Introduction
Thyroid cancer is the most prevalent endocrine
malignancy (1). Its incidence has
been increasing over the past forty years in every part of the
universe including Saudi Arabia (2). Mitogen-activated protein kinase (MAPK)
and phosphatidylinositol-3 kinase/AKT (PI3K/AKT) pathways are the
two most genetically deregulated pathways in follicular
cell-derived thyroid cancer. Aberrant activation of those vital
signaling promotes uncontrolled cell division, proliferation,
growth, invasion, and metastasis that collectively lead to thyroid
tumorigenesis (3). Differentiated
thyroid cancer (DTC) is the most commonly diagnosed type of thyroid
cancer. DTC refers to papillary (PTC) and follicular thyroid cancer
(FTC). Two other more aggressive subtypes of thyroid cancer include
the poorly differentiated (PDTC) and anaplastic thyroid cancer
(ATC) (4). Genetic alterations in
DTC involve many genes and include RET/PTC, BRAF,
RAS, PAX8/PPAR-γ, EGFR, EIF1AX, PPM1D,
CHEK2, with a low prevalence of PIK3CA and
PTEN genes (5-11).
TERT promoter mutations have been demonstrated to be a major
determinant of poor outcomes in DTCs (12-14).
High prevalence mutations of ALK (anaplastic
lymphoma kinase), IDH1 (isocitrate dehydrogenase 1),
IDH2 (isocitrate dehydrogenase 2), and MMP8 (matrix
metalloproteinase 8) genes have been reported in diverse human
cancers (15-17).
ALK is a receptor tyrosine kinase that belongs to the insulin
receptor subfamily. Initially, ALK has been identified as
part of various oncogenic fusion genes (18,19).
ALK mutations were found both in familial and sporadic
neuroblastomas (6-14%). Most of the ALK mutations were
identified within the catalytic domain of ALK. Some of its kinase
domain mutations were demonstrated to be oncogenic. The ALK mutants
F1174L and K1062M were shown to confer enhanced tyrosine kinase
activity and promote cell transformation, focus formation, and
tumor formation in nude mice (15).
Mutations of the IDH1 gene were frequently
detected at high frequency in secondary glioblastomas (>70%).
IDH plays a key role within the Krebs cycle and produces
α-ketoglutarate (α-KG) by catalyzing the oxidative decarboxylation
of isocitrate. The IDH activity is exclusively dependent on
nicotinamide adenine dinucleotide phosphate (NADP+) which is
catalyzed by IDH1 to produce NADPH that is involved in controlling
oxidative damage of the cell (20).
The IDH1 mutations were shown to occur mainly in the hotspot
arginine at codon R132 (R132H/S/C/G). The IDH2 mutations
were identified in codon 172 and tumors without mutations in
IDH1 often harbor mutations in the analogous amino acid
arginine (R) at 172 of the IDH2 gene. All the codon R132
IDH1 mutants have been shown to have decreased enzymatic activity
(16).
The MMPs are calcium-dependent zinc-containing
proteolytic enzymes that play a vital role in the extracellular
environment particularly in degrading the extracellular matrix
(ECM) and non-matrix protein. Essentially, they are involved in
morphogenesis, wound healing, tissue repair, and remodeling
(21). MMP8 gene mutations
have been frequently reported in melanoma (17). The majority of MMP8 mutations
have been identified in exon 2. This gene was characterized as a
tumor-suppressor as the wild-type MMP8 could inhibit cell
proliferation on soft-agar, cell invasion, and tumor formation in
the immunocompromised nude mice. Various mutations (S50F, P78S,
K87N, and G104R) found in the MMP8 gene were demonstrated to
be invasive and tumorigenic (17).
Common mutations of the ALK, IDH1,
IDH2, and MMP8 genes have previously been found in
anaplastic thyroid cancers (22-25).
Furthermore, these genes were also implicated in the activation of
MAPK, PI3K/AKT, and metabolic pathways to promote proliferation and
invasion (15-21).
However, the rates of ALK, IDH1, IDH2, and MMP8
mutations have not been examined specifically in differentiated
thyroid cancer (DTC), particularly in Saudi Arabia, where the
incidence of the DTC is within the top two cancers in Saudi women.
Given the important role of these genes in anaplastic thyroid
cancers and a prominent role in activating thyroid
cancer-associated MAPK, PI3K/AKT pathways, we aimed to determine
whether ALK, IDH1, IDH2, and MMP8 genes
could carry somatic mutations in DTCs.
Materials and methods
Tumor samples and DNA extraction
Unselected malignant thyroid tumor tissues which
were fixed in formaldehyde and embedded in paraffin, dissected
using a microtome, selected without normal cell contamination,
deparaffinized with xylene and subjected to genomic DNA isolation.
This formalin-fixed paraffin-embedded (FFPE) DTC samples with a
total of 126 for ALK, 271 for IDH1 and IDH2,
and 50 for MMP8 were used in this study. Non-cancer samples
consisting of 17 multinodular goiters (MNGs) were also included for
mutational analysis of the above-indicated genes. Somatic mutations
of each of these genes were analyzed at different time periods
caused to have a different cohort with variable sample size. Our
inclusion criterion is to consider only malignant thyroid tumors
and hence samples we obtained after confirming malignancy by the
pathologist in a particular period of study. Therefore, these
samples were unselected and used in an unbiased manner for each
study. In Saudi population, the aggressive thyroid cancer subtypes
(ATCs and PDTCs) are rare. Therefore, we did not use these as
exclusion criteria. Baseline demographic and clinical
characteristics for each cohort are listed in Table I. This research work was approved
(RAC-2130015) by the Institutional Review Board (IRB) of King
Faisal Specialist Hospital and Research Centre (KFSH & RC),
Riyadh, Saudi Arabia. Samples were carefully examined by an
experienced pathologist (H.A.) and dissected with ~10-micron
thickness from FFPE tissue. Genomic DNA was isolated from the FFPE
tissue by a commercially available kit (Gentra Puregene; Qiagen)
per the manufacturer's instruction as previously described
(26).
 | Table ICharacteristics of DTCs used for
mutational analysis of ALK, IDH1, IDH2 and
MMP8 genes. |
Table I
Characteristics of DTCs used for
mutational analysis of ALK, IDH1, IDH2 and
MMP8 genes.
| Characteristic | ALK | IDH1 and
IDH2 | MMP8 |
|---|
| Number of patients
(%) | 126 (100.0) | 271 (100.0) | 50 (100.0) |
| Sex, n (%) | | | |
|
Male | 23 (18.3) | 62 (22.9) | 15 (30.0) |
|
Female | 89 (70.6) | 209 (77.1) | 31 (62.0) |
|
N/A | 14 (11.1) | 0 (0.0) | 4 (8.0) |
| Age, years | | | |
|
Range | 11-71 | 9-75 | 21-75 |
|
Median
age | 42 | 42 | 48 |
| Tumor types, n
(%) | | | |
|
PTC | | | |
|
CPTC | 70 (55.6) | 142 (52.4) | 31 (62.0) |
|
FV
PTC | 24 (19.0) | 67 (24.7) | 10 (20.0) |
|
TC
PTC | 16 (12.7) | 29 (10.7) | 1 (2.0) |
|
DSV
PTC | 2 (1.6) | 4 (1.5) | 1 (2.0) |
|
CCV
PTC | 2 (1.6) | 4 (1.5) | 1 (2.0) |
|
OV
PTC | 1 (0.8) | 5 (1.8) | 1 (2.0) |
|
HCC | 1 (0.8) | 3 (1.1) | 1 (2.0) |
|
FTC | 4 (3.2) | 7 (2.6) | 0 (0.0) |
|
N/A | 6 (4.8) | 6 (2.2) | 4 (8.0) |
| Tumor size, n
(%) | | | |
|
>4
cm | 16 (12.7) | 54 (19.9) | 11 (22.0) |
|
1-4 cm | 89 (70.6) | 196 (72.3) | 32 (64.0) |
|
<1
cm | 6 (4.8) | 14 (5.1) | 3 (6.0) |
|
N/A | 15 (11.9) | 7 (2.6) | 4 (8.0) |
| TNM stage, n
(%) | | | |
|
<I-II | 92 (73.0) | 211 (77.9) | 32 (64.0) |
|
III-IV | 19 (15.0) | 57 (21.0) | 14 (28.0) |
|
N/A | 15 (11.9) | 3 (1.1) | 4 (8.0) |
PCR amplification and sequencing
Exons 23, 24, and 25 of the ALK gene were
amplified in 126 DTCs and exon 4 of the IDH1 and IDH2
gene was amplified in 271 DTCs. Exons 1-10 of the MMP8 gene
were amplified in 50 DTCs. Primers (sense and antisense) and PCR
conditions for the ALK, IDH1, IDH2, and
MMP8 gene amplification were used as exactly described
before (15-17).
The amplified PCR products (amplicons) were directly sequenced
using the BigDye terminator v3.1 cycle sequencing ready reaction
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). All the
identified genetic alterations were ascertained in both sense and
antisense sequencing. The related sequencing results were analyzed
against the appropriate gene. GeneBank accession no: ALK
(NM_005296.2), IDH1 (NM_005896.2), IDH2
(NM_002168.2), and MMP8 (NM_002424.2).
Analyses of the mutational rates of
ALK, IDH1, IDH2, and MMP8 genes in differentiated thyroid cancers
(DTCs)
The TCGA data comprising 496 DTCs (well
differentiated papillary thyroid carcinoma) were analyzed for the
mutational frequencies of ALK, IDH1, IDH2, and
MMP8 genes. Mutations/deletions were included and copy
number variations (CNVs) were omitted in this study (9).
Analyses of the mutational rates of
ALK, IDH1, IDH2, and MMP8 genes in aggressive thyroid cancers (PDTC
and ATC)
The next-generation sequencing data of 117
aggressive thyroid cancer samples [84 poorly differentiated (PDTC)
and 33 anaplastic thyroid cancer (ATC)] from the MSKCC cohort were
analyzed in this study (27). We
excluded CNVs while only the mutations/deletions were included.
TCGA and MSKCC data were analyzed with the tools incorporated
within the cBioPortal for Cancer Genomics (www.cbioportal.org).
Statistical analysis
In this study, various basic statistical analyses,
including percentage, histogram and median, were performed using
GraphPad Prism (v8.0.2; GraphPad Software, Inc.).
Results
No somatic mutations were identified in the examined
exons of ALK, IDH1, IDH2, and MMP8 genes in DTCs and MNGs (Table II). Mutations of the ALK
(exons 23, 24, and 25), IDH1, IDH2 (exon 4), and
MMP8 (all exons 1-10) genes were analyzed by PCR
amplification followed by direct Sanger sequencing. We selectively
analyzed the indicated exons because they harbored the majority of
the reported mutations in these genes. Although no somatic mutation
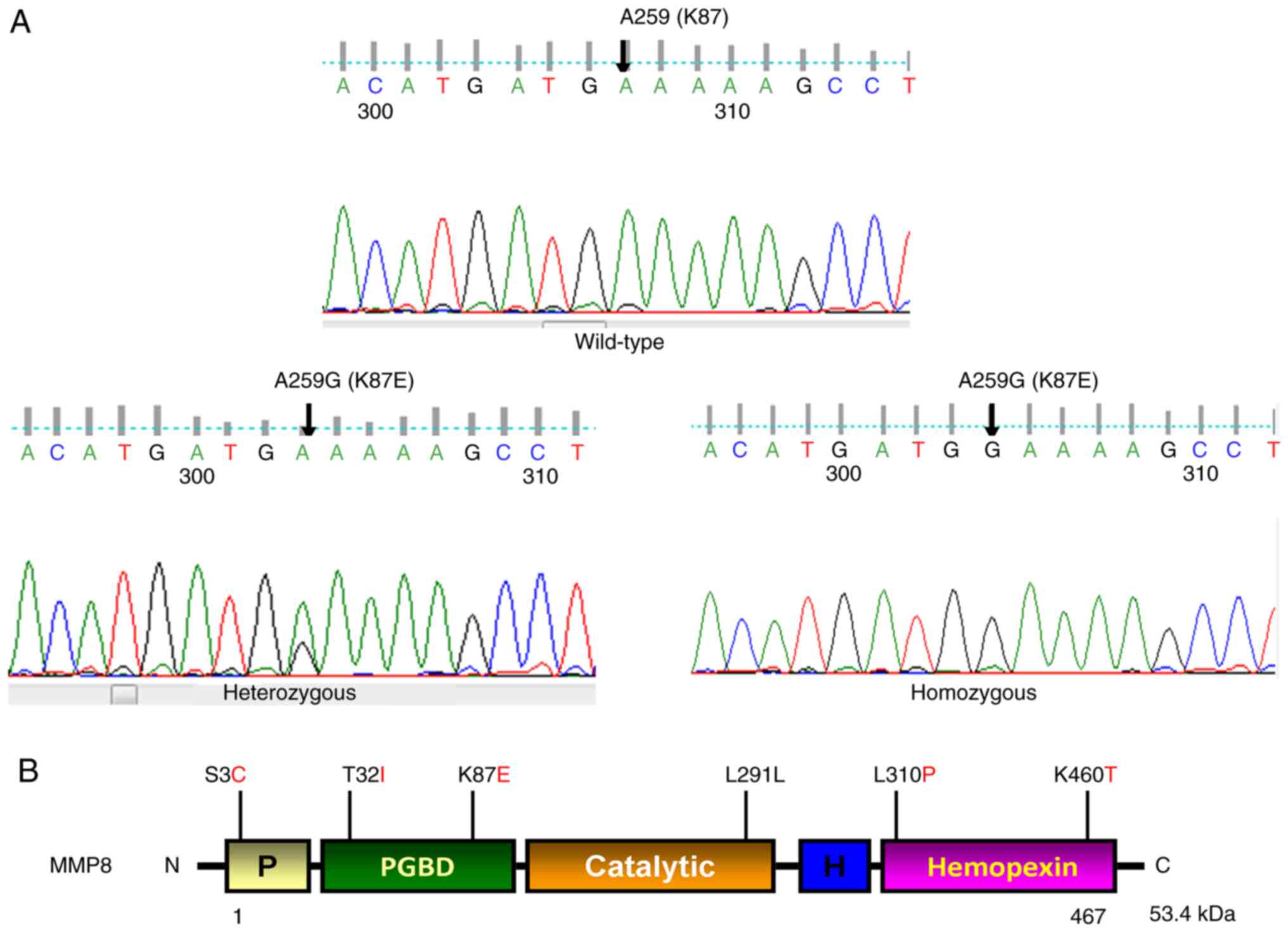
was found in MMP8, as illustrated in Fig. 1A and B, we found five previously reported
non-synonymous single nucleotide polymorphisms (SNPs) in this gene
[S3C (rs17099450), 1/50 (2%)], [T32I (rs3765620), 4/50 (8%)] in
exon 1, [K87E (rs1940475) 43/50 (86%)] in exon 2, [L310P
(rs61753779) 1/50 (2%)] in exon 7, and [K460T (rs35866072) 9/50
(18%)] in exon 10, and a synonymous SNP [L291L (rs61753779) 2/50
(4%)] in exon 6 of the MMP8 gene. We also observed a high
rate of [86% (43/50)] heterozygous/homozygous A>G transition at
nucleotide position 259, resulting in codon 87 changing from AAA to
GAA, lysine to glutamic acid (K87E) in exon 2 of MMP8. At
nucleotide 259 position, we observed GG in 48% (24/50), AG in 38%
(19/50), and AA in 14% (7/50). The allele frequency of A=0.14 in
this study and it varies greatly (A=0.25-0.499) across world
regions (https://www.ncbi.nlm.nih.gov/snp/rs1940475). All these
SNPs were documented in the SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/).
 | Table IIPrevalence of ALK, IDH1,
IDH2 and MMP8 gene somatic mutations in DTCs, ATCs and
PDTCs. |
Table II
Prevalence of ALK, IDH1,
IDH2 and MMP8 gene somatic mutations in DTCs, ATCs and
PDTCs.
| Cohort | Thyroid cancer
subtypes | Genes | Alterations
(mutations/fusions) | Prevalence [altered
cases/total cases analyzed (%)] |
|---|
| Current study | DTC | ALK | (0) | 0/126 (0.0) |
| | | IDH1 | (0) | 0/271 (0.0) |
| | | IDH2 | (0) | 0/271 (0.0) |
| | | MMP8 | (0) | 0/50 (0.0) |
| TCGA | DTC | ALK | (4) P693S (FV PTC);
ALK-STRN (CPTC); ALK-EML4 (CPTC) GTF2IRD1-ALK
(CPTC) | 4/414 (1.0) |
| | | IDH1 | (0) | 0/414 (0.0) |
| | | IDH2 | (0) | 0/414 (0.0) |
| | | MMP8 | (1) A444S
(CPTC) | 1/414 (0.2) |
| MSKCC | ATC | ALK | (1) K1079N | 1/33 (3.0) |
| | | IDH1 | (0) | 0/33 (0.0) |
| | | IDH2 | (1) T435M | 1/33 (3.0) |
| | | MMP8 | (0) | 0/33 (0.0) |
| | PDTC | ALK | (3) EML4-ALK;
STRN-ALK; CCDC149-ALK | 3/84 (3.6) |
| | | IDH1 | (1) V178I | 1/84 (1.2) |
| | | IDH2 | (0) | 0/84 (0.0) |
| | | MMP8 | (0) | 0/84 (0.0) |
Analyses of TCGA data revealed rare
mutations of the ALK and MMP8 and no mutation of IDH1 and IDH2
genes in DTCs
To test whether our results corroborate TCGA data,
which is mostly derived from the Western population, we analyzed
TCGA data of DTCs (6). As shown in
Fig. 2A, although 496 samples were
included in the study, only 414 samples included data on the
ALK, IDH1, IDH2, and MMP8 genes.
Genetic alterations of ALK were found in 1% (4/414) of DTCs.
Of 4 ALK-altered cases, 1 follicular variant papillary
thyroid cancer (FV PTC) case had a mutation (ALK, P693S) and the
other 3 conventional PTCs (CPTCs) had ALK fusions
(ALK-STRN, ALK-EML4, and GTF2IRD1-ALK). Only
one MMP8 mutation (MMP8, A444S) was observed (in CPTC) out
of 414 DTC samples (0.24%). No IDH1 and IDH2 gene
mutations were identified in the DTCs of TCGA (Fig. 2B-F; Table II). These findings suggest that
only a small subset of DTCs harbors ALK and MMP8
genetic alterations but not IDH1 and IDH2
mutations.
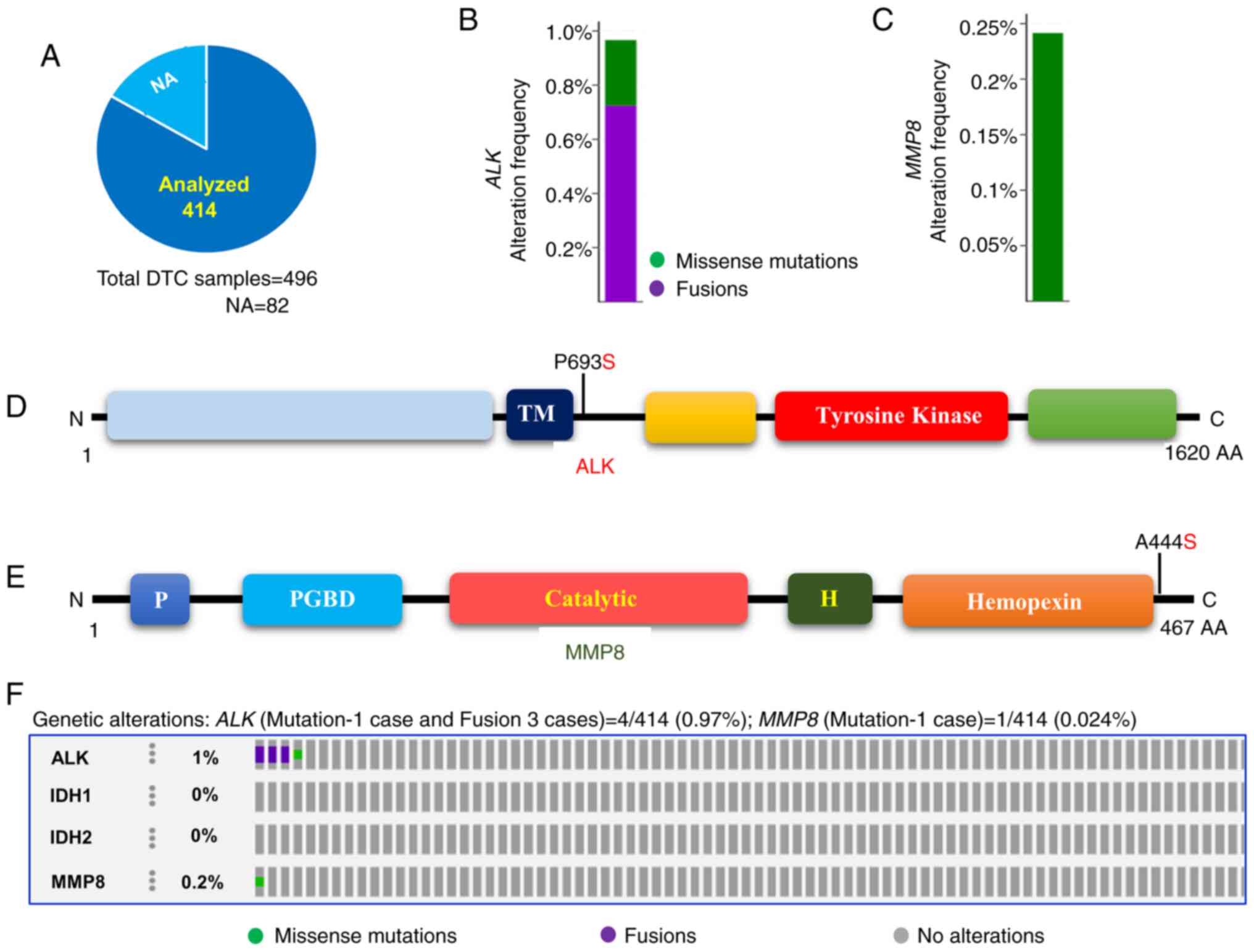 | Figure 2Prevalence of ALK, IDH1, IDH2
and MMP8 mutations in DTCs. (A) Pie chart of DTCs. The chart
indicates the type and number of tumor samples analyzed from the
DTCs of TCGA. (B) Histogram showing the genetic alterations of
ALK in DTCs. The bar indicates both the mutation and fusion
of ALK in 0.97% of DTCs. (C) Histogram showing the somatic
mutations of the MMP8 gene. The bar indicates the somatic
mutation of MMP8 (0.24%) in DTCs. (D) Mutation tab.
Schematic illustration showing ALK protein and its respective
domains with indicated mutation. (E) Mutation tab. Schematic
diagram shows MMP8 protein and its respective domains with
indicated mutation. The frequently mutated residue is depicted in
the diagram. (F) OncoPrint tab. The tab indicates the ALK,
IDH1, IDH2 and MMP8 mutations across the DTCs
(TCGA). ALK, anaplastic lymphoma kinase; TM, transmembrane domain;
Tyrosine kinase, tyrosine kinase domain; IDH1, isocitrate
dehydrogenase 1; IDH2, isocitrate dehydrogenase 2; MMP8, matrix
metalloproteinase 8; P, propeptide; PGBD, proteoglycan binding
domain; catalytic, catalytic domain; Hemopexin, hemopexin-like
domain; DTC, differentiated thyroid cancer; TCGA, The Cancer Genome
Atlas; NA, not available. |
Analyses of aggressive thyroid cancers
(PDTC and ATC) showed common somatic mutations in ALK, IDH1, and
IDH2 genes but not in the MMP8 gene
We found a low rate of ALK, IDH1,
IDH2, and MMP8 mutations in DTCs including our study
and TCGA. We, therefore, analyzed the next-generation sequencing
data of 84 PDTC and 33 ATC from the MSKCC cohort to examine whether
these mutations play any role in the more aggressive subtypes of
thyroid cancer. As shown in Fig.
3A, B, E and H,
the ALK mutation (K1079N) was found in 3% (1/33) of ATC and
various fusions (EML4-ALK, STRN-ALK, and
CCDC149-ALK) were identified in 3.6% (3/84) of PDTC. The
IDH1 (V178I) and IDH2 (T435M) mutations were found in
1.2% (1/84) of PDTC and 3% (1/33) of ATC, respectively (Fig. 3C, D
and F-H; Table II). No IDH1 and IDH2
mutations were found in ATC and PDTC, respectively. None of the ATC
and PDTC samples harbored MMP8 mutations (Fig. 3H). These results suggest that,
unlike the MMP8 gene, the ALK, IDH1, and
IDH2 are likely to have a role in aggressive subtypes of
thyroid cancer (ATC and PDTC).
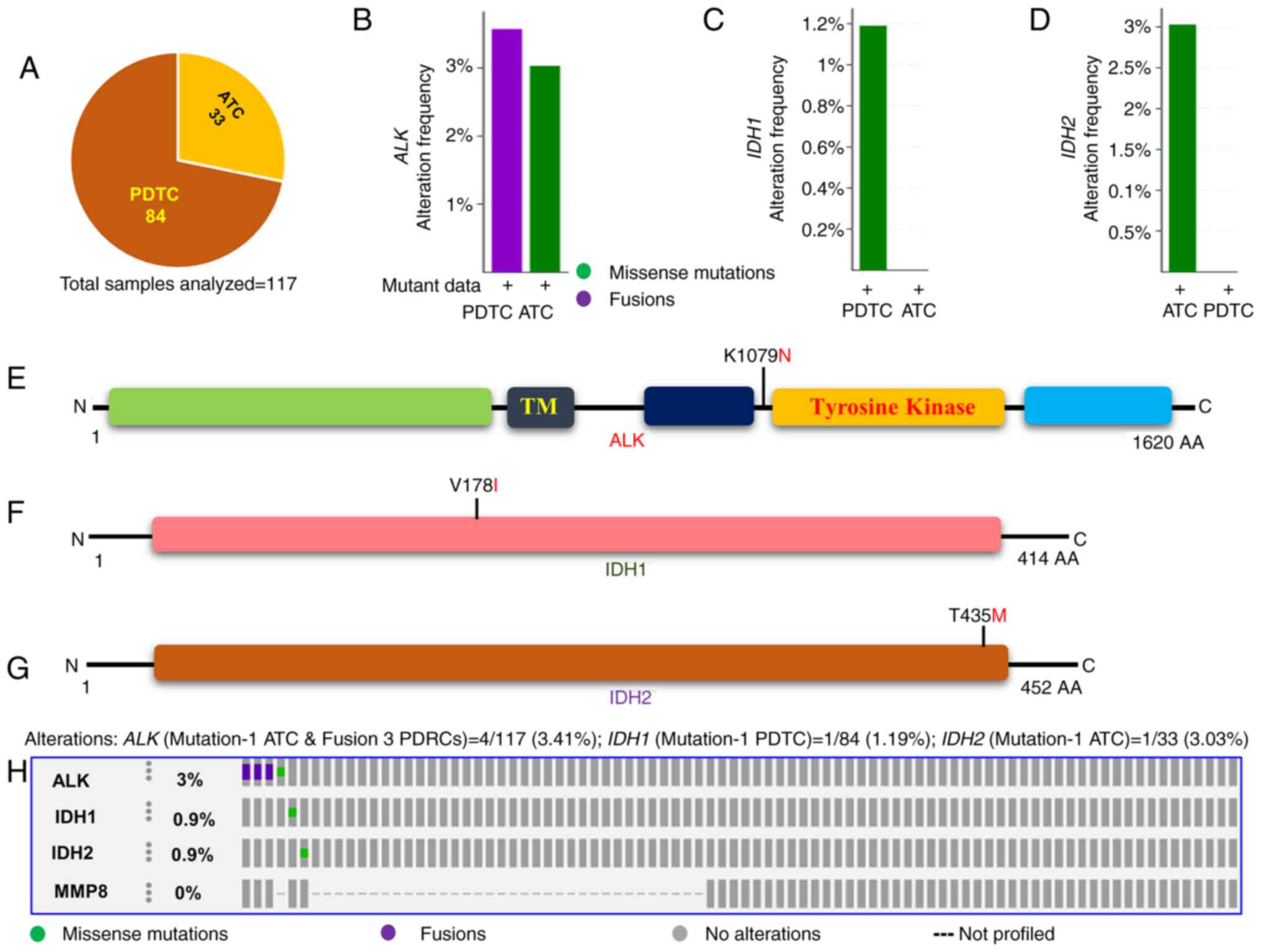 | Figure 3Prevalence of ALK,
IDH1, IDH2 and MMP8 mutations in aggressive
thyroid cancers (PDTC and ATC). (A) Pie chart of aggressive thyroid
cancers. The chart displays the type of tumors, the number of
samples in each type and the total number of aggressive thyroid
cancer samples analyzed. (B) Histogram showing the genetic
alterations of ALK in PDTC and ATC. The bar indicates the
overall frequency of ALK fusions and mutations in PDTC
(3.6%) and ATC (3.03%), respectively. (C) Histogram indicating the
mutation rate of IDH1. The bar indicates 1.2% IDH1
mutations in PDTC. (D) Histogram showing the mutation rate of
IDH2. The bar indicates 3.03% IDH2 mutations in ATC.
(E) Mutation tab. The schematic diagram displays ALK protein and
its domains indicating the position of the detected mutation. (F)
Mutation tab. The schematic diagram shows IDH1 protein and
indicating the position of the detected mutation. (G) Mutation tab.
The schematic diagram shows IDH2 protein indicating the position of
the detected mutation. (H) OncoPrint tab. The tab indicates the
ALK, IDH1, IDH1 and MMP8 mutations
across the aggressive thyroid cancers, PDTC and ATC. Each row
represents a particular gene and each column displays a tumor
sample. The non-synonymous mutations are indicated as green square
plots on the columns. ALK, anaplastic lymphoma kinase; IDH1,
isocitrate dehydrogenase 1; IDH2, isocitrate dehydrogenase 2; MMP8,
matrix metalloproteinase 8; PDTC, poorly differentiated thyroid
cancer; ATC, anaplastic thyroid cancer. |
Discussion
The ALK, IDH1, IDH2, and
MMP8 gene mutations were recurrently reported in human
cancer. Particularly, point mutations of these genes were
demonstrated to be major therapeutic targets and important
prognostic markers. However, to date, the prevalence of somatic
mutations of these genes has never been examined in DTC from Saudi
Arabia, a highly consanguineous society with high prevalence of
thyroid cancer. We, therefore, studied somatic point mutations of
these genes in samples of DTC from this population.
In this study, we found five non-synonymous SNPs
(S3C, T32I, K87E, L310P, and K460T) and a synonymous SNP (L291L) of
the MMP8 gene. We found no ALK, IDH1,
IDH2, and MMP8 gene mutations in DTC and benign
goiters. The MMP8 SNP, S3C (rs17099450), has been shown to
be one of the significant genetic determinants of allergic
sensitization to cockroach allergens in children. However, the role
of this SNP in cancer is not fully investigated (28). The MMP8 SNP K87E (rs1940475)
has been shown to have a differential effect on human cancers. For
example, rs1940475 was reported to be associated with an enhanced
risk of bladder cancer in never smokers while it was shown to
protect from the invasive type in former smokers of this malignancy
(29). Moreover, rs1940475 has been
reported to be significantly associated with a higher risk for
recurrence, reduced overall survival, recurrence-free survival, and
disease-free survival in gastric adenocarcinoma (30). Further, this SNP was described to
have a reduced risk for basal cell carcinoma (BCC) while exhibited
no effect in squamous cell carcinoma (SCC) and melanoma (31). Conversely, a recent meta-analysis of
several MMP8 polymorphisms showed that the K460T
(rs35866072) and K87E (rs1940475) variants were not significantly
associated with cancer susceptibility (32). Nevertheless, this SNP was not
substantially studied despite its higher prevalence in PTCs of the
Western population (80.6%) (8) and
DTCs in our study (86%) suggesting that a future study with a large
number of samples is warranted to discover the role of this SNP in
thyroid cancer. The MMP8 SNP K460T (rs35866072) was shown to
have no association with cancer risk including leukemia, head and
neck, lung, breast, and bladder (33). The importance of other rare
MMP8 SNPs (rs3765620 and rs61753779) remains unknown in
cancers including thyroid cancer.
Besides, while our study revealed no mutations in
ALK, IDH1, IDH2, and MMP8 genes in
DTCs, we comprehensively analyzed the TCGA data from a completely
distinct ethnic background, a large Western cohort for DTCs
(n=414). Consistent with our data, we found a rare incidence of
ALK (0.97%) and MMP8 (0.24%) genetic alterations and
no incidence of IDH1 and IDH2 mutations. Our study
and TCGA data result collectively suggest that somatic mutations of
the ALK, IDH1, IDH2, and MMP8 are
uncommon in DTCs and hence play a pivotal role in a small portion
of DTC pathogenesis.
Aggressive subtypes of thyroid cancers including
PDTC and ATC are generally rare yet they are deadly. Notably, ATC
has <5 months of median survival from the initial detection
(3). Therefore, we were inquisitive
whether these genes play any role in aggressive thyroid cancers
(PDTC and ATC). Analysis of ALK, IDH1, IDH2,
and MMP8 gene mutations in 117 aggressive thyroid cancers
from MSKCC data revealed 3% of ALK genetic alterations
including 3% mutations in ATC and 3.6% fusions in PDTC.
Consistently, two previous studies also independently showed
identification of ALK somatic mutations in ATCs ~10%
(22,23) which is much higher than the
currently analyzed aggressive thyroid cancer data (3%) from MSKCC
(27). IDH1 was found in
1.2% of PDTC while no IDH1 mutation was detected in ATC. The
IDH2 mutation was observed in 3.03% of ATC but not in PDTC.
Interestingly, similar to ALK mutations, a high frequency of
IDH1 mutations has previously been reported both in ATCs
(11%) and undifferentiated thyroid cancers (33%) (24,25).
The prevalence of IDH1 mutations in the previously reported
cases was relatively higher when compared with the current analysis
of aggressive thyroid cancer (ATC and PDTC) data derived from MSKCC
(27). The incidence of the
ALK and IDH1 and IDH2 mutation in aggressive
thyroid cancer cases varies among different studies, likely because
of the selection of tumor tissue, tissue preservation, mode of
tissue dissection, tumor tissue with normal cell contamination,
number of samples, and sequencing methods that could greatly
influence the incidence of mutation (34). Collectively, data from MSKCC and
other previous studies strongly suggest that the ALK,
IDH1, and IDH2 are likely to play an important role
in the pathogenesis of some cases of aggressive thyroid cancers
including ATCs and PDTCs. Consistent with a previous study
(8), somatic mutation of the
MMP8 was not found in the current analysis of aggressive
thyroid cancer (PDTC and ATC) and this was also observed in both
DTCs of our study and only one case in TCGA (9) suggesting that the MMP8 somatic
mutations are rare in thyroid cancer regardless of its
subtypes.
Moreover, the mutational incidence of DTC-specific
genes including BRAF, TERT, RAS and
PIK3CA, in the Saudi Arabian cohort was comparable to that
of the incidence of the Western cases (10,11,35).
The limitation of this study is a failure to analyze the ALK
gene fusion which is considerably detected in thyroid cancers
(9,27).
More than 85% of DTCs are treated and cured with
surgical methods, radioactive iodine, and TSH suppression. About
50-60% of DTCs harbor the BRAFV600E mutation
(10). Two BRAF inhibitors
(vemurafenib and dabrafenib) are in clinical use. The vemurafenib
is used for BRAFV600E mutated thyroid cancer with
radioactive iodine-refractory phenotype and the dabrafenib along
with tramatenib, a MEK inhibitor is used for
BRAFV600E-mutated ATC (36). Rare DTC cases harboring ALK
alterations may benefit from a range of first (crizotinib), second
(alectinib and brigatinib), and third (lorlatinib) generation ALK
inhibitors, and they are currently approved for ALK-positive
non-small cell lung cancers (37).
Similarly, IDH1-mutant cases could be treated with ivosidenib or
recently developed vaccine targeting IDH1 mutants (38). Although MMP inhibitors were
developed a few decades ago, their therapeutic impact on cancer was
not that potent as expected in the beginning; yet, thyroid cancers
with genetic alterations in MMPs are likely to benefit from
BRAF inhibitors as they mediate signals downstream of BRAF
(39). Given the availability of
these therapeutic agents, tumors bearing these gene alterations may
have the advantage of being treated precisely and more
effectively.
In conclusion, both the data derived from our study
and the TCGA revealed a rare incidence of ALK, IDH1,
IDH2, and MMP8 gene mutations in DTCs and a higher
prevalence of mutations of these genes in ATCs and PDTCs. The
findings suggest that these mutations are rare and could play a
role in the pathogenesis of a small subset of DTCs but are more
likely to have a role in the aggressive tumor subtypes and may
serve as potential therapeutic targets at least in a portion of
PDTCs and ATCs.
Acknowledgements
Not applicable.
Funding
The present study was supported by King Abdulaziz City for
Science and Technology (KACST), Riyadh, Saudi Arabia (grant no.
12-BIO2952-20).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request. In addition, the mutation rate datasets
generated and/or analyzed during the current study are available in
the cBioPortal repository (https://www.cbioportal.org/study/summary?id=thca_tcga_pub
and https://www.cbioportal.org/study/summary?id=thyroid_mskcc_2016).
Authors' contributions
AKM and EQ performed the experiments and analyzed
the data. AKM wrote the manuscript. HAH carefully identified and
selected the samples and was involved in interpretation of data.
ASA contributed to conception and design, critically reviewed the
data and revised the manuscript. AKM and ASA confirm the
authenticity of all the raw data. All the authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The present study was approved (RAC-2130015) by the
Institutional Review Board of King Faisal Specialist Hospital and
Research Centre, Riyadh, Saudi Arabia. Written informed consent was
obtained from all individual participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Furuya-Kanamori L, Sedrakyan A, Onitilo
AA, Bagheri N, Glasziou P and Doi SAR: Differentiated thyroid
cancer: Millions spent with no tangible gain? Endocr Relat Cancer.
25:51–57. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alzahrani AS, Alomar H and Alzahrani N:
Thyroid cancer in Saudi Arabia: A histopathological and outcome
study. Int J Endocrinol. 2017(8423147)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fagin JA and Wells SA Jr: Biologic and
clinical perspectives on thyroid cancer. N Engl J Med.
375:1054–1067. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grieco M, Santoro M, Berlingieri MT,
Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G,
Fusco A and Vecchio G: PTC is a novel rearranged form of the ret
proto-oncogene and is frequently detected in vivo in human thyroid
papillary carcinomas. Cell. 60:557–563. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nikiforova MN, Biddinger PW, Caudill CM,
Kroll TG and Nikiforov YE: PAX8-PPARgamma rearrangement in thyroid
tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol.
26:1016–1023. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Murugan AK, Dong J, Xie J and Xing M:
Uncommon GNAQ, MMP8, AKT3, EGFR, and PIK3R1 mutations in thyroid
cancers. Endocr Pathol. 22:97–102. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cancer Genome Atlas Research Network.
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Murugan AK, Qasem E, Al-Hindi H, Shi Y and
Alzahrani AS: Classical V600E and other non-hotspot BRAF mutations
in adult differentiated thyroid cancer. J Transl Med.
14(204)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Murugan AK, Qasem E, Al-Hindi H and
Alzahrani AS: GPCR-mediated PI3K pathway mutations in pediatric and
adult thyroid cancer. Oncotarget. 10:4107–4124. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Melo M, da Rocha AG, Vinagre J, Batista R,
Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, et
al: TERT promoter mutations are a major indicator of poor outcome
in differentiated thyroid carcinomas. J Clin Endocrinol Metab.
99:E754–E765. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xing M, Liu R, Liu X, Murugan AK, Zhu G,
Zeiger MA, Pai S and Bishop J: BRAF V600E and TERT promoter
mutations cooperatively identify the most aggressive papillary
thyroid cancer with highest recurrence. J Clin Oncol. 32:2718–2726.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen Y, Takita J, Choi YL, Kato M, Ohira
M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, et al:
Oncogenic mutations of ALK kinase in neuroblastoma. Nature.
455:971–974. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palavalli LH, Prickett TD, Wunderlich JR,
Wei X, Burrell AS, Porter-Gill P, Davis S, Wang C, Cronin JC,
Agrawal NS, et al: Analysis of the matrix metalloproteinase family
reveals that MMP8 is often mutated in melanoma. Nat Genet.
41:518–520. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Morris SW, Kirstein MN, Valentine MB,
Dittmer KG, Shapiro DN, Saltman DL and Look AT: Fusion of a kinase
gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's
lymphoma. Science. 263:1281–1284. 1994.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Geisbrecht BV and Gould SJ: The human PICD
gene encodes a cytoplasmic and peroxisomal NADP(+)-dependent
isocitrate dehydrogenase. J Biol Chem. 274:30527–30533.
1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Quintero-Fabián S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9(1370)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Murugan AK and Xing M: Anaplastic thyroid
cancers harbor novel oncogenic mutations of the ALK gene. Cancer
Res. 71:4403–4411. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Latteyer S, Tiedje V, König K, Ting S,
Heukamp LC, Meder L, Schmid KW, Führer D and Moeller LC: Targeted
next-generation sequencing for TP53, RAS, BRAF, ALK and NF1
mutations in anaplastic thyroid cancer. Endocrine. 54:733–741.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Murugan AK, Bojdani E and Xing M:
Identification and functional characterization of isocitrate
dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys
Res Commun. 393:555–559. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hemerly JP, Bastos AU and Cerutti JM:
Identification of several novel non-p.R132 IDH1 variants in thyroid
carcinomas. Eur J Endocrinol. 163:747–755. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Murugan AK, Humudh EA, Qasem E, Al-Hindi
H, Almohanna M, Hassan ZK and Alzahrani AS: Absence of somatic
mutations of the mTOR gene in differentiated thyroid cancer. Meta
Gene. 6:69–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Landa I, Ibrahimpasic T, Boucai L, Sinha
R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP,
Xu B, et al: Genomic and transcriptomic hallmarks of poorly
differentiated and anaplastic thyroid cancers. J Clin Invest.
126:1052–1066. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tripathi P, Hong X, Caruso D, Gao P and
Wang X: Genetic determinants in the development of sensitization to
environmental allergens in early childhood. Immun Inflamm Dis.
2:193–204. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kader AK, Liu J, Shao L, Dinney CP, Lin J,
Wang Y, Gu J, Grossman HB and Wu X: Matrix metalloproteinase
polymorphisms are associated with bladder cancer invasiveness. Clin
Cancer Res. 13:2614–2620. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin Y, Liu J, Jin L and Jiang Y:
Polymorphisms in matrix metalloproteinases 2, 3, and 8 increase
recurrence and mortality risk by regulating enzyme activity in
gastric adenocarcinoma. Oncotarget. 8:105971–105983.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nan H, Niu T, Hunter DJ and Han J:
Missense polymorphisms in matrix metalloproteinase genes and skin
cancer risk. Cancer Epidemiol Biomarkers Prev. 17:3551–3557.
2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang LF, Zhu LJ and Zhang W, Yuan W, Song
NH, Zuo L, Mi YY, Wang ZJ and Zhang W: MMP-8 C-799 T, Lys460Thr,
and Lys87Glu variants are not related to risk of cancer. BMC Med
Genet. 20(162)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Juurikka K, Butler GS, Salo T, Nyberg P
and Åström P: The role of MMP8 in cancer: A systematic review. Int
J Mol Sci. 20(4506)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Murugan AK, Munirajan AK and Tsuchida N:
Ras oncogenes in oral cancer: The past 20 years. Oral Oncol.
48:383–392. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zou M, Baitei EY, Alzahrani AS, BinHumaid
FS, Alkhafaji D, Al-Rijjal RA, Meyer BF and Shi Y: Concomitant RAS,
RET/PTC, or BRAF mutations in advanced stage of papillary thyroid
carcinoma. Thyroid. 24:1256–1266. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Roskoski R Jr: Properties of FDA-approved
small molecule protein kinase inhibitors: A 2021 update. Pharmacol
Res. 165(105463)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Naito T, Shiraishi H and Fujiwara Y:
Brigatinib and lorlatinib: Their effect on ALK inhibitors in NSCLC
focusing on resistant mutations and central nervous system
metastases. Jpn J Clin Oncol. 51:37–44. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Platten M, Bunse L, Wick A, Bunse T, Le
Cornet L, Harting I, Sahm F, Sanghvi K, Tan CL, Poschke I, et al: A
vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature.
592:463–468. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Murugan AK, Al-Amr A, Al-Ansari MM,
Manogaran PS, Al-Hindi H and Alzahrani AS: Single nucleotide
polymorphisms in matrix metalloproteinase 2 (MMP2) enhance
BRAFV600E mutation-mediated oncogenicity and invasiveness of
papillary thyroid cancer cells. Endocr Relat Cancer. 28:273–289.
2021.PubMed/NCBI View Article : Google Scholar
|