Introduction
Metastatic pancreatic ductal adenocarcinoma (PDAC)
is a serious disease with a 5-year survival rate of 3% (1). Therefore, clinical trials using
innovative therapies are urgently required. In recent years,
molecular profiling and next-generation sequencing of archived or
resected tumor samples have been developed, and certain genetic
mutations in tumors have predicted favorable responses to
gene-targeted inhibitor therapies (2). A unique patient with chemoresistant
PDAC metastatic to the liver, lymph nodes and peritoneum is
reported in the present study because she participated in a phase
I/II clinical trial that used DeltaRex-G, the first-in-human
intravenously (i.v.) administered tumor-targeted gene therapy
approach to stage 4 pancreatic cancer (3), has survived beyond the median survival
time of 8 months reported for the optimal first-line therapy
(gemcitabine and nab-paclitaxel) (4) and beyond the 5-year survival time
reported for metastatic PDAC (1),
with no evidence of cancer or delayed therapy-related adverse
events for >12 years. Furthermore, her tumor harbored a genetic
mutation that could favorably broaden the limited range of
treatments available for the otherwise lethal prognosis of stage 4
pancreatic cancer.
DeltaRex-G (formerly Mx-dnG1, dnG1 or Rexin-G) is a
replication-incompetent tumor-targeted retroviral vector that
displays a collagen matrix (Signature, SIG)-binding decapeptide for
targeting anaplastic collagenous (SIG) proteins exposed by the
invading tumor and encodes a dominant negative mutant construct of
the cyclin G1 (CCNG1) gene that is devoid of its N-terminus
and the first two helical segments (α1 andα2) of the definitive
cyclin (proteolytic processing). The cytocidal dnG1 protein, which
induces apoptosis in proliferating cells, retains the
cyclin-dependent kinase (CDK) contact points (helices
α3* and α5*) and the structural domains for
serine/threonine protein phosphatase subunit designated 2A (PP2A),
β' and Mdm2 binding, ultimately blocking CCNG1 function and
proliferative cell competence and survival through the commanding
CCNG1/CDK/Myc/Mdm2/p53 axis (5).
Case report
Patient information and clinical
findings
In late 2006, the patient was initially diagnosed
with localized, poorly differentiated PDAC, underwent a Whipple's
procedure with postoperative radiation therapy, and received
fluorouracil chemoradiotherapy and external beam radiation,
followed by gemcitabine. In 2008, the patient presented with
hepatic and lymph node metastases, and peritoneal carcinomatosis
based on abnormalities on the fluorodeoxyglucose-positron emission
tomography scan and elevated serum carbohydrate antigen (CA)19-9
levels. At that time, the patient refused further chemotherapy and
decided to participate in a phase I/II study using DeltaRex-G, a
tumor-targeted retrovector encoding and expressing a truncated
cytocidal construct of the CCNG1 oncogene, which blocks
CCNG1 function in the malignant cell cycle (3).
Therapeutic intervention
The advanced phase I/II clinical trial (NCT00504998)
was a dose-seeking study that incorporated a modified cohort-of-3
design (3). Increasing doses of
DeltaRex-G [1.0-3.0 x1011] colony-forming units
(cfu)/dose] were administered i.v. two/three times per week for 4
weeks with a 2-week rest period, which comprised one treatment
cycle. Treatment cycles were repeated if grade ≤1 toxicity was
observed. Treatment response was evaluated based on the Response
Evaluation Criteria in Solid Tumors (v1.0). Safety and efficacy
analyses were conducted by the Site Principal Investigators
(clinical sites: Santa Monica, Brooklyn and Durham, USA). The
clinical protocol was reviewed and approved by the Western
Institutional Review Board (Olympia, WA, USA). The patients were
recruited on a first-come, first-served basis, and written informed
consent was obtained from each patient at the time of enrollment.
All personnel who handled and disposed of the vector complied to
biosafety level 2 requirements in accordance with the NIH
Guidelines for Research Involving Recombinant or Synthetic Nucleic
Acid Molecules.
Follow-up and outcomes
The patient received dose level 3, which consisted
of 3x1011 cfu DeltaRex-G/dose three times per week for 4
weeks with a 2-week rest period (one treatment cycle) for 1.5
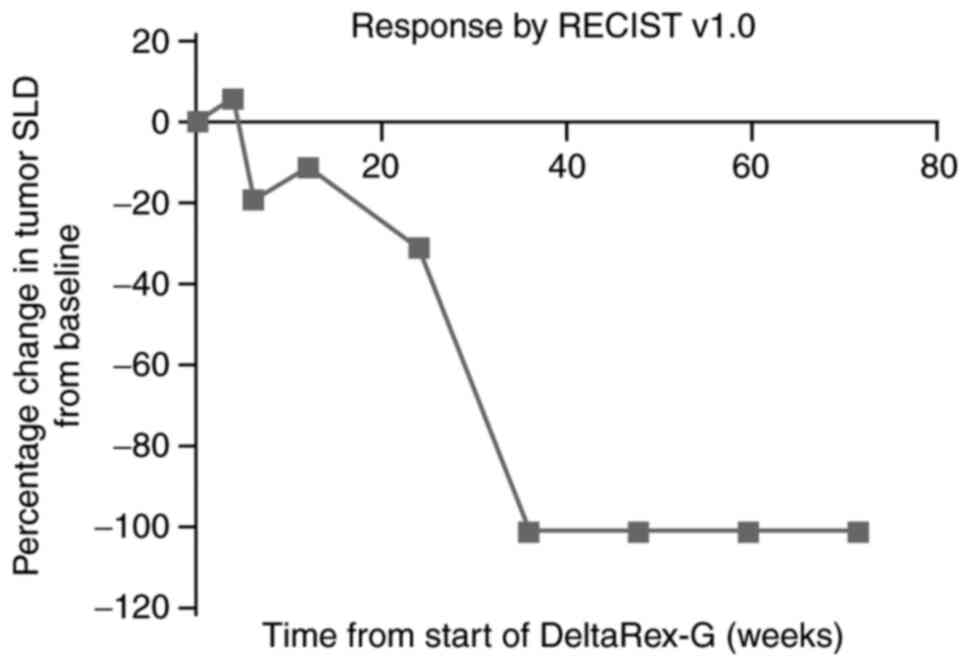
years. The progressive reduction in the sum of the longest tumor
diameters over time is shown in Fig.
1. The patient achieved complete remission with minimal
toxicity (grade 2 fatigue) after 8 months of therapy (Fig. 2). She received no further treatment
following the completion of the study and has achieved a sustained
remission with normal serum CA19-9 levels (32 ng/ml), and no
late-onset treatment-related adverse events as of the last
follow-up in April 2021.
Diagnostic assessment
‘FoundationOne®CDx is performed
exclusively as a laboratory service using DNA extracted from
formalin-fixed, paraffin-embedded (FFPE) tumor samples. The assay
involves a single DNA extraction from routine FFPE biopsy or
surgical resection specimens; 50-1,000 ng DNA then undergoes
whole-genome shotgun library construction and hybridization-based
capture of all coding exons from 309 cancer-related genes, one
promoter region, one non-coding RNA and selected intronic regions
from 34 commonly rearranged genes, 21 of which also include the
coding exons. In total, the assay detects alterations in a total of
324 genes. Using the Illumina® HiSeq 4000 platform
(Illumina, Inc.), hybrid capture-selected libraries are sequenced
to high uniform depth (targeting >500X median coverage with
>99% of exons at a coverage of >100X). Sequence data are then
processed using a customized analysis pipeline designed to detect
all classes of genomic alterations, including base substitutions,
indels, copy number alterations (amplifications and homozygous gene
deletions), and select genomic rearrangements (e.g., gene
fusions)’.
Retrospective RNA sequence analysis of this
patient's archived tumor samples, performed by Foundation
One®CDx, showed two clinically significant genetic
mutations: KRAS proto-oncogene, GTPase (KRAS; G12R) and
tumor protein p53 (TP53; G199V). In addition, the patient
had simultaneous U2 small nuclear RNA auxiliary factor 1 (U2AF1;
S34F) gene expression, which is considered to be involved in
epithelial-to-mesenchymal transition (EMT) and increased tumor cell
invasion (6). While KRAS and TP53
mutations are frequently found in PDAC (7) and are associated with poor prognosis,
these genetic alterations have not, thus far, been targetable.
Discussion
DeltaRex-G is an immunologically stealth (repeatedly
injectable) retrovector displaying a Signature
collagen-matrix-binding targeting peptide on its gp70 Env protein
and encoding a cytocidal dominant negative CCNG1 inhibitor
gene, which blocks the executive CCNG1 axis. When injected
i.v., the DeltaRex-G nanoparticles (~100 nm in diameter) seek out
the tumor and accumulate in the tumor microenvironment (TME), where
anaplastic collagenous proteins secreted by tumor-associated
fibroblasts (TAFs) constitute an abnormal finding, thus increasing
the effective drug concentration in the TME in the vicinity of
proliferating cancer cells. The vector then enters the cancer cell
and delivers its cytocidal genetic construct into the nucleus of
rapidly dividing cancer cells, TAFs and neoangiogenic cells,
causing apoptosis by blocking the G1 phase of the cell division
cycle (8). The 10 steps of the
DeltaRex-G function are shown in Fig.
3.
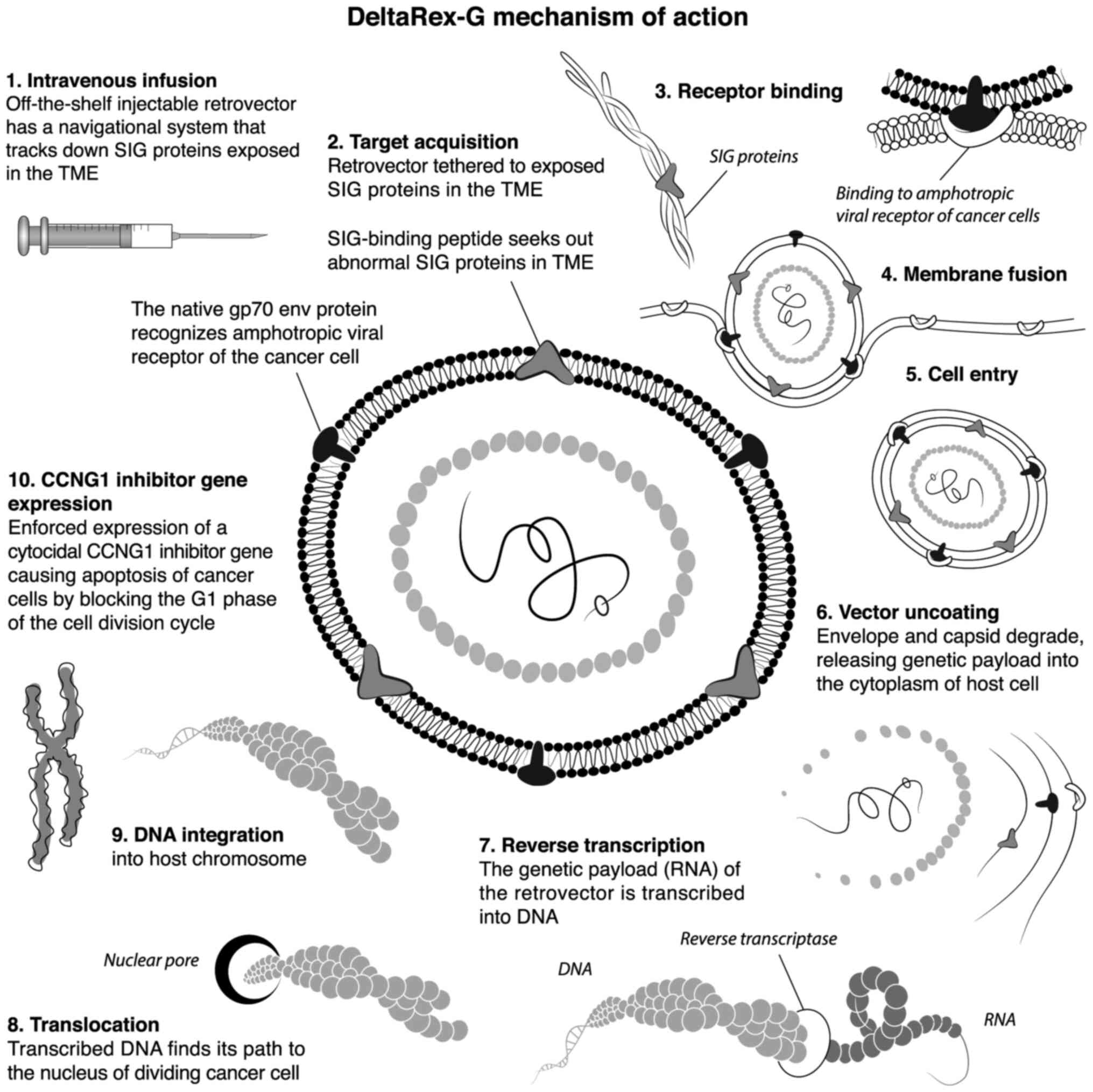 | Figure 3Ten-step illustration of DeltaRex-G
mechanism of action. The DeltaRex-G nanoparticle displays a
collagen matrix (SIG)-binding peptide derived from coagulation vWF
on its gp70 envelope protein. When injected i.v., DeltaRex-G seeks
out the tumors and accumulates in cancerous lesions by binding to
abnormal collagenous SIG proteins exposed in the TME as a result of
tumor invasion. This chimeric retrovector has the innate property
of binding to the natural amphotropic viral/cell receptor, fusing,
entering, uncoating and integrating randomly into the chromosomes
of only actively dividing cells (i.e., cancer cells), sparing
normal cells. DeltaRex-G bears a cytocidal CCNG1 inhibitor
gene, which causes cell death through apoptosis. CCNG1,
cyclin G1; SIG, abnormal signature; vWF, von Willebrand factor;
TME, tumor microenvironment. |
The discovery that CCNG1 is physically
associated with both PP2A and Mdm2, and that this physical
association regulates the accumulation and degradation of the p53
protein, has provided new and important insights into the oncogenic
function of CCNG1, and suggests that a major role of
CCNG1 is to activate the Mdm2 oncoprotein to override the
cell cycle checkpoint control functions of p53(8). The loss of p53-mediated tumor
suppression in addition to the mutational activation of the KRAS
oncogene was found to drive multiple oncogenic signaling cascades
(Fig. 4), including
mitogen-activated protein kinase, phosphoinositol-3 kinase and
transforming growth-factor-β pathways governing cancer stem cell
survival, proliferation and metastatic behavior (EMT and U2AF1 S34F
gene expression). These findings may uncover a potential mechanism
for CCNG1-related growth promotion, rather than simply
p53-mediated growth arrest (9-11).
This hypothesis of the pro-survival and pro-growth function of the
CCNG1 oncogene is further supported by the reduced incidence
of hepatic tumors in CCNG1 knockout mice upon exposure to
hepatocarcinogens followed by partial hepatectomy (12). The decrease in tumor predisposition
associated with the loss of CCNG1 function during
embryogenesis was partially due to a consequential increase in p53
levels and p53 tumor suppressor activity (9). In combination, these findings may
provide evidence to support a unifying molecular genetic
hypothesis: That the strategic modulation of CCNG1
function(s) observed in the commanding
CCNG1/CDK2/Myc/Mdm2/p53 axis (Fig. 4) may guide the development of novel,
precise targeted anticancer agents, such as DeltaRex-G (10), as well as for combinatorial
approaches.
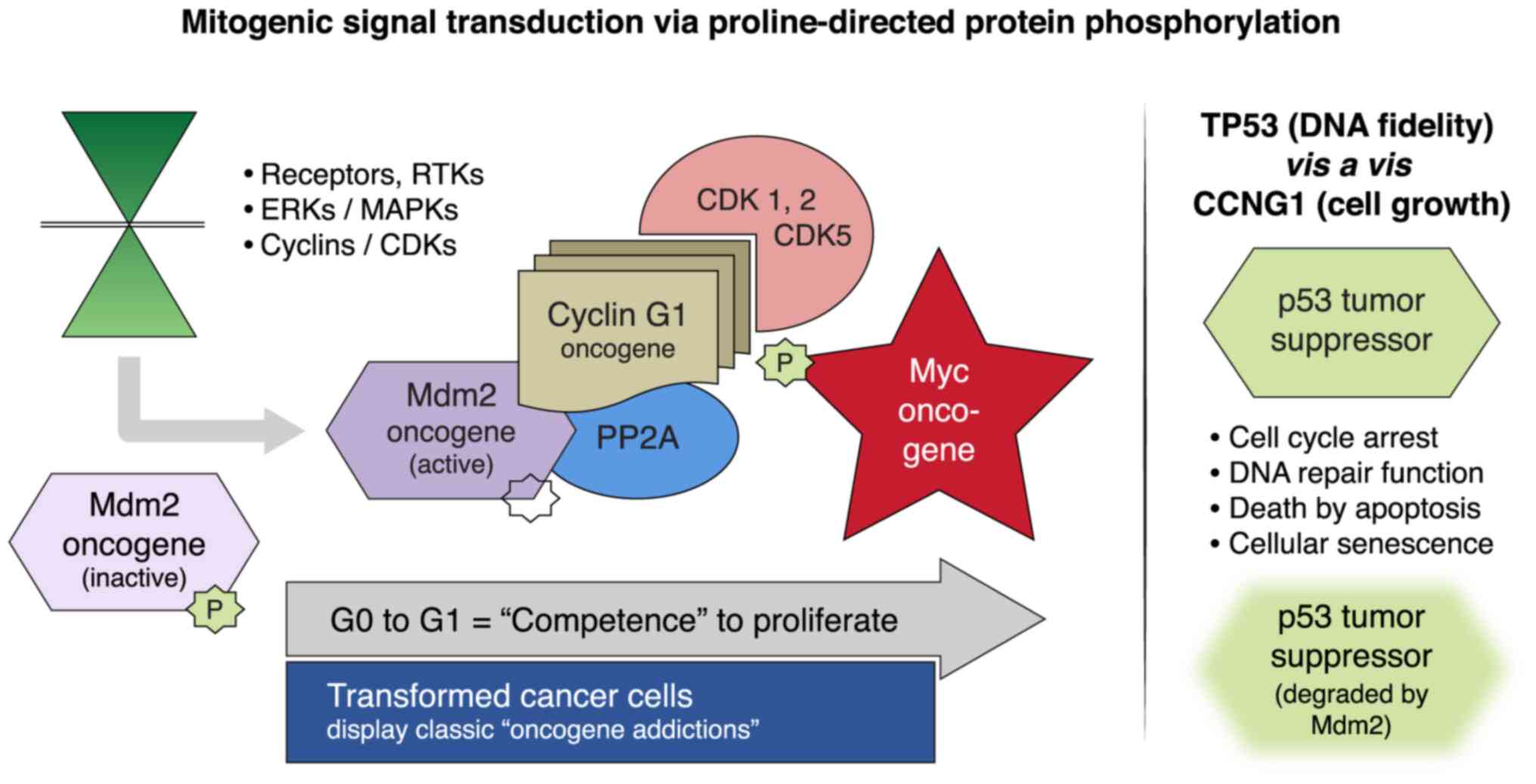 | Figure 4Mitogenic signaling pathways and the
human CCNG1 gene. Left panel, RTKs, MAPKs/ERKs and CDK
complexes control the progressive phases of the cell division
cycle. CCNG1 physically binds to the PP2A to activate a key
regulatory oncoprotein, Mdm2. The Mdm2 oncoprotein forms a physical
complex with the p53 tumor suppressor, thus inactivating its tumor
suppressor function, while also acting as a specific E3 ubiquitin
ligase responsible for the ubiquitination and degradation of the
p53 tumor suppressor protein. This dephosphorylation event is
CCNG1-dependent. CCNG1 also activates CDK5 and CDK1/2 to
target/activate the c-Myc oncoprotein. Right panel, TP53 tumor
suppressor functions are presented as opposing to the CCNG1
growth-promoting function. CCNG1, cyclin G1; RTK, receptor
tyrosine kinase; MAPKs, mitogen-activated protein kinases; ERKs,
extracellular-signal-regulated kinases; CDK, cyclin-dependent
kinase; PP2A, serine/threonine protein phosphatase subunit
designated 2A; Mdm2, mouse double minute 2 homolog; TP53, tumor
protein p53. |
Consistent with this hypothesis, MiaPaca-2 cell
lines (p.R248W; American Tissue Culture Collection) and human
xenograft murine models of pancreatic cancer expressing TP53
hot-spot mutations continue to demonstrate significant sensitivity
to CCNG1 inhibitor treatment (13,14).
High-level transduction efficiency and cytocidal activity of
DeltaRex-G vector have been reported in MiaPaca-2 cells in
vitro (14). Furthermore, the
systemic delivery of DeltaRex-G was found to inhibit the growth of
liver metastasis in vivo in a nude mouse model of p53
mutated pancreatic cancer, likely through apoptosis-mediated
pathways (14). Finally,
intravenous infusions of DeltaRex-G inhibited tumor growth in
vivo in a subcutaneous human xenograft model of pancreatic
cancer expressing TP53 hot-spot mutations (15).
In conclusion, DeltaRex-G seeks out tumors, inhibits
tumor growth and eradicates metastatic tumors and, plausibly,
cancer stem cells, by precisely blocking the proliferative
competence of cancer cells with CCNG1 oncogene-targeted
therapy for a prolonged period of time. An interesting emerging
concept is that patients with advanced oncogene-addicted tumors,
even those harboring TP53 mutation/loss, may still respond
favorably to DeltaRex-G gene-targeted therapy, while the cytocidal
CCNG1 inhibitor expressed by DeltaRex-G is itself lethal in
the presence or absence of a functional p53 gene, and the
inhibition of CCNG1 by complementary molecular genetic
approaches may indirectly (through Mdm2) restore the
tumor-suppressive function of p53, highlighting DeltaRex-G as an
optimal targeted therapy for pancreatic adenocarcinoma with a
prevalence of TP53 mutations (13).
Studies are planned to determine whether oncogenic drivers along
the CCNG1 pathway (5) could
be exploited to achieve effective therapies for pancreatic
adenocarcinoma, sarcoma and other solid tumors, since DeltaRex-G
has achieved long-term survival (>12 years) in a number of
patients with chemotherapy-resistant hard-to-treat stage 4 solid as
well as hematological malignancies (Table I).
 | Table IDetails of US-based clinical trials
using DeltaRex-G as monotherapy for chemotherapy resistant solid
malignancies. |
Table I
Details of US-based clinical trials
using DeltaRex-G as monotherapy for chemotherapy resistant solid
malignancies.
| First author,
Year | Clinical trial NCT
no. | Phase | Dose level | Clinical Site:
Principal Investigator/s | Type of cancer | Number of
patients | Overall survival | (Refs.) |
|---|
| Galanis et al,
2008 | NCT00121745 | 1 | -3 to -1 | Rochester MN: E.
Galanis | Pancreatic
adenocarcinoma, gemcitabine-resistant | 12 | 1-year OS: 0% | (16) |
| Chawla et al,
2019 | NCT00504998 | 1/2 | 1 to 3 | Santa Monica CA: SP
Chawla Manhattan NY: HW Bruckner Durham NC: MA Morse | Pancreatic
adenocarcinoma, gemcitabine-resistant | 20 | 1-year OS: 28.6%
1.5-year OS: 21.4% 1 alive in sustained remission, >12
years | (4) |
| Chawla et al,
2009 and 2016 | NCT00505713 | 1/2 | 1 to 4 | Santa Monica CA: SP
Chawla, PI | Bone and soft tissue
sarcoma, chemotherapy-resistant | 36 | 1-year OS: 38.5%
2-year OS: 31% 2 alive, with no active disease, >12 years | (17,18) |
| Bruckner et
al, 2019 | NCT00505271 | 1/2 | 1 to 4 | Santa Monica CA: SP
Chawla, PI Manhattan NY: HW Bruckner, PI | Breast cancer,
chemotherapy-resistant | 20 | 1-year OS: 60% 1
alive, >12 years | (19) |
| Chawla et al,
2009 | NCT00572130 | 2 | 1 to 2 | Santa Monica CA: SP
Chawla, PI | Osteosarcoma,
chemotherapy-resistant | 22 | 1-year OS: 27.3%
2-year OS: 22.7% 1 alive in sustained remission, >12 years | (17) |
Acknowledgements
The authors would like to thank Heather Gordon,
Director of Operations at Aveni Foundation (Santa Monica, CA, USA)
for the graphic illustrations.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAM, SPC and HWB were the principal investigators of
the clinical trial, conducted the study and evaluated the patients'
tumor responses, survival and safety, wrote parts of the
manuscript, as well as reviewed and edited the final manuscript.
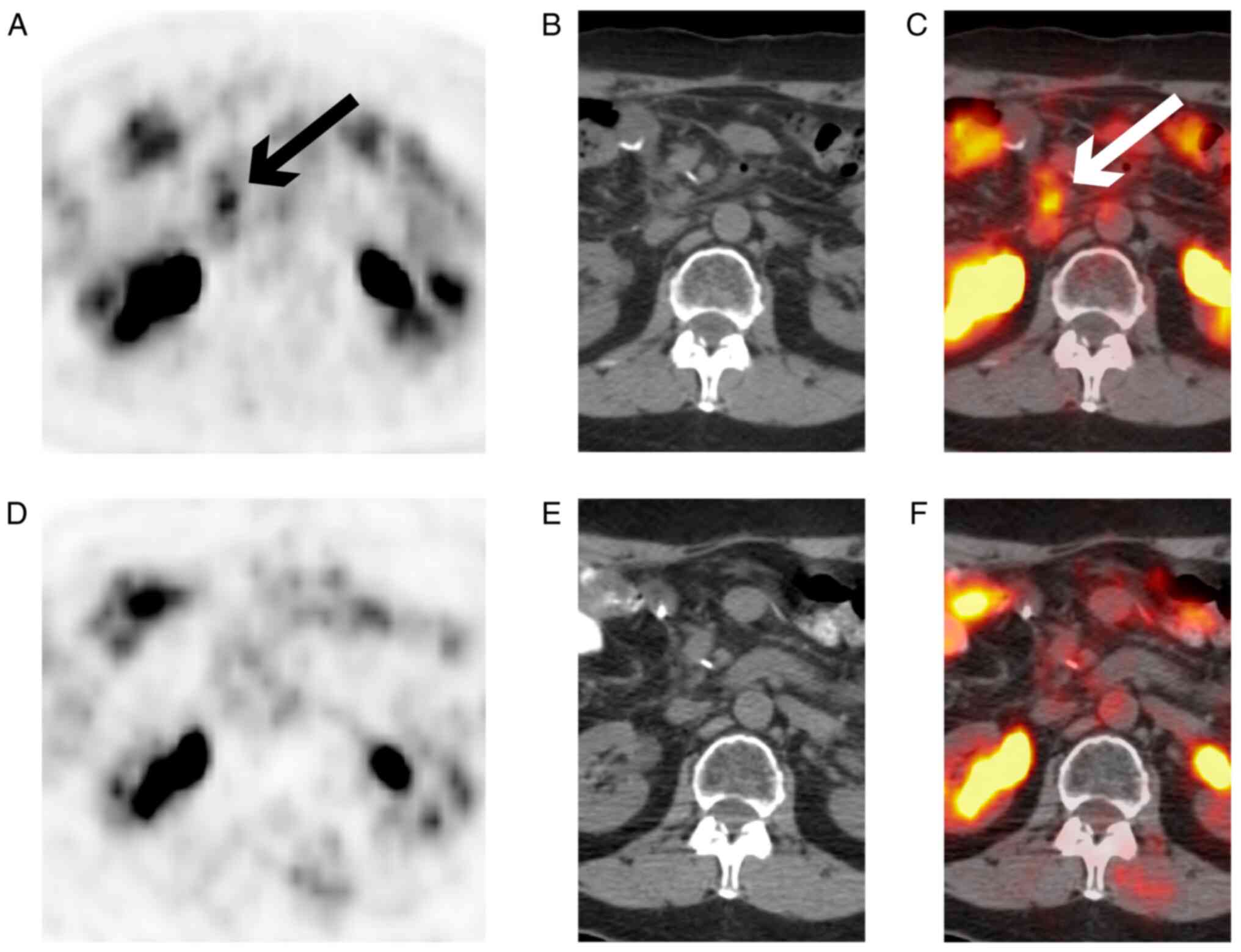
MAM and SPC assessed the authenticity of all the raw data. TZW
evaluated and provided the PET CT images, and reviewed and edited
the manuscript. EMG and FLH designed the clinical protocol and
informed consent, submitted the Investigational New Drug
application to the US Food and Drug Administration, wrote parts of
the manuscript, reviewed the published literature, oversaw the
clinical trial, as well as reviewed and edited the final
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The clinical protocol was approved by the US Food
and Drug Administration, the Western Institutional Review Board and
the Institutional Biosafety Committee for the Cancer Center of
Southern California and Bruckner Oncology, and by the Institutional
Review Board and Institutional Biosafety Committee of Duke
University Medical Center. Written informed consent was obtained
from each patient prior to DeltaRex-G treatment.
Patient consent for publication
The patient signed a written informed consent form
to use the data provided in this manuscript.
Competing interests
MAM, SPC, TZW and HWB have no competing interests.
EMG and FLH are co-inventors of DeltaRex-G, including its targeted
gene delivery system, which was originally developed at the
University of Southern California Keck School of Medicine (Los
Angeles, CA, USA) and are co-founders of Delta Next-Gene, LLC
(Santa Monica, CA, USA). Patent applications are being prosecuted
by Delta Next-Gene, LLC. EMG is the founder and president of the
Aveni Foundation, an IRS approved 501(c)(3) public charity (Seattle, WA, USA).
References
|
1
|
American Cancer Society: Survival Rates
for Pancreatic Cancer. American Cancer Society. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html.
Acessed February 12, 2021.
|
|
2
|
Malone ER, Oliva M, Sabatini PJB, Stockley
TL and Siu LL: Molecular profiling for precision cancer therapies.
Genome Med. 12(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
G, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus Gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chawla SP, Bruckner H, Morse MA, Assudani
N, Hall FL and Gordon EM: A phase I/II clinical study on the
safety, efficacy, and therapeutic potential of intravenous
DeltaRex-G: A tumor-targeted retrovector encoding a
dominant-negative cyclin G1 inhibitor for advanced pancreatic
cancer. Mol Ther Oncol. 12:56–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al-Shihabi A, Chawla SP, Hall FL and
Gordon EM: Exploiting oncogenic drivers along the CCNG1 pathway for
cancer therapy and gene therapy. Mol Ther Oncolytics. 11:122–126.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Esfahani MS, Lee LJ, Jeon YJ, Flynn RA,
Stehr H, Hui AB, Ishisoko N, Kildebeck E, Newman AM, Bratman SV, et
al: Functional significance of U2AF1 S34F mutations in lung
adenocarcinomas. Nat Commun. 10(5712)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cicenas J, Kvederaviciute K, Meskinyte I,
Meskinyte-Kausiliene E, Skeberdyte A and Cicenas J: KRAS, TP53,
CDKN2A, SMAD4, BRCA1, and BRCA2 mutations in pancreatic cancer.
Cancers (Basel). 9(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kimura SH and Nojima H: Cyclin G1
associates with MDM2 and regulates accumulation and degradation of
p53 protein. Genes Cells. 7:869–880. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Giono LE and Manfredim JJ: The p53 tumor
suppressor participates in multiple cell cycle checkpoints. J Cell
Physiol. 209:13–20. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gordon EM, Ravicz JR, Liu S, Chawla SP and
Hall FL: Cell cycle checkpoint control: The cyclin G1/Mdm2/p53 axis
emerges as a strategic target for broad-spectrum cancer gene
therapy-a review of molecular mechanisms for oncologists. Mol Clin
Oncol. 9:115–134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen X: Cyclin G: A regulator of the
p53-Mdm2 network. Dev Cell. 2:518–519. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jensen MR, Factor VM, Fantozzi A, Helin K,
Huh CG and Thorgeirsson SS: Reduced hepatic tumor incidence in
Cyclin G1-deficient mice. Hepatology. 37:862–870. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Olivier M, Hollstein M and Hainau P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2(a001008)2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gordon EM, Liu PX, Chen ZH, Liu L, Whitley
MD, Gee C, Groshen S, Hinton DR, Beart RW and Hall FL: Inhibition
of metastatic tumor growth in nude mice by portal vein infusions of
matrix-targeted retroviral vectors bearing a cytocidal cyclin G1
construct. Cancer Res. 60:3343–3347. 2000.PubMed/NCBI
|
|
15
|
Gordon EM, Chen ZH, Liu L, Whitley M, Liu
L, Wei D, Groshen S, Hinton DR, Anderson WF, Beart RW Jr and Hall
FL: Systemic administration of a matrix-targeted retroviral vector
is efficacious for cancer gene therapy in mice. Hum Gene Ther.
12:193–204. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Galanis E, Carlson SK, Foster NR, Lowe V,
Quevedo F, McWilliams RR, Grothey A, Jatoi A, Alberts SR and Rubin
J: Phase I trial of a pathotropic retroviral vector expressing a
cytocidal cyclin G1 construct (Rexin-G) in patients with advanced
pancreatic cancer. Mol Ther. 16:979–984. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chawla SP, Chua VS, Fernandez L, Quon D,
Saralou A, Blackwelder WC, Hall FL and Gordon EM: Phase I/II and
phase II studies of targeted gene delivery in vivo: Intravenous
Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol
Ther. 17:1651–1657. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chawla SP, Chawla NS, Quon D, Chua-Alcala
V, Blackwelder WC, Hall FL and Gordon EM: An advanced phase 1/2
study using an XC-targeted gene therapy vector for chemotherapy
resistant sarcoma. Sarcoma Res Int. 3(1024)2016.
|
|
19
|
Bruckner H, Chawla SP, Liu S, Assudani N,
Hall FL and Gordon EM: Phase I-II study using Rexin-G, a
tumor-targeted retrovector encoding a cyclin G1 inhibitor for
metastatic carcinoma of breast: A ten-year follow-up. Presented at
the 2019 American Society of Gene and Cell Therapy (ASGCT) Annual
Meeting. ASGCT, Washington, DC, Abstract 273, 2019.
|