Introduction
Tumors of the head and neck region are a
heterogeneous group of neoplasms and are among the most common
cancer worldwide (1). With a
proportion of 90%, squamous cell carcinoma (SCC) is the most common
histological type (2,3). The main risk factors for the
development of oral squamous cell carcinoma (OSCC) are chronic
alcohol and/or nicotine abuse with synergistic potentiation
(4). Surgical therapy for extensive
OSCC is associated with a functional loss in stomatology, aesthetic
restrictions, and a deterioration in a patient's health-related
quality of life (HRQOL). HRQOL evaluation is a multidimensional
concept comprising positive and negative components (5-7).
Besides physical, psychological, and social dimensions, marital
status and spiritual well-being are also important influencing
factors (8,9). Various questionnaires have been
developed to assess HRQOL in different dimensions (10-13).
Among them, the University of Washington Quality of Life
Questionnaire version 4 (UW-QoL V4) is a widely used tool for HRQOL
evaluation in patients with OSCC (14), and is routinely used in many centers
worldwide during postoperative follow-up care.
Cancer diagnosis and treatment are traumatic life
events that can trigger post-traumatic stress disorder (PTSD)
(15,16), but can also lead to subjectively
positive effects, such as post-traumatic growth (PTG) (17,18).
PTG does not refer to the traumatic event itself, but the
subjectively perceived positive change in the way in which the
traumatic experience is dealt with (17). Many sociodemographic factors, such
as age, sex, social support, religion, educational level, income,
resilience, HRQOL, and disease severity, have been discussed to
affect PTG. PTG can be assessed using the PTG-inventory (PTGI)
questionnaire developed by R. Tedeschi and L. Calhoun (19).
Both HRQOL and PTG are important influencing factors
during post-therapy cancer follow-up care. For this reason, a
better understanding of the relationships between HRQOL, PTG, and
influencing clinical factors is important in specifically
addressing individual patient needs and improving quality of
treatment. To the authors' knowledge, this study is the first to
report a combined HRQOL and PTG evaluation in patients with
OSCC.
Materials and methods
Patients and data acquisition
Coherent clinical data (seamless data acquisition
over the entire observation period) of 15 patients with OSCC mainly
treated surgically between January 2011 and June 2012 were
retrospectively analyzed. Data included patient characteristics,
such as age, sex, marital status, and denominational affiliation;
pathological characteristics, such as localization of the primary
tumor and TNM classification; and therapy characteristics, such as
surgical reconstruction modality and adjuvant therapy. Clinical
data of patients can be found in Table
I. HRQOL and PTG were recorded using the UW-QoL V4 and PTGI
questionnaires (14,19,20).
UW-QoL V4 was completed one day preoperatively and during
postoperative tumor follow-ups (after 1/2, 1, 3, 6, 9, and 12
months); and it consisted of 12 questions concerning the domains of
pain, appearance, activity, recreation, swallowing, chewing,
speech, shoulder function, taste, saliva, mood, and anxiety. PTGI
was completed after 1, 6, and 12 months postoperatively; and it
consisted of 21 questions with six possible answers to evaluate the
five subscales [‘relating to others’ (RO), ‘new
possibilities’ (NP), ‘personal strength’ (PS),
‘appreciation of life’ (AOL), and ‘spiritual change’
(SC)]. Of the 30 patients with OSCC who initially agreed to
participate in the trial, 15 completed all questionnaires (n=15)
over the one-year study period and were included in the final
statistical evaluation (attrition rate, n=15).
 | Table IPatient clinical characteristics. |
Table I
Patient clinical characteristics.
| Sex | Age | Profession | Religion | Marital status | Tumor
localization | pT | pN | pM | Reconstruction |
|---|
| Male | 48 | Gardener | None | Not married | Anterior mouth
floor | 4 | 2 | 0 | Distant flap |
| Female | 48 |
Psychotherapist | Evangelic | Divorced | Lateral upper
alveolar process | 2 | 0 | 0 | Local |
| Male | 67 | Baker | Catholic | Not married | Lateral mouth
floor | 1 | 0 | 0 | Local |
| Male | 47 | Warehouse
worker | Muslim | Not married | Cheek | 1 | 0 | 0 | Distant flap |
| Female | 94 | Secretary | Evangelic | Widowed | Lateral tongue | Cis | 0 | 0 | Local |
| Male | 48 | Educator | Catholic | Not married | Palate | 1 | 0 | 0 | Local |
| Male | 67 | Manager | Evangelic | Married | Anterior mouth
floor | 2 | 0 | 0 | Distant flap |
| Male | 63 | Caregiver | Catholic | Married | Lateral lower
alveolar process | 4 | 0 | 0 | Distant flap |
| Male | 64 | Caregiver | Catholic | Married | Lateral lower
alveolar process | 4 | 0 | 0 | Distant flap |
| Female | 46 | Banker | None | Married | Lateral upper
alveolar process | 4 | 0 | 0 | Distant flap |
| Male | 56 | Bricklayer | None | Not married | Lateral tongue | 2 | 0 | 0 | Local |
| Male | 56 | Gardener | None | Not married | Cheek | 2 | 0 | 0 | Distant flap |
| Male | 54 | Machinist | Evangelic | Married | Anterior mouth
floor | 1 | 0 | 0 | Distant flap |
| Female | 67 | Secretary | Catholic | Married | Lower lip | 1 | 0 | 0 | Local |
| Female | 76 | Artist | None | Married | Lateral tongue | 1 | 0 | 0 | Local |
Statistical evaluation
All HRQOL and PTG subscales were analyzed using a
Friedman test. Significant test scores were further examined with a
pairwise comparison using the Bonferroni-Dunn post hoc test. To
investigate all possible influencing factors (e.g., sex, age,
religious denomination, and marital status) on PTG, further
correlation tests were performed. Mann-Whitney U tests for
independent samples, and Wilcoxon tests for paired samples, were
used to compare two groups. In addition, a descriptive statistical
evaluation of pre- and postoperative HRQOL, as well as presentation
of the different influencing variables on HRQOL, was performed. A
Spearman's correlation analysis was performed to describe the
relationship of HRQOL and PTG. All analyses were performed using
SAS 9.4-(SAS Institute, Inc), Statistica 10.0 (Hamburg, Germany)
and SPSS 24 (IBM; SPSS, Inc.) software. All tests resulting in a
P-value <0.05 were considered statistically significant.
Results
Health-related quality of life
(HRQOL)
HRQOL evaluation revealed that both physical and
socio-emotional HRQOL decreased in a pre- to-postoperative manner
in all patients (Fig. 1A and
B).
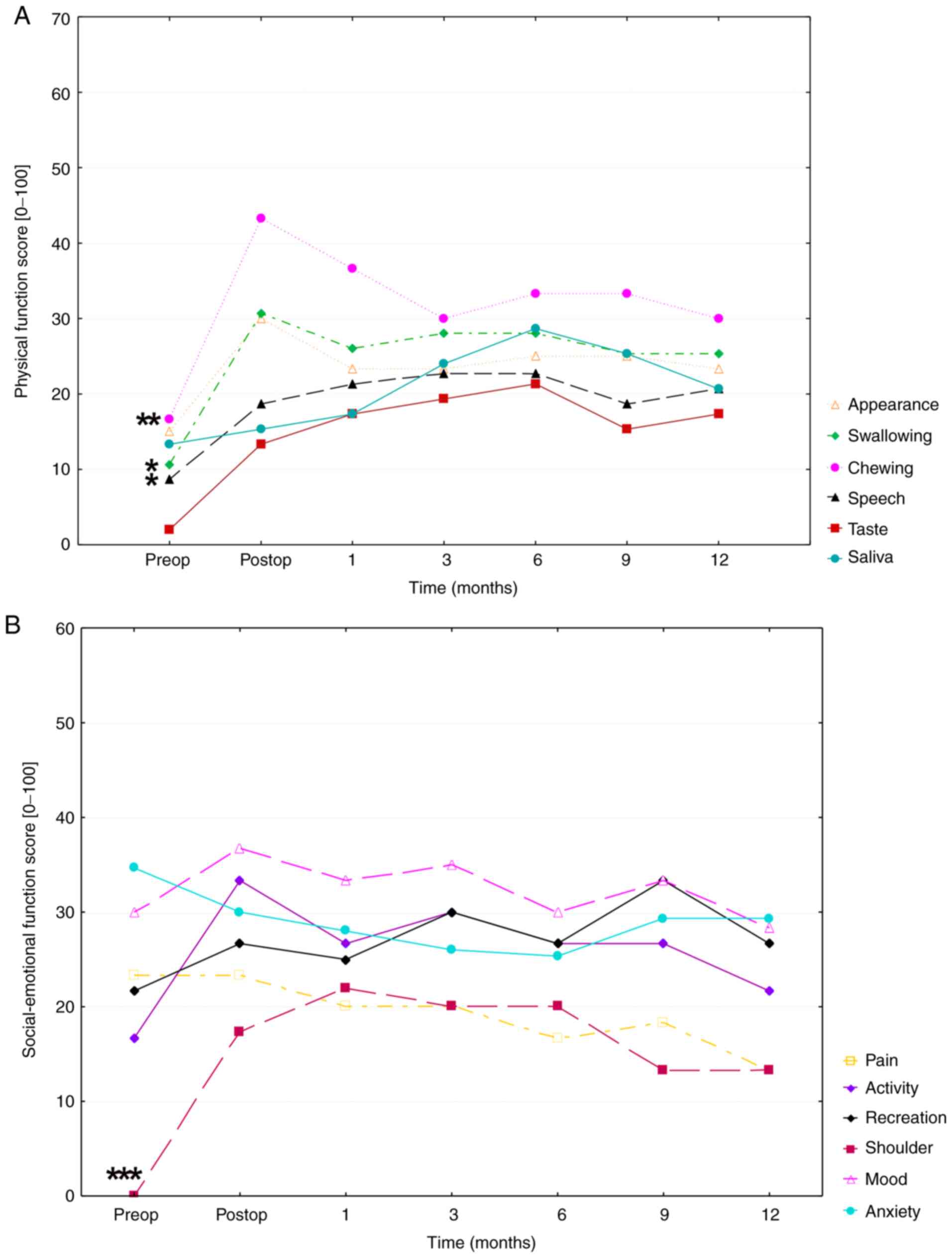 | Figure 1(A) Physical function score over time
of the UW-QOL v4 questionnaire. The time of the mean scores of
appearance, swallowing, chewing, speech, taste and saliva (physical
subscales of the UW-QOL v4 questionnaire) are presented over a
period from pre-surgery to 12 months post-surgery. High scores in
the figure indicate poor HRQOL, whereas low scores indicate good
HRQOL. (B) Social-emotional function score over time of the mean
scores of pain, activity, shoulder, mood, anxiety and recreation
(social-emotional subscales of the UW-QOL v4 questionnaire) over a
period from pre-surgery to 12 months post-surgery. A Friedman test
with Bonferroni-Dunn post hoc analysis was performed to compare
differences between preoperative and mean postoperative physical
and social-emotional function score values, respectively.
Significances are indicated as asterisks (*P<0.05,
**P<0.01 and ***P<0.001). HRQOL,
health-related quality of life. UW-QOL, University of Washington
Quality of Life Version 4. |
The mean UW-QoL v4 value in the physical function
increased from 11 points preoperatively to 25 points
postoperatively, indicating a decrease in physical HRQOL. In the
area of physical function score, swallowing (MV, 24.9±25.6 SD;
P=0.025), chewing (MV, 31.926.9 SD; P=0.002) and speech (MV,
19.1±20.7 SD; P=0.016) were the main factors responsible for
postoperative deterioration in HRQOL. The mean value of the
socio-emotional function increased from the preoperative (21
points) to postoperative (28 points) stage. Socio-emotional HRQOL
was strongly influenced by patients' shoulder function (MV,
15.1±23.0; P<0.0001), and mood (mean, 32.4±25.2) which showed
high mean values over the entire study period and was only subject
to a few fluctuations. This result, however, was not statistically
significant (P=0.88).
Statistical analysis of the preoperative UW-QoL v4
data with the one-year postoperative data indicated a significant
decrease in the physical (P=0.011; 11 points preop, 23 points after
1 year) but not in the socio-emotional HRQOL (P=0.972; 21 points
preop, 22 points after 1 year) over the one-year study period.
Correlation analysis results between various
clinical baseline characteristics and the physical and
socio-emotional HRQOL revealed that sex had a nearly significant
effect on HRQOL (P=0.058). In general, men indicated a poorer HRQOL
than women. On average, single patients reported a slightly poorer
overall HRQOL than married patients. However, this difference was
not significant (P=0.113). Furthermore, no significant effect of
denominational affiliation (P=0.908) and patient's age (P=0.261) on
HRQOL could be observed.
On average, patients with tumors classified as
T1/T2, free microvascular flap reconstruction, and no additional
radiotherapy (RT) were characterized by the poorest HRQOL scores.
Patients (T1/T2) who received local defect reconstruction reported
a better HRQOL. In T1/T2 carcinomas, socio-emotional HRQOL was
rated as worse than physical HRQOL, regardless of the type of
therapy used. In patients without free microvascular flap
reconstruction and RT, no major difference could be observed
between physical and socio-emotional HRQOL. In general, patients
with T3/T4 carcinomas rated their physical HRQOL worse than those
of other groups.
Post-traumatic growth (PTG)
For PTG evaluation, three postoperative
questionnaires (1, 6, and 12 months postoperative) were available
for each patient. Mean PTG values per question (MV) for all
patients are shown in Fig. 2A. The
five subscales: ‘Relating to others’ (RO), ‘new
possibilities’ (NP), ‘personal strength’ (PS),
‘appreciation of life’ (AOL), and ‘spiritual change’
(SC), composed of different number of questions, were separately
evaluated. The mean values were used for comparison. The mean
overall PTG score was 72.2±35.7 (95% CI, 69.9-74.5).
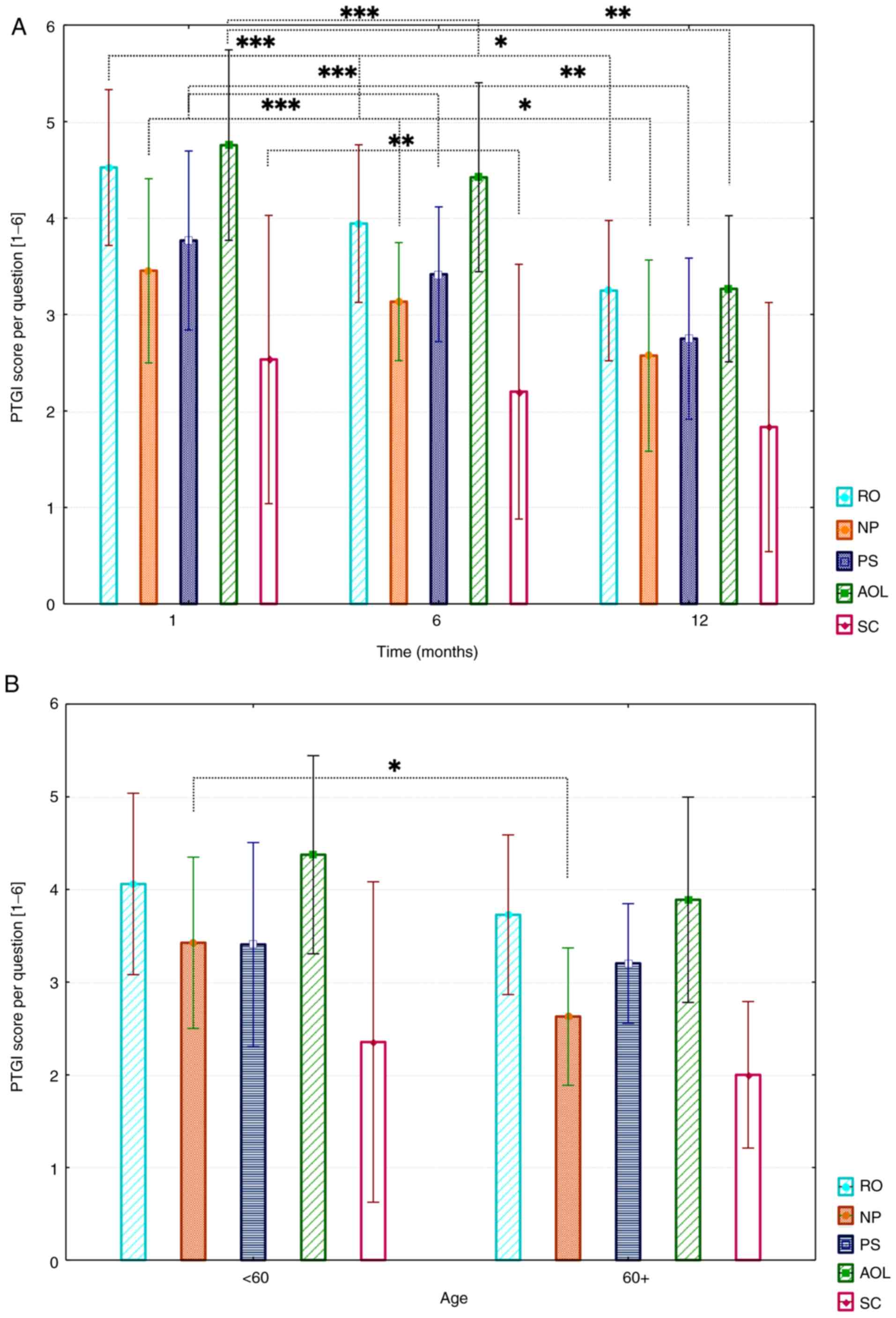 | Figure 2(A) Results of the PTGI questionnaire
divided by subscales. MVs and SDs are demonstrated per question. A
Friedman test with Bonferroni-Dunn post-hoc analysis was performed.
*P<0.05, **P<0.01 and
***P<0.001. (B) Influence of age on overall PTG (1-,
6- and 12-months), MVs and SDs are presented. A Mann-Whitney U test
was performed to determine statistical significance.
*P<0.05, **P<0.01 and
***P<0.001 as indicated. PTGI, Posttraumatic Growth
Inventory; MV, mean values; RO, relating to others; NP, new
possibilities; PS, personal strength; AOL, appreciation of life;
SC, spiritual change. |
Results show that patients most strongly felt PTG
shortly after surgery (1 month postoperatively). Mean values of all
subscales revealed a PTG decrease over the study period, which was
also observed uniformly for all subscales. The biggest changes were
found in ‘appreciation of life’ (MV, 4.1±1.8; 95% CI,
3.6-4.6) and ‘relating to others’ (MV, 3.9±1.5; 95% CI,
3.5-4.3). ‘Personal strength’ (MV, 3.3±1.6; 95% CI, 2.8-3.8)
and ‘new possibilities’ (MV, 3.0±1.6; 95% CI, 2.5-3.5)
values slightly decreased in comparison. The lowest values were
found in the ‘spiritual change’ subscale (MV,
2.2±2.7; 95% CI, 1.4-3.0). ‘Appreciation of life’ showed the
highest decrease over all three timepoints (1 to 12 months
postoperatively). Initially, a moderate decrease (MV, 4.8 to 4.4)
from 1 to 6 months postoperatively was found, whereas a strong
decrease from 6 months to 12 months postoperatively (MV 4.4 to MV
3.2) was observed. Additionally, for ‘new possibilities’ and
‘personal strength,’ such decrease was, however, found with
initial lower values (NP MV 3.4 to MV 2.5; PS MV 3.8 to MV.8).
‘Relating to others’ showed a strong decrease from 1 month
to 6 months postoperatively (RO MV 4.5 to MV 3.9). The global
analysis showed a significant trend for all subscales (in
particular: RO, P<0.001; NP, P<0.001; AOL, P<0.001; SC,
P=0.001, and PS, P<0.001). Pairwise comparisons revealed
significant differences between PTG subscales. For RO, between 1
month and 1 year (P<0.0001) and 6 months and 1 year (P=0.024).
For NP, between 1 month and 1 year (P<0.0001) and 6 months and 1
year (P=0.019). For PS, between 1 month and 1 year (P<0.0001)
and 6 months and 1 year (P=0.003). Also, significant differences
could be observed between AOL subscale after 1 month and 1 year
(P<0.0001) and 6 months and 1 year (P=0.002), and SC between 1
month and 1 year (P=0.006).
Analysis revealed slightly higher mean values in all
five subscales for patients younger than 60 years of age (MV, 3.5)
than those over 60 years (MV, 3.0). However, just for the ‘new
possibilities’ subscale, the difference was statistically
significant (P=0.040). A graphical representation is shown in
Fig. 2B.
No significant correlation between PTG and sex or
PTG and marital status has been found. The P-value from the
‘relating to others’ scale (P=0.04) indicates a significant
correlation between the intensification of ‘relating to others’ and
‘spiritual change.’
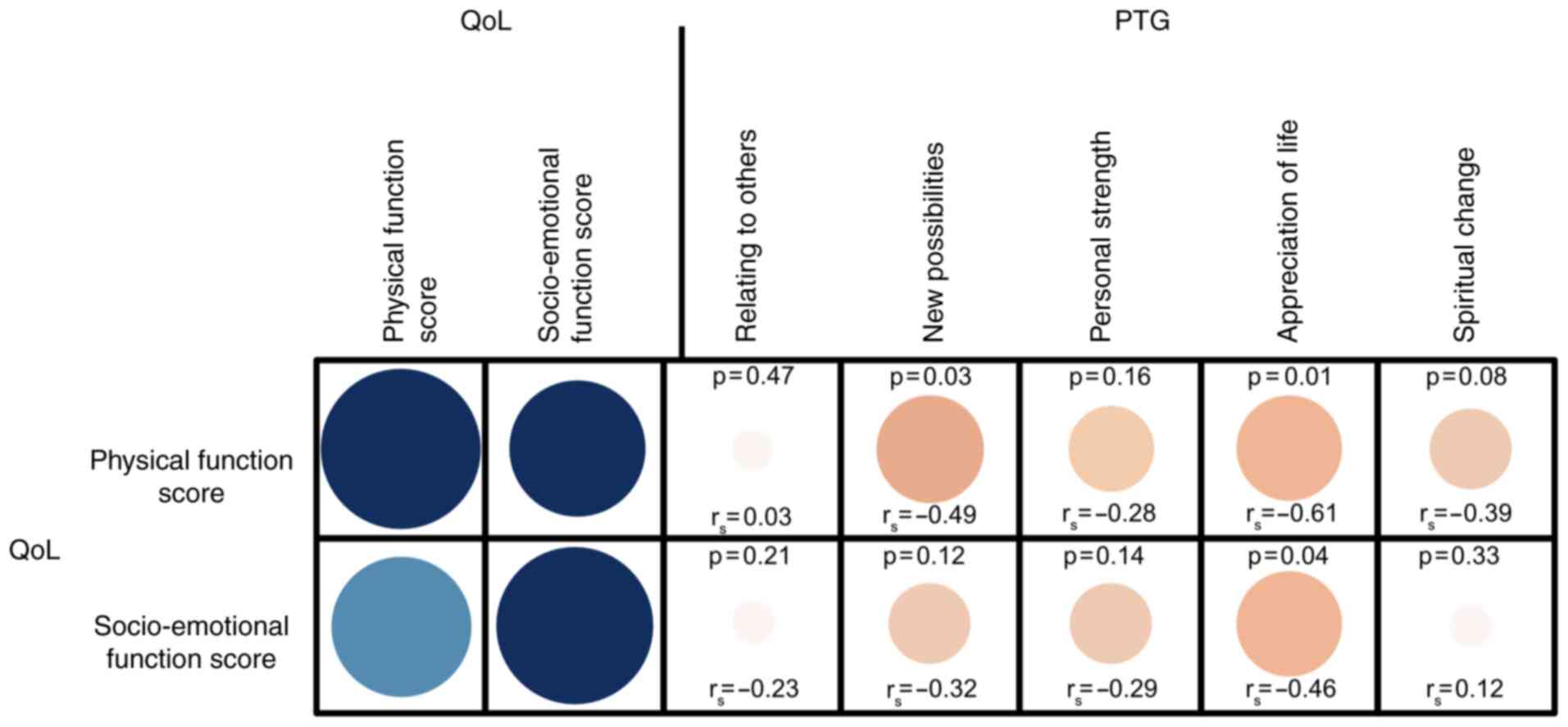
Correlation between HRQOL and PTG
Statistical analyses results revealed a significant
negative correlation between physical and socio-emotional HRQOL and
the PTG subscales, ‘new possibilities’, and ‘appreciation
of life’ (all P-values <0.05). All other correlation tests
were not significant, as depicted in Fig. 3.
Discussion
Head and neck cancers continue to be characterized
by poor survival outcome and reduced HRQOL of patients (21,22).
However, life-threatening diseases, such as cancer, are possible
traumas that can trigger PTG (17).
Many factors, such as hope, optimism, carer, and coping, may
influence HRQOL and PTG. In this study, we characterize the
relationship between influencing clinical factors, HRQOL, and PTG
after extensive surgical OSCC treatment, and focus on data
analyzable with available clinical standard questionnaires.
As already described in literature (23), the present analysis results showed a
reduced physical- and socio-emotional HRQOL in all patients with
OSCC. Comparable results, also achieved with the UW-QoL v4
questionnaire, were described one year postoperatively in patients
with oropharyngeal carcinoma by Biazevic et al (24).
Chewing, swallowing, and speech were found to be the
significant factors responsible for reduced postoperative physical
HRQOL in this study. Most likely, these results are mainly due to
the extensive intraoral OSCC resection and are comparable to the
evaluation of Villaret et al (25).
The comparison of patients with tumors classified as
T1/T2 showed a better HRQOL in those with local plastic defect
reconstruction than in patients who received free microvascular
flap reconstruction. In this context, it is important to note that
there are important differences between the extents of tumor
infiltration. Depending on the location, different surgical and
reconstructive strategies are necessary. Interestingly, more than
50% of patients rated their health-related and overall HRQOL as
‘good’ or ‘satisfying,’ suggesting that despite the postoperatively
reduced physical and socio-emotional function scores, the overall
assessment was multifactorial influenced.
In this study, no sociodemographic factor, such as
age, religion, and marital status, revealed a significant effect on
HRQOL. This could be due to the small number of cases. Due to the
burden of this dramatic life event, many patients who were
preoperatively included in this trial showed poor postoperative
compliance and were no longer willing to participate in the
evaluation (drop-out rate, n=15). However, the particular value of
the recent study lies in the complete dataset with no missing
values over the entire study period. This is an important
difference from many other studies where data are incomplete. Men
rated their HRQOL worse than women, which, however, was on
borderline significance (P=0.058). The influence of sex on
postoperative HRQOL is debatable. While Rogers et al
(26) reported a better HRQOL in
women, other studies did not demonstrate a significant difference
between the sexes or report contrarily (23,27).
Social support facilitates the coping process and
reduces emotional discomfort. The ability to trust others and the
feeling of support, understanding, and acceptance are considered
important prerequisites for the emergence of PTG (19). The opportunity to express thoughts
and fears gives patients the opportunity to complete mental
processing (28,29).
In this study, all patients with OSCC reported a
postoperative PTG. PTG was most strongly felt immediately after
treatment (one month) and decreased over the one-year study period.
The data in the literature on the development of PTG in the
follow-up interval are not definite (30).
Thus far, the PTG experience is unclear as to
whether it is real or just a self-calming defensive illusion in the
course of mental processing (31-33).
The question of the objectivity of the subjectively perceived PTG
has already been a focus of research and is still being discussed
controversially (34,35). In this context, the objectively
collected PTGI values in this study should be noted to still
indicate a positive perception even after one year. These results
are consistent with those of the longitudinal study by Sharp et
al (36). Additionally, the
authors used the PTGI questionnaire by Tedeschi and Calhoun for PTG
evaluation (17). ‘Appreciation of
life’ had the highest mean value in this study, followed by the
‘relating to others’ and ‘personal strength’ subscales. In contrast
to our results, however, ‘spiritual change’ occupied a stronger
position among patients. The feeling of having ‘new possibilities’
was least felt by patients with head and neck cancers.
The findings of the study revealed slightly higher
mean PTG values in all five subscales for younger patients than
older patients. However, for the ‘new possibilities’ scale,
the difference was statistically significant (P=0.040). Sharp et
al (36) and others (37) reported similar results and assumed
that older patients had a different perception due to earlier life
experiences.
Although numerous studies have highlighted the
effect of sociodemographic factors on PTG (30,37),
no significant correlation between PTG, sex, marital status, or
religious affiliation could be observed in this study. Despite the
lack of significance, women tended to show higher values in all
five PTG subscales, as also described by others (35,36,38,39).
Although patients who identified themselves as non-denominational
reported higher average values in three of the five subscales,
spirituality did not show any significant effect on PTG in this
study. In contrast, other studies reported that people with strong
beliefs had less difficulty in coping with trauma (39,40).
A higher level of psychological distress is
generally assumed to correlate with a higher level of PTG (41). We found a significant negative
correlation between HRQOL and PTG in patients with OSCC. Patients
reporting a poor HRQOL also reported poorer PTG values. This is
corroborated by other studies (34,36,42).
However, so far, no consistent relationship between HRQOL and PTG
has been found (37).
After extensive tumor surgery, postoperative HRQOL
and PTG are the most important factors for patients dealing with
this traumatic life event. Many individually different factors can
affect HRQOL and PTG, which can be assessed with available
standardized questionnaires. Due to the suspicion of coherence in
patients with OSCC, these should be used in clinical practice for
postoperative combined screening to address individual patient
needs and improve cancer follow-up care.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RMG and JJL conceived the current study. GH, JJL and
BW collected all data. RMG and PB evaluated and interpreted all
data and confirm the authenticity of all the raw data. GH, RMG and
PB were major contributors in writing the manuscript. BS, PK made
substantial contributions to the analysis and interpretation of
data. HS made substantial contributions to the conception and
design of the study and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
ethical standards (Declaration of Helsinki) approved by the local
ethics committee of the University Medical Center Goettingen
(approval no. 27/04/06 and DOK_93_2013). Informed consent for
participation the trial was obtained from patients.
Patient consent for publication
All patients gave their consent for publication of
patient data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lo WL, Kao SY, Chi LY, Wong YK and Chang
RC: Outcomes of oral squamous cell carcinoma in Taiwan after
surgical therapy: Factors affecting survival. J Oral Maxillofac
Surg. 61:751–758. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Howaldt HP, Vorast H, Blecher JC,
Reicherts M and Kainz M: Results of the DOSAK tumor register. Mund
Kiefer Gesichtschir. 4 (Suppl 1):S216–S225. 2000.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
4
|
Figuero Ruiz E, Carretero Pelaez MA,
Cerero Lapiedra R, Esparza Gomez G and Moreno Lopez LA: Effects of
the consumption of alcohol in the oral cavity: Relationship with
oral cancer. Med Oral. 9:14–23. 2004.PubMed/NCBI(In English, Spanish).
|
|
5
|
Ferrans CE: Quality of life: Conceptual
issues. Semin Oncol Nurs. 6:248–254. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Morton RP and Izzard ME: Quality-of-life
outcomes in head and neck cancer patients. World J Surg.
27:884–889. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Calman KC: Quality of life in cancer
patients-an hypothesis. J Med Ethics. 10:124–127. 1984.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Han KT, Park EC, Kim JH, Kim SJ and Park
S: Is marital status associated with quality of life? Health Qual
Life Outcomes. 12(109)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Reis LB, Leles CR and Freire MC:
Associations of religiosity and spiritual well-being with
appearance concerns after head and neck cancer surgery. Community
Dent Oral Epidemiol: Dec 28, 2020 (Epub ahead of print). doi:
10.1111/cdoe.12615.
|
|
10
|
Rogers SN, Fisher SE and Woolgar JA: A
review of quality of life assessment in oral cancer. Int J Oral
Maxillofac Surg. 28:99–117. 1999.PubMed/NCBI
|
|
11
|
Rathod S, Livergant J, Klein J, Witterick
I and Ringash J: A systematic review of quality of life in head and
neck cancer treated with surgery with or without adjuvant
treatment. Oral Oncol. 51:888–900. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pusic A, Liu JC, Chen CM, Cano S, Davidge
K, Klassen A, Branski R, Patel S, Kraus D and Cordeiro PG: A
systematic review of patient-reported outcome measures in head and
neck cancer surgery. Otolaryngol Head Neck Surg. 136:525–535.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rogers SN, Ahad SA and Murphy AP: A
structured review and theme analysis of papers published on
‘quality of life’ in head and neck cancer: 2000-2005. Oral Oncol.
43:843–868. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rogers SN, Lowe D, Yueh B and Weymuller EA
Jr: The physical function and social-emotional function subscales
of the university of washington quality of life questionnaire. Arch
Otolaryngol Head Neck Surg. 136:352–357. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moschopoulou E, Hutchison I, Bhui K and
Korszun A: Post-traumatic stress in head and neck cancer survivors
and their partners. Support Care Cancer. 26:3003–3011.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shakespeare-Finch J and Lurie-Beck J: A
meta-analytic clarification of the relationship between
posttraumatic growth and symptoms of posttraumatic distress
disorder. J Anxiety Disord. 28:223–229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tedeschi RG and Calhoun LG: Posttraumatic
Growth: Conceptual foundations and empirical evidence. Psychol
Inquiry. 15:1–18. 2004.
|
|
18
|
Harding S, Sanipour F and Moss T:
Existence of benefit finding and posttraumatic growth in people
treated for head and neck cancer: A systematic review. PeerJ.
2(e256)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tedeschi RG and Calhoun LG: The
posttraumatic growth inventory: Measuring the positive legacy of
trauma. J Trauma Stress. 9:455–471. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Maercker A and Langner R: Persönliche
Reifung (Personal Growth) durch Belastungen und Traumata.
Diagnostica. 47:153–162. 2001.
|
|
21
|
So WK, Chan RJ, Chan DN, Hughes BG, Chair
SY, Choi KC and Chan CW: Quality-of-life among head and neck cancer
survivors at one year after treatment-a systematic review. Eur J
Cancer. 48:2391–2408. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Borggreven PA, Verdonck-de Leeuw IM,
Muller MJ, Heiligers ML, de Bree R, Aaronson NK and Leemans CR:
Quality of life and functional status in patients with cancer of
the oral cavity and oropharynx: Pretreatment values of a
prospective study. Eur Arch Otorhinolaryngol. 264:651–657.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schliephake H and Jamil MU: Prospective
evaluation of quality of life after oncologic surgery for oral
cancer. Int J Oral Maxillofac Surg. 31:427–433. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Biazevic MG, Antunes JL, Togni J, de
Andrade FP, de Carvalho MB and Wunsch-Filho V: Survival and quality
of life of patients with oral and oropharyngeal cancer at 1-year
follow-up of tumor resection. J Appl Oral Sci. 18:279–284.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Villaret AB, Cappiello J, Piazza C,
Pedruzzi B and Nicolai P: Quality of life in patients treated for
cancer of the oral cavity requiring reconstruction: A prospective
study. Acta Otorhinolaryngol Ital. 28:120–125. 2008.PubMed/NCBI
|
|
26
|
Rogers SN, Humphris G, Lowe D, Brown JS
and Vaughan ED: The impact of surgery for oral cancer on quality of
life as measured by the Medical Outcomes Short Form 36. Oral Oncol.
34:171–179. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vartanian JG, Carvalho AL, Yueh B, Priante
AV, de Melo RL, Correia LM, Köhler HF, Toyota J, Kowalski IS and
Kowalski LP: Long-term quality-of-life evaluation after head and
neck cancer treatment in a developing country. Arch Otolaryngol
Head Neck Surg. 130:1209–1213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lepore SJ, Silver RC, Wortman CB and
Wayment HA: Social constraints, intrusive thoughts, and depressive
symptoms among bereaved mothers. J Pers Soc Psychol. 70:271–282.
1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vishnevsky T, Cann A, Calhoun LG, Tedeschi
RG and Demakis GJ: Gender differences in self-reported
posttraumatic growth: A meta-analysis. Psychology Women Qrterly.
34:110–120. 2010.
|
|
30
|
Casellas-Grau A, Ochoa C and Ruini C:
Psychological and clinical correlates of posttraumatic growth in
cancer: A systematic and critical review. Psychooncology.
26:2007–2018. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Zoellner T and Maercker A: Posttraumatic
growth in clinical psychology-a critical review and introduction of
a two component model. Clin Psychol Rev. 26:626–653.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ho S, Rajandram RK, Chan N, Samman N,
McGrath C and Zwahlen RA: The roles of hope and optimism on
posttraumatic growth in oral cavity cancer patients. Oral Oncol.
47:121–124. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rajandram RK, Jenewein J, McGrath C and
Zwahlen RA: Coping processes relevant to posttraumatic growth: An
evidence-based review. Support Care Cancer. 19:583–589.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Menger F, Patterson J, O'Hara J and Sharp
L: Research priorities on post-traumatic growth: Where next for the
benefit of cancer survivors? Psychooncology. 29:1968–1970.
2020.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
McFarland C and Alvaro C: The impact of
motivation on temporal comparisons: Coping with traumatic events by
perceiving personal growth. J Pers Soc Psychol. 79:327–343.
2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sharp L, Redfearn D, Timmons A, Balfe M
and Patterson J: Posttraumatic growth in head and neck cancer
survivors: Is it possible and what are the correlates?
Psychooncology. 27:1517–1523. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Shand LK, Cowlishaw S, Brooker JE, Burney
S and Ricciardelli LA: Correlates of post-traumatic stress symptoms
and growth in cancer patients: A systematic review and
meta-analysis. Psychooncology. 24:624–634. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Ruf M, Buchi S, Moergeli H, Zwahlen RA and
Jenewein J: Positive personal changes in the aftermath of head and
neck cancer diagnosis: A qualitative study in patients and their
spouses. Head Neck. 31:513–520. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Linley PA and Joseph S: Positive change
following trauma and adversity: A review. J Trauma Stress.
17:11–21. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schreiber JA and Brockopp DY: Twenty-five
years later-what do we know about religion/spirituality and
psychological well-being among breast cancer survivors? A
systematic review. J Cancer Surviv. 6:82–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tomich PL and Helgeson VS: Posttraumatic
growth following cancer: Links to quality of life. J Trauma Stress.
25:567–573. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sim BY, Lee YW, Kim H and Kim SH:
Post-traumatic growth in stomach cancer survivors: Prevalence,
correlates and relationship with health-related quality of life.
Eur J Oncol Nurs. 19:230–236. 2015.PubMed/NCBI View Article : Google Scholar
|