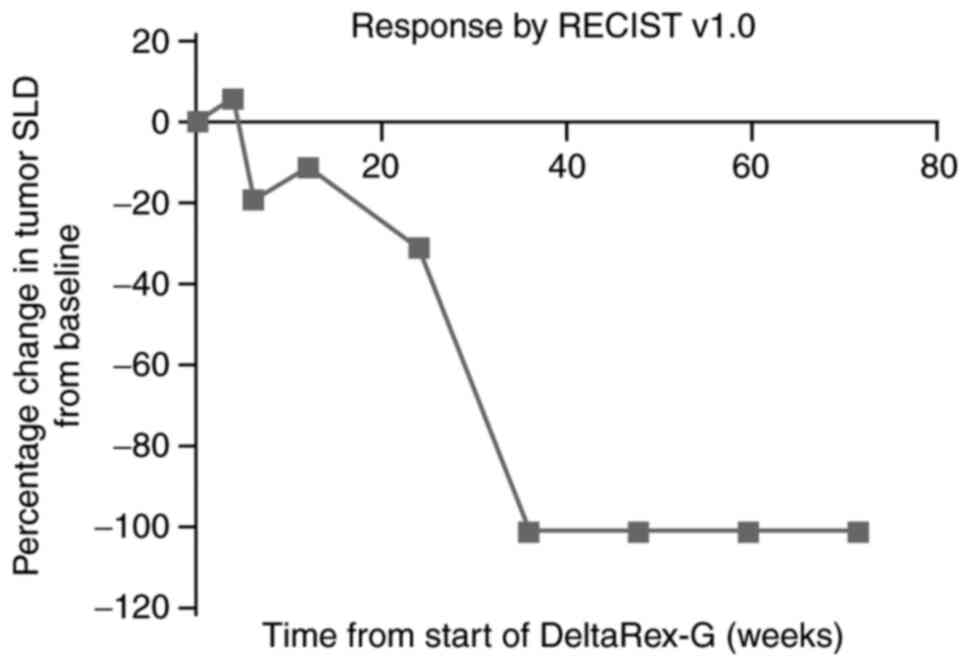
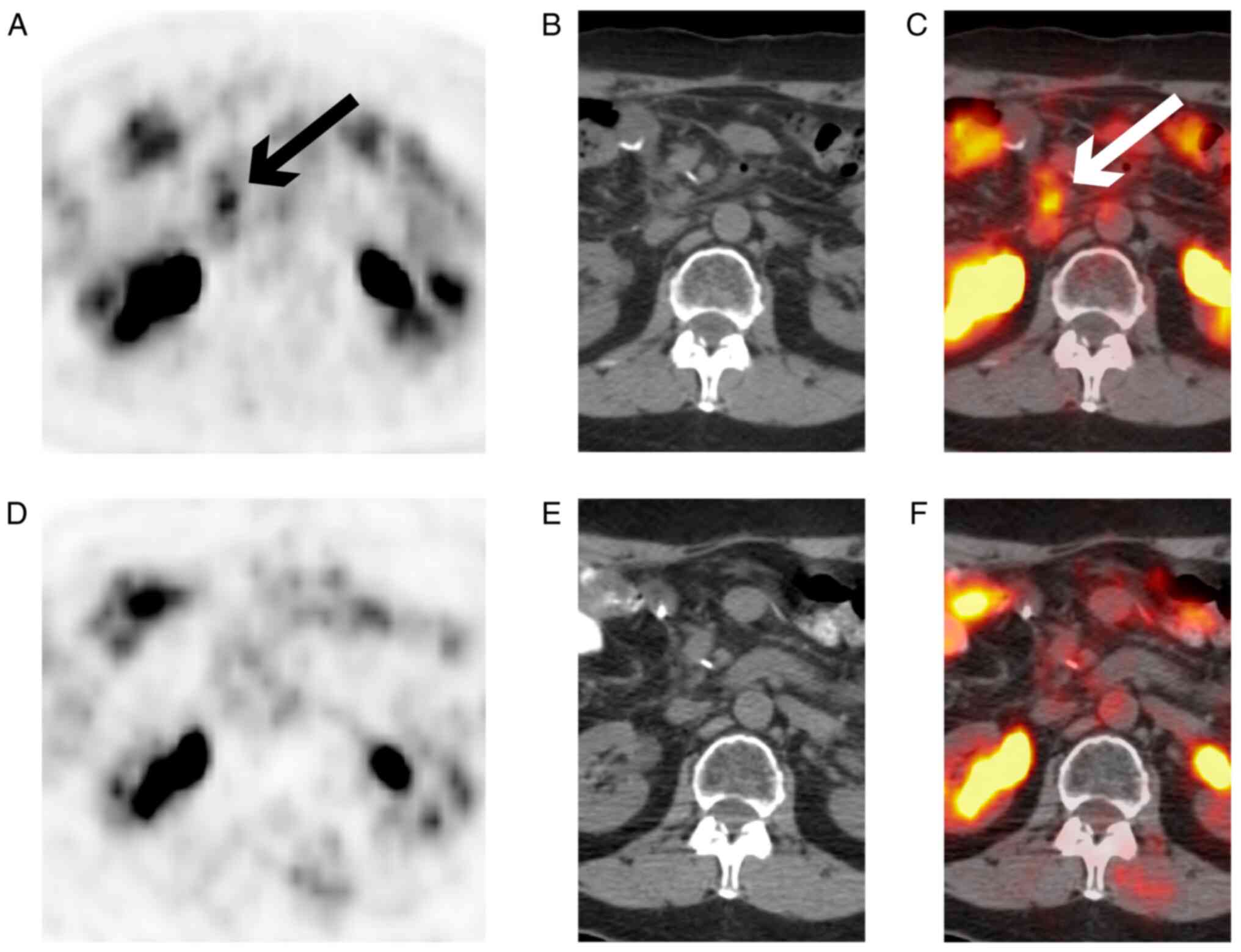
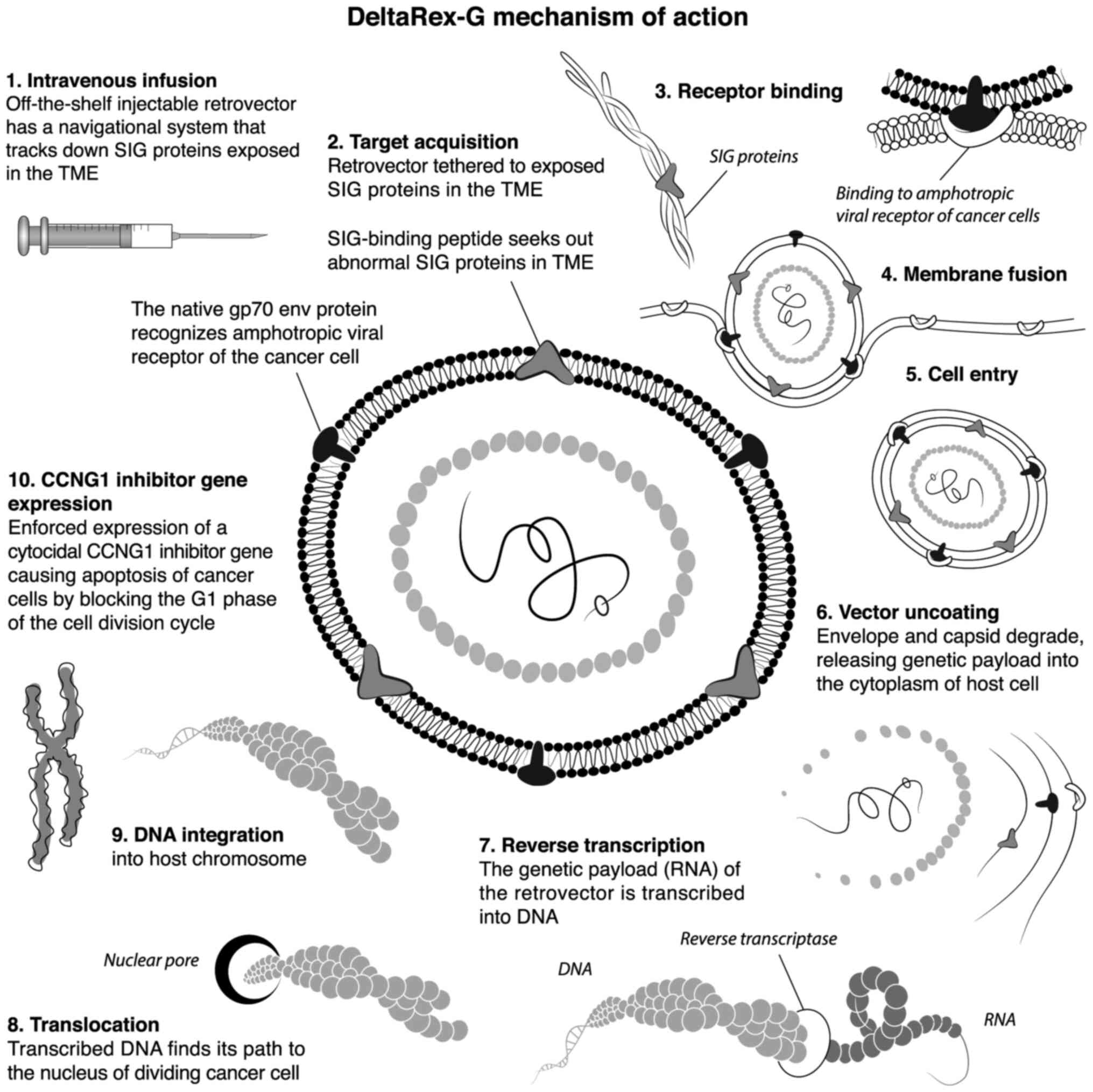
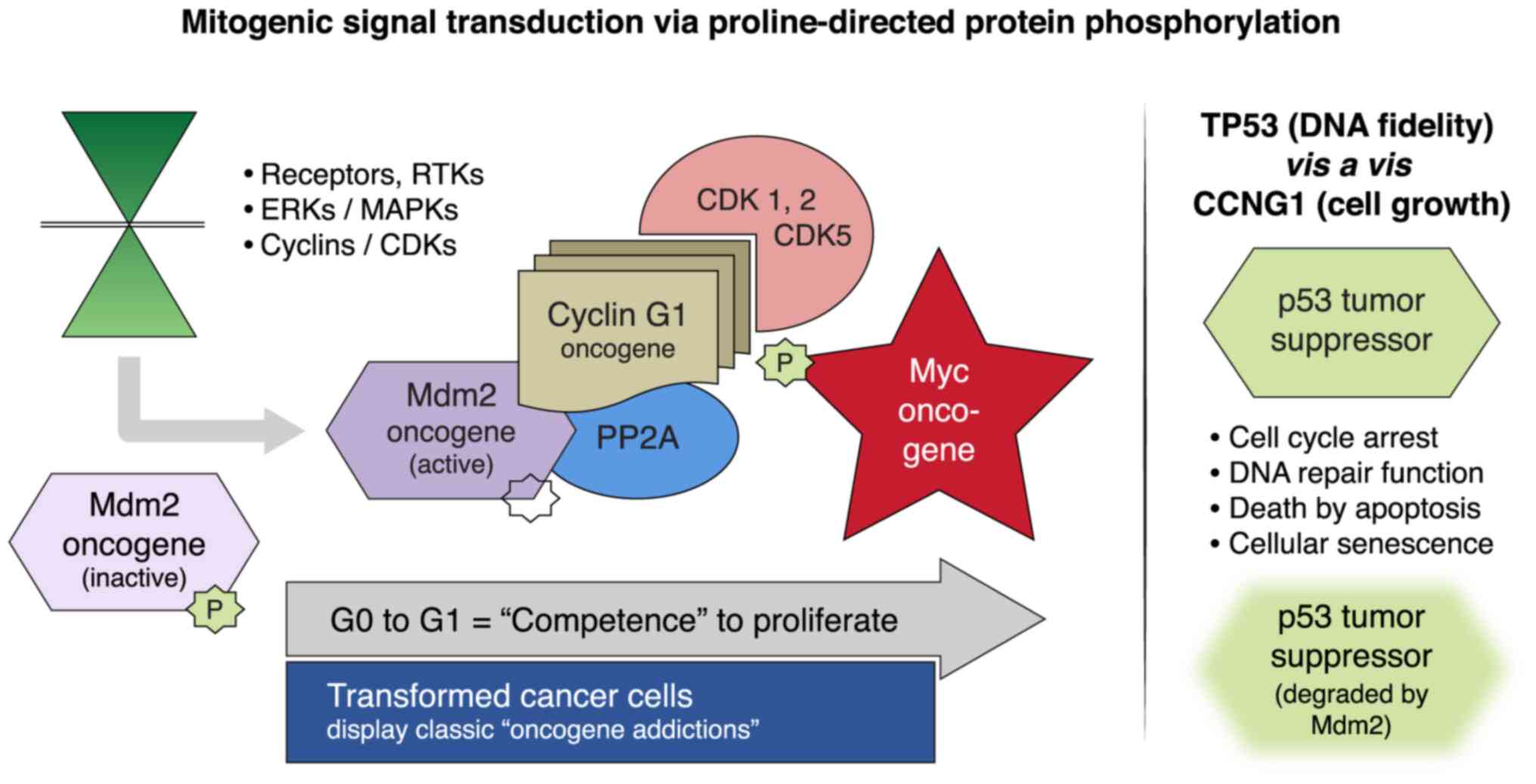
|
1
|
American Cancer Society: Survival Rates
for Pancreatic Cancer. American Cancer Society. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html.
Acessed February 12, 2021.
|
|
2
|
Malone ER, Oliva M, Sabatini PJB, Stockley
TL and Siu LL: Molecular profiling for precision cancer therapies.
Genome Med. 12(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
G, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus Gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chawla SP, Bruckner H, Morse MA, Assudani
N, Hall FL and Gordon EM: A phase I/II clinical study on the
safety, efficacy, and therapeutic potential of intravenous
DeltaRex-G: A tumor-targeted retrovector encoding a
dominant-negative cyclin G1 inhibitor for advanced pancreatic
cancer. Mol Ther Oncol. 12:56–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al-Shihabi A, Chawla SP, Hall FL and
Gordon EM: Exploiting oncogenic drivers along the CCNG1 pathway for
cancer therapy and gene therapy. Mol Ther Oncolytics. 11:122–126.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Esfahani MS, Lee LJ, Jeon YJ, Flynn RA,
Stehr H, Hui AB, Ishisoko N, Kildebeck E, Newman AM, Bratman SV, et
al: Functional significance of U2AF1 S34F mutations in lung
adenocarcinomas. Nat Commun. 10(5712)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cicenas J, Kvederaviciute K, Meskinyte I,
Meskinyte-Kausiliene E, Skeberdyte A and Cicenas J: KRAS, TP53,
CDKN2A, SMAD4, BRCA1, and BRCA2 mutations in pancreatic cancer.
Cancers (Basel). 9(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kimura SH and Nojima H: Cyclin G1
associates with MDM2 and regulates accumulation and degradation of
p53 protein. Genes Cells. 7:869–880. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Giono LE and Manfredim JJ: The p53 tumor
suppressor participates in multiple cell cycle checkpoints. J Cell
Physiol. 209:13–20. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gordon EM, Ravicz JR, Liu S, Chawla SP and
Hall FL: Cell cycle checkpoint control: The cyclin G1/Mdm2/p53 axis
emerges as a strategic target for broad-spectrum cancer gene
therapy-a review of molecular mechanisms for oncologists. Mol Clin
Oncol. 9:115–134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen X: Cyclin G: A regulator of the
p53-Mdm2 network. Dev Cell. 2:518–519. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jensen MR, Factor VM, Fantozzi A, Helin K,
Huh CG and Thorgeirsson SS: Reduced hepatic tumor incidence in
Cyclin G1-deficient mice. Hepatology. 37:862–870. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Olivier M, Hollstein M and Hainau P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2(a001008)2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gordon EM, Liu PX, Chen ZH, Liu L, Whitley
MD, Gee C, Groshen S, Hinton DR, Beart RW and Hall FL: Inhibition
of metastatic tumor growth in nude mice by portal vein infusions of
matrix-targeted retroviral vectors bearing a cytocidal cyclin G1
construct. Cancer Res. 60:3343–3347. 2000.PubMed/NCBI
|
|
15
|
Gordon EM, Chen ZH, Liu L, Whitley M, Liu
L, Wei D, Groshen S, Hinton DR, Anderson WF, Beart RW Jr and Hall
FL: Systemic administration of a matrix-targeted retroviral vector
is efficacious for cancer gene therapy in mice. Hum Gene Ther.
12:193–204. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Galanis E, Carlson SK, Foster NR, Lowe V,
Quevedo F, McWilliams RR, Grothey A, Jatoi A, Alberts SR and Rubin
J: Phase I trial of a pathotropic retroviral vector expressing a
cytocidal cyclin G1 construct (Rexin-G) in patients with advanced
pancreatic cancer. Mol Ther. 16:979–984. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chawla SP, Chua VS, Fernandez L, Quon D,
Saralou A, Blackwelder WC, Hall FL and Gordon EM: Phase I/II and
phase II studies of targeted gene delivery in vivo: Intravenous
Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol
Ther. 17:1651–1657. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chawla SP, Chawla NS, Quon D, Chua-Alcala
V, Blackwelder WC, Hall FL and Gordon EM: An advanced phase 1/2
study using an XC-targeted gene therapy vector for chemotherapy
resistant sarcoma. Sarcoma Res Int. 3(1024)2016.
|
|
19
|
Bruckner H, Chawla SP, Liu S, Assudani N,
Hall FL and Gordon EM: Phase I-II study using Rexin-G, a
tumor-targeted retrovector encoding a cyclin G1 inhibitor for
metastatic carcinoma of breast: A ten-year follow-up. Presented at
the 2019 American Society of Gene and Cell Therapy (ASGCT) Annual
Meeting. ASGCT, Washington, DC, Abstract 273, 2019.
|