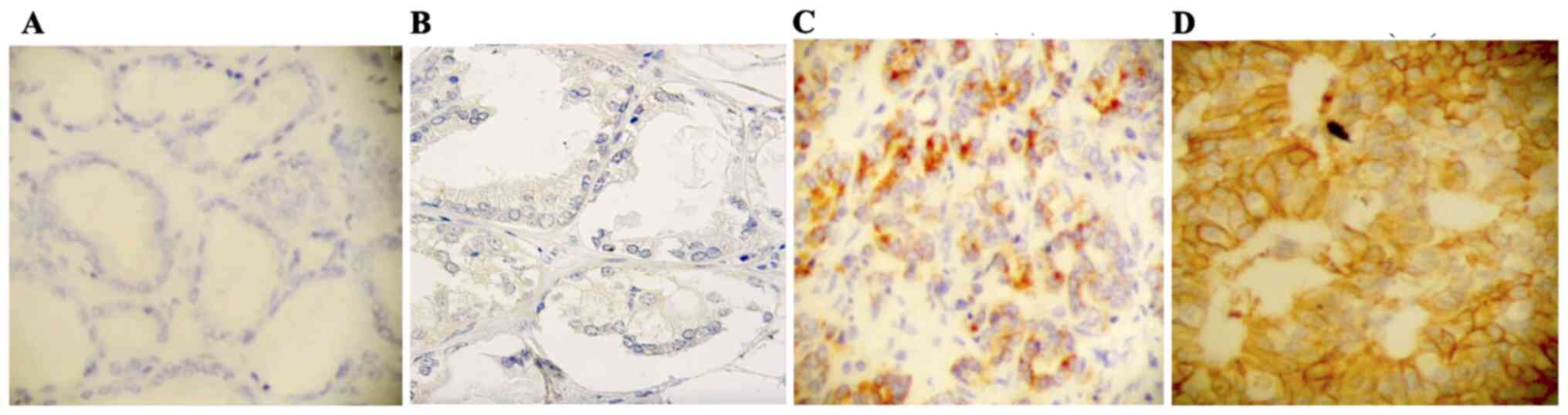
HER2 expression was observed in 85.7% of specimens
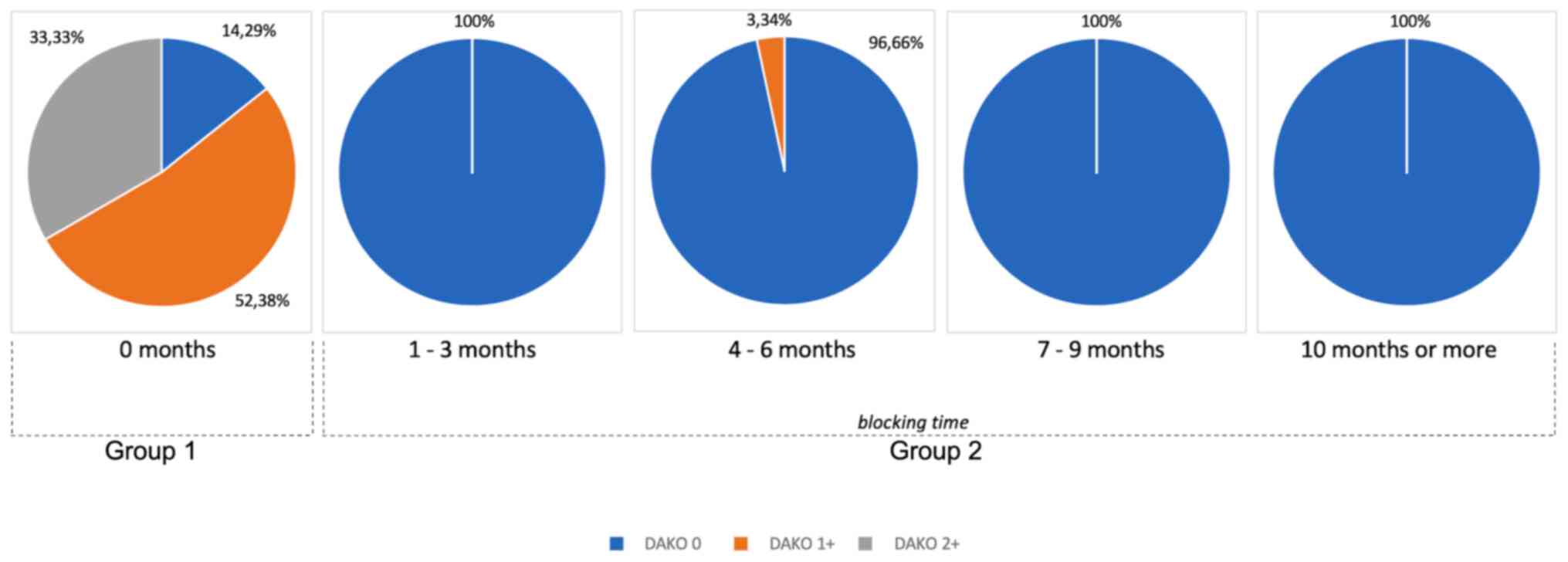
in group 1 and only in 1% of group 2 (Table II) (Fig. 1). Fig.
2 shows the expression of HER2 over time. In group 2, HER2
expression was subdivided into periods of exposure to hormonal
therapy with cyproterone. Even after short periods of exposure to
therapy, its expression was completely suppressed. While the cancer
was sensitive to hormone therapy, HER2 expression was not detected
(Fig. 2).
 | Table IIDistribution of HER2 expression in
each group (P<0.001). |
Table II
Distribution of HER2 expression in
each group (P<0.001).
| HER2
expression | group 1 N (%) | group 2 N (%) | Total N (%) |
|---|
| 0 | 6 (14.29) | 103 (99.04) | 109 (74.66) |
| 1+ | 22 (52.38) | 1 (0.96) | 23 (15.75) |
| 2+ | 14 (33.33) | 0 (0.00) | 14 (9.59) |
| 3+ | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Total | 42 (100.00) | 104 (100.00) | 146 (100.00) |
When considering only patients without neoadjuvant
ADT (group 1), univariate regression analysis showed an association
between ISUP and HER2 expression (P=0.018). However, multivariate
regression analysis showed that perineural invasion, PSA, ISUP,
angiolymphatic invasion, positive margin, and T stage had no
significant effect on HER2 expression (P>0.05) (Table III).
 | Table IIIUnivariate and multivariate ordinal
logistic regression for HER2 expression in group 1. |
Table III
Univariate and multivariate ordinal
logistic regression for HER2 expression in group 1.
| | Univariate
regression | Multivariate
regression |
|---|
| Parameter | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| Age | 1.00
(0.93-1.09) | 0.924 | - | - |
| Perineural
invasion | | | | 0.134 |
|
No | Reference | - | Reference | |
|
Yes | 0.81
(0.25-2.65) | 0.731 | 0.33
(0.0-1.41) | |
| PSA | 1.07
(0.87-1.33) | 0.511 | 1.04
(0.81-1.34) | 0.733 |
| ISUP | | | | |
|
1-2 | Reference | - | Reference | - |
|
3-5 | 5.33
(1.33-21.27) | 0.018 | 1.26
(0.30-5.33) | 0.757 |
| Angiolymphatic
invasion | | | | |
|
No | Reference | - | Reference | - |
|
Yes | 3.38
(0.96-11.90) | 0.058 | 3.87
(0.81-1.34) | 0.090 |
| Surgical
margins | | | | |
|
No | Reference | - | Reference | - |
|
Yes | 2.76
(0.82-9.31) | 0.102 | 2.19
(0.54-8.83) | 0.271 |
| T stage | | | | |
|
T1-T2A | Reference | - | Reference | - |
|
T2B | 1.00
(0.06-15.31) | 0.999 | 0.86
(0.05-14.70) | 0.918 |
|
>T2C | 1.97
(0.25-15.28) | 0.514 | 0.97
(0.10-9.36) | 0.980 |
| Diabetes | | | | |
|
No | Reference | - | - | - |
|
Yes | 1.40
(0.17-11.89) | 0.755 | - | - |
| Hypertension | | | | |
|
No | Reference | - | - | - |
|
Yes | 0.48
(0.15-1.60) | 0.236 | - | - |
| Smoking | | | | |
|
No | Reference | - | - | - |
|
Yes | 1.45
(0.30-7.07) | 0.645 | - | - |
| Ethnicity | | | | |
|
White | Reference | - | - | - |
|
Black | 2.23
(0.29-17.23) | 0.443 | - | - |
|
Mixed ethnic
ancestries | 0.76
(0.22-2.61) | 0.666 | - | - |
When comparing both groups, the univariate
regression analysis indicated that perineural invasion, PSA, ISUP,
angiolymphatic invasion, margin, T stage, and neoadjuvant ADT
correlated with HER2 expression. Nevertheless, ordinal regression
analysis, including all cited variables, indicated a significant
effect on HER2 expression only for neoadjuvant ADT (P<0.001).
Similarly, regression analysis indicated a statistically
significant effect of neoadjuvant ADT alone on HER2 expression
(OR=0.01; 95% CI: 0.00. 0.02; P<0.001) (Table IV).
 | Table IVUnivariate and multivariate ordinal
logistic regression for HER2 expression in all groups. |
Table IV
Univariate and multivariate ordinal
logistic regression for HER2 expression in all groups.
| | Univariate
regression | Multivariate
regression |
|---|
| Parameter | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| Age | 0.99
(0.94-1.04) | 0.733 | - | - |
| Perineural
invasion | | | | |
|
No | Reference | - | Reference | - |
|
Yes | 2.15
(1.01-4.54) | 0.046 | 0.31
(0.08-1.28) | 0.106 |
| PSA | 0.88
(0.81-0.96) | 0.003 | 0.96
(0.81-1.15) | 0.694 |
| ISUP | | | | |
|
1-2 | Reference | - | Reference | - |
|
3-5 | 3.81
(1.74-8.33) | 0.001 | 1.73
(0.45-6.68) | 0.425 |
| Angiolymphatic
invasion | | | | |
|
No | Reference | - | Reference | - |
|
Yes | 4.71
(2.04-10.86) | <0.001 | 3.43
(0.78-15.16) | 0.104 |
| Surgical
margins | | | | |
|
No | Reference | - | Reference | - |
|
Yes | 7.54
(3.21-17.75) | <0.001 | 1.88
(0.50-7.10) | 0.349 |
| T stage | | | | |
|
T1-T2A | Reference | - | Reference | - |
|
T2B | 0.76
(0.14-4.07) | 0.413 | 0.71
(0.06-8.98) | 0.790 |
|
>T2C | 5.04
(1.41-18.02) | 0.013 | 1.35
(0.17-10.64) | 0.776 |
| Neoadjuvant
ADT | | | | |
|
No | Reference | - | Reference | - |
|
Yes | 0.01
(0.00-0.01) | <0.001 | 0.01
(0.00-0.02) | <0.001 |
| Diabetes | | | | |
|
No | Reference | - | - | - |
|
Yes | 2.76
(0.60-12.78) | 0.193 | - | - |
| Hypertension | | | | |
|
No | Reference | - | - | - |
|
Yes | 2.61
(1.20-5.67) | 0.016 | - | - |
| Smoking | | | | |
|
No | Reference | - | - | - |
|
Yes | 1.82
(0.63-5.24) | 0.268 | - | - |
| Ethnicity | | | | |
|
White | Reference | - | - | - |
|
Black | 3.79
(0.96-14.98) | 0.057 | - | - |
|
Mixed ethnic
ancestries | 2.92
(1.30-6.58) | 0.010 | - | - |
Discussion
The epidermal growth factor receptor (EGFR) family
consists of four members: EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3, and
HER4/ErbB4. These are activated by ligand binding (except for
HER2), followed by dimerization and phosphorylation (16). HER2 is the preferred dimerization
partner for EGFR, and both regulate cell proliferation,
differentiation, angiogenesis, and survival (17). Nevertheless, the role of ErbB-2 vs.
EGFR in androgen-stimulated proliferation is still not fully
understood; this is partially due to the lack of suitable cell
models (18). In the present study,
we evaluated, for the first time, the effect of neoadjuvant ADT on
HER2 expression.
According to our results, the expression of HER2
occurred at distinct levels in a significant number of cases and
was not associated with any prognostic factors. Various
immunohistochemical methods have been used to examine the
relationship between HER2 expression and PCa. Significant
heterogeneity in HER2 expression has been noted in these previous
studies (19-21),
which is partially explained by discrepancy between methods, lack
of measurement standardization, and heterogeneity of PCa itself
(22). An important example is the
study by Sanchez et al, who used two different evaluation
techniques: The standard and modified HercepTest™ (23). This approach was necessary to
improve the quality of HER2 analysis in patients with PCa. HER2
overexpression was found to be related to tumor stage and Gleason
score. Our decision to use the standard HercepTest™ as a means of
immunohistochemical interpretation was based on the literature and
availability of kits in our institution's laboratories.
The introduction of neoadjuvant ADT was sufficient
to suppress HER2 expression (P<0.001). This suppression was so
relevant that individuals who received neoadjuvant ADT had a 0.01
chance of HER2 expression compared to individuals who did not
receive neoadjuvant ADT (OR=0.01; 95% CI, 0.00, 0.02; P<0.001).
Similarly, Muniyan et al observed that a HER2 inhibitor
blocked androgen-induced activation and cell growth (24). These results are consistent with
previous observations that HER2 activation plays an essential role
in regulating the androgen-stimulated proliferation of PCa cells
(25). This pharmacological
inhibition revealed that basal and androgen-induced ERK1/2 and p38
MAPK were significantly inhibited, which correlated with abolished
cell growth. In our study, the suppression of HER2 caused by
neoadjuvant ADT occurred as soon as one month after the initiation
of therapy and was maintained thereafter. This suppression seemed
to be maintained throughout the period that PCa was shown to be
sensitive to hormone therapy.
We observed a higher percentage of HER2 expression
in group 1 (85.7%). A significant impact of neoadjuvant ADT was
noted; only 1% of group 2 patients presented with HER2 expression.
In addition, the effect was noted regardless of the time of
analysis (1-24 months). Even a short period of neoadjuvant ADT
suppressed HER2 expression. The study results highlight an exciting
correlation between HER2, PCa, and ADT.
Interestingly, Chen et al demonstrated that
dual inhibition of EGFR/HER2 with ADT resulted in the apoptosis of
PCa cells (26). This could be an
alternative, especially for castration-resistant prostate cancer
(CRPC). In another study, Di Lorenzo et al observed a
significant association between HER2, high levels of PSA, and a
high Gleason score in patients with metastatic CRPC, contributing
to the hypothesis of the association between HER2 and PCa
aggressiveness. PCa recurrence also correlated significantly with
c-erB2 levels in 60% of cases (27).
Significant efforts have been made to determine
whether neoadjuvant treatment improves clinical outcomes in PCa
(27). For radiation therapy,
numerous studies have shown benefits with the addition of
neoadjuvant, concurrent, and adjuvant ADT in treating intermediate-
and high-risk diseases (28,29).
In contrast, the benefits of neoadjuvant therapy before RP (both
ADT and chemotherapy) have not yet been determined. In addition, no
significant improvement in progression-free survival and overall
survival (OS) has been demonstrated in several trials (30). Despite this, neoadjuvant therapy
before RP provides a unique opportunity to clarify the effects of
treatment on the tumor microenvironment. Access to material from an
old study in which patients underwent neoadjuvant hormone therapy
offered a unique opportunity to study the effects of this type of
treatment on HER2 expression; this is the reason why we included
group 2 patients in this study. The suppression of HER2 observed in
our study may be one of the mechanisms related to the response of
tumors to ADT.
Some studies have suggested that HER2 acts as a
co-receptor in the cell response mediated by HER substrates
(31-33).
In addition, overexpression of HER2 could increase the rate of cell
transformation, one of the pathways involved in
castration-resistant prostate adenocarcinoma. The specific
activation of HER2 induces many independent signaling pathways,
such as phospholipase C (PLC), phosphatidylinositol 3-kinase
(PI3K), the JAK-STAT pathway, mitogen-activated protein kinases
(MAPKs), and proteins activated by stress (27). These pathways activate
proto-oncogenes such as c-fos, c-jun, and c-Src, which could lead
to cell proliferation even in the absence of testosterone.
Signaling of the PI3K pathway by HER2 also induces
phosphorylation and inactivation of glycogen synthase kinase-3
(GSK3), resulting in increased nuclear levels of β-catenin, which
in turn increases the activity of the androgen receptor (AR) and,
consequently, stimulates the growth and survival of prostate cells.
These findings delineate the mechanism by which HER2 and AR
regulate the androgen pathway during prostate cell growth and
survival (34).
In metastatic PCa, circulating levels of HER2 have
often been used as predictive markers of progression (35,36).
Jathal et al demonstrated that the failure of lapatinib in
clinical trials of CRPC was due to its ability to significantly
increase HER2 levels, which consequently led to increased protein
synthesis rates. This resulted in the accumulation of excess HER2
in the plasma membrane, the formation of EGFR/HER2 dimers, and the
transmission of signals to downstream targets that prevent loss of
cell viability (36). Similarly,
Tome-Garcia et al demonstrated that overexpression of the
constitutively activated form of HER2 increases the metastatic
potential of androgen-insensitive human PCa cell lines, but not of
androgen-sensitive PCa cell lines (37). All these results can lead to the
hypothesis that the moment of transformation of PCa in CRPC could
be correlated with the moment of increased HER2 expression after
inhibition by ADT.
Future studies will examine whether suppression of
HER2 transcription results in the cellular transformation of PCa,
mainly in CRPC. The data reported herein suggest a possible
association between ADT and the inhibition of HER2 expression while
the tumor was hormone-sensitive.
Our study has certain limitations. First, as in
several other studies, we performed immunohistochemical analysis to
evaluate HER2 expression in prostate specimens. However, while
immunohistochemistry has an established track record for evaluating
the expression of HER2 in breast cancer, it has not been used as
definitively in PCa. The HerceptTest™ technique we used has
specific instructions only for breast and gastric cancers, and not
PCa. In addition, it did not show classic HER2 overexpression (3+)
in any of the 146 cases studied. According to the HercepTest™
Interpretation Manual, the specific result ‘HER2 +2’ could be
analyzed later with fluorescent in situ hybridization
(FISH), which was not available in our laboratories (13). The use of FISH has also been
suggested to solve the potential problem of inconsistent results
whenever different antibodies are used in immunohistochemical
testing. Another limitation was the use of medications for androgen
deprivation. Despite these limitations, which could limit the
clinical significance of our findings, our material is unique and
provides valuable insights for research purposes, as well as
suggesting possible directions for further research using different
methods, such as FISH.
The data reported here suggest a possible
association between testosterone-suppressing hormone therapy and
inhibition of HER2 receptor expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
All authors contributed to data collection and
analysis. GAP, FK, MGDC and LHSS drafted the manuscript. CLP, TFNL,
MLW, NMC, MTM and SG edited the manuscript. All authors have read
and approved the final version of the manuscript. GAP and FK
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Centro Universitario FMABC and Santa Casa of São Paulo
Hospital (approval nos. 84427718.0.0000.0082 and
06937412.0.1001.0082, respectively). Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rimawi MF, Mayer IA, Forero A, Nanda R,
Goetz MP, Rodriguez AA, Pavlick AC, Wang T, Hilsenbeck SG,
Gutierrez C, et al: Multicenter phase II study of neoadjuvant
lapatinib and trastuzumab with hormonal therapy and without
chemotherapy in patients with human epidermal growth factor
receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol.
31:1726–1731. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Koeppen HK, Wright BD, Burt AD, Quirke P,
McNicol AM, Dybdal NO, Sliwkowski MX and Hillan KJ: Overexpression
of HER2/neu in solid tumours: An immunohistochemical survey.
Histopathology. 38:96–104. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cobleigh MA, Vogel CL, Tripathy D, Robert
NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman
G and Slamon DJ: Multinational study of the efficacy and safety of
humanized anti-HER2 monoclonal antibody in women who have
HER2-overexpressing metastatic breast cancer that has progressed
after chemotherapy for metastatic disease. J Clin Oncol.
17:2639–2648. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Neto AS, Tobias-Machado M, Wroclawski ML,
Fonseca FL, Teixeira GK, Amarante RD, Wroclawski ER and Del Giglio
A: Her-2/neu expression in prostate adenocarcinoma: A systematic
review and meta-analysis. J Urol. 184:842–850. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Minner S, Jessen B, Stiedenroth L, Burandt
E, Köllermann J, Mirlacher M, Erbersdobler A, Eichelberg C, Fisch
M, Brümmendorf TH, et al: Low level HER2 overexpression is
associated with rapid tumor cell proliferation and poor prognosis
in prostate cancer. Clin Cancer Res. 16:1553–1560. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carrión-Salip D, Panosa C, Menendez JA,
Puig T, Oliveras G, Pandiella A, De Llorens R and Massaguer A:
Androgen-independent prostate cancer cells circumvent EGFR
inhibition by overexpression of alternative HER receptors and
ligands. Int J Oncol. 41:1128–1138. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Craft N, Shostak Y, Carey M and Sawyers
CL: A mechanism for hormone-independent prostate cancer through
modulation of androgen receptor signaling by the HER-2/neu tyrosine
kinase. Nat Med. 5:280–285. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Miyamoto H, Hernandez DJ and Epstein JI: A
pathological reassessment of organ-confined, Gleason score 6
prostatic adenocarcinomas that progress after radical
prostatectomy. Hum Pathol. 40:1693–1698. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kobayashi M, Ishida H, Shindo T, Niwa S,
Kino M, Kawamura K, Kamiya N, Imamoto T, Suzuki H, Hirokawa Y, et
al: Molecular analysis of multifocal prostate cancer by comparative
genomic hybridization. Prostate. 68:1715–1724. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lepor H and Donin NM: Gleason 6 prostate
cancer: Serious malignancy or toothless lion? Oncology (Williston
Park). 28:16–22. 2014.PubMed/NCBI
|
|
12
|
Korkes F, de Castro MG, de Cassio Zequi S,
Nardi L, Del Giglio A and de Lima Pompeo AC: Hyaluronan-mediated
motility receptor (RHAMM) immunohistochemical expression and
androgen deprivation in normal peritumoral, hyperplasic and
neoplastic prostate tissue. BJU Int. 113:822–829. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Espinoza F and Thompson J: HercepTest™
interpretation manual. Dako, 2010.
|
|
14
|
Egevad L, Delahunt B, Srigley JR and
Samaratunga H: International society of urological pathology (ISUP)
grading of prostate cancer-an ISUP consensus on contemporary
grading. APMIS. 124:433–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM classification of malignant tumors. UICC
International Union Against Cancer, 2017.
|
|
16
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Di Lorenzo G, Tortora G, D'Armiento FP, De
Rosa G, Staibano S, Autorino R, D'Armiento M, De Laurentiis M, De
Placido S, Catalano G, et al: Expression of epidermal growth factor
receptor correlates with disease relapse and progression to
androgen-independence in human prostate cancer. Clin Cancer Res.
8:3438–3444. 2002.PubMed/NCBI
|
|
19
|
Rao K, Gaughan L, Robson C and McCracken
S: The role of the HER2 and HER3 in prostate cancer and their
potential as therapeutic targets. Eur J Cancer. 61 (Suppl
1)(S177)2016.
|
|
20
|
Baek KH, Hong ME, Jung YY, Lee CH, Lee TJ,
Park ES, Kim MK, Yoo JH and Lee SW: Correlation of AR, EGFR, and
HER2 expression levels in prostate cancer: Immunohistochemical
analysis and chromogenic in situ hybridization. Cancer Res Treat.
44:50–56. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yan M, Schwaederle M, Arguello D, Millis
SZ, Gatalica Z and Kurzrock R: HER2 expression status in diverse
cancers: Review of results from 37,992 patients. Cancer Metastasis
Rev. 34:157–164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lara PN Jr, Meyers FJ, Gray CR, Edwards
RG, Gumerlock PH, Kauderer C, Tichauer G, Twardowski P, Doroshow JH
and Gandara DR: HER-2/neu is overexpressed infrequently in patients
with prostate carcinoma. Results from the California cancer
consortium screening trial. Cancer. 94:2584–2589. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sanchez KM, Sweeney CJ, Mass R, Koch MO,
Eckert GJ, Geary WA, Baldridge LA, Zhang S, Eble JN and Cheng L:
Evaluation of HER-2/neu expression in prostatic adenocarcinoma: A
requested for a standardized, organ specific methodology. Cancer.
95:1650–1655. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Muniyan S, Chen SJ, Lin FF, Wang Z, Mehta
PP, Batra SK and Lin M-F: ErbB-2 signaling plays a critical role in
regulating androgen-sensitive and castration-resistant androgen
receptor-positive prostate cancer. cells. 27:2261–2271.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Di Lorenzo G, Autorino R, De Laurentiis M,
Cindolo L, D'Armiento M, Bianco AR and De Placido S: HER-2/neu
receptor in prostate cancer development and progression to androgen
independence. Tumori. 90:163–170. 2004.PubMed/NCBI
|
|
26
|
Chen L, Mooso BA, Jathal MK, Madhav A,
Johnson SD, van Spyk E, Mikhailova M, Zierenberg-Ripoll A, Xue L,
Vinall RL, et al: Dual EGFR/HER2 inhibition sensitizes prostate
cancer cells to androgen withdrawal by suppressing ErbB3. Clin
Cancer Res. 17:6218–6228. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meng TC, Lee MS and Lin MF: Interaction
between protein tyrosine phosphatase and protein tyrosine kinase is
involved in androgen-promoted growth of human prostate cancer
cells. Oncogene. 19:2664–2677. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bolla M, Van Tienhoven G, Warde P, Dubois
JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C,
Billiet I, et al: External irradiation with or without long-term
androgen suppression for prostate cancer with high metastatic risk:
10-year results of an EORTC randomised study. Lancet Oncol.
11:1066–1073. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Denham JW, Steigler A, Lamb DS, Joseph D,
Turner S, Matthews J, Atkinson C, North J, Christie D, Spry NA, et
al: Short-term neoadjuvant androgen deprivation and radiotherapy
for locally advanced prostate cancer: 10-year data from the TROG
96.01 randomised trial. Lancet Oncol. 12:451–459. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pendleton J, Pisters LL, Nakamura K, Anai
S and Rosser CJ: Neoadjuvant therapy before radical prostatectomy:
Where have we been? Where are we going? Urol Oncol. 25:11–18.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wilson KJ, Gilmore JL, Foley J, Lemmon MA
and Riese DJ II: Functional selectivity of EGF family peptide
growth factors: Implications for cancer. Pharmacol Ther. 122:1–8.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Furrer D, Paquet C, Jacob S and Diorio C:
The human epidermal growth factor receptor 2 (HER2) as a prognostic
and predictive biomarker: Molecular insights into HER2 activation
and diagnostic implications. Cancer Prognosis: pp11-13, 2018.
|
|
33
|
Ding L, Tian C, Feng S, Fida G, Zhang C,
Ma Y, Ai G, Achilefu S and Gu Y: Small sized EGFR1 and HER2
specific bifunctional antibody for targeted cancer therapy.
Theranostics. 5:378–398. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tambo M, Higashihara E, Terado Y, Nutahara
K and Okegawa T: Comparison of serum HER2/neu with
immunohistochemical HER2/neu expression for the prediction of
biochemical progression in metastatic prostate cancer. Int J Urol.
16:369–374. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Domingo-Domenech J, Fernandez PL, Filella
X, Martinez-Fernandez A, Molina R, Fernandez E, Alcaraz A, Codony
J, Gascon P and Mellado B: Serum HER2 extracellular domain predicts
an aggressive clinical outcome and biological PSA response in
hormone-independent prostate cancer patients treated with
docetaxel. Ann Oncol. 19:269–275. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jathal MK, Steele TM, Siddiqui S, Mooso
BA, D'Abronzo LS, Drake CM, Whang YE and Ghosh PM: Dacomitinib, but
not lapatinib, suppressed progression in castration-resistant
prostate cancer models by preventing HER2 increase. Br J Cancer.
121:237–248. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tome-Garcia J, Li D, Ghazaryan S, Shu L
and Wu L: ERBB2 increases metastatic potentials specifically in
androgen-insensitive prostate cancer cells. PLoS One.
9(e99525)2014.PubMed/NCBI View Article : Google Scholar
|