Introduction
Novel insights into gene mutations in lung
adenocarcinoma have led to the molecular-stratified therapy of the
disease. Fewer than 5% of patients with lung adenocarcinoma harbor
HER2 (also known as ErbB2) alterations. Those with HER2 mutations
have poorer survival outcomes compared with lung adenocarcinoma
harboring other gene mutations, mandating tailored HER2-directed
therapies in this subset of patients (1-3).
The majority of HER2 mutations occur in HER2 exon 20 as a
duplication or insertion mutation (4). The overall response rate of anti-HER2
therapy with HER2 inhibitors, including poziotinib, pyrotinib, and
afatinib, ranges from 30 to 50% (5-7).
In addition to HER2 gene mutations, the mechanisms of HER2
activation include HER2 amplification or overexpression. Despite
the success of anti-HER2 therapy in patients with breast cancer
with HER2 amplification or overexpression, the results of clinical
trials on anti-HER2 therapy for patients with lung cancer have
failed (8).
The aim of the present study was to report a case of
stage IIIA lung adenocarcinoma in a non-smoker female patient with
primary chemoresistance who achieved partial response (PR) with
anti-HER2 therapy using trastuzumab and lapatinib and complete
response (CR) with pyrotinib after disease progression.
Case report
A 53-year-old Chinese, non-smoker, female patient
with no family history of cancer was diagnosed pathologically with
lung adenocarcinoma after undergoing thoracoscopic left upper
lobectomy and hilar and mediastinal lymph node dissection at Daping
Hospital (Chongqing, China) in March 2018. The TNM classification
of the disease was T2N2M0 and stage IIIA. A CT scan after two
cycles of pemetrexed (500 mg/m2, day 1) plus platinum
(75 mg/m2, day 1, every 3 weeks) revealed mediastinal
lymph node and liver metastases. Magnetic resonance imaging of the
brain revealed no intracranial metastasis. The CEA level increased
to 180 ng/ml (normal reference value range: 0-5 ng/ml). The patient
was switched to a second-line regimen with albumin-conjugated
paclitaxel (260 mg/m2, once every 3 weeks), platinum and
bevacizumab (7.5 mg/kg, once every 3 weeks). However, the CEA level
continued to increase (414 ng/ml). After one treatment cycle, the
CT examination revealed a new liver lesion. Next-generation
sequencing (NGS) of the surgical specimen revealed HER2
amplification (gene copy number: 11), HER2 mutation (1.46%; F616L
is not an exon 20 mutation), ErbB4 mutation (24.41%), TP53 mutation
(33.57%), and other mutations (Table
I). The tumor mutation burden was 17.9/Mb. Supplementary
immunohistochemistry (IHC) examination revealed HER2 overexpression
(3+; Fig. S1). The third-line
regimen was commenced in June 2018 with four courses of lapatinib
(1,250 mg/day) and one course of trastuzumab (75 mg/m2,
once every 3 weeks), in addition to docetaxel (75 mg/m2,
once every 3 weeks), platinum, vinorelbine (25 mg/m2,
days 1 and 8, every 3 weeks) and capecitabine (1,250
mg/m2 twice a day, days 1-14, every 3 weeks). The
patient achieved PR. The disease progressed with left axillary
lymph node metastasis detected on CT scan in November 2018. The
fourth-line regimen was then initiated, with pyrotinib (400 mg/day)
combined with irinotecan (220 mg/m2, once every 3
weeks), and oxaliplatin (130 mg/m2, once every 3 weeks).
After two cycles, the regimen was changed to pyrotinib monotherapy
at 400 mg/day. The patient achieved CR in February 2019. The
metastatic foci in the liver and mediastinal lymph nodes
disappeared, and the CEA levels returned to normal. The main
treatment-associated side effect was grade 2 diarrhea.
 | Table IGene mutational profile in a patient
with lung adenocarcinoma by next-generation sequencing of DNA
obtained from surgical specimens. |
Table I
Gene mutational profile in a patient
with lung adenocarcinoma by next-generation sequencing of DNA
obtained from surgical specimens.
| Genes | Mutation sites | Frequency, % | Amplification (copy
no.) |
|---|
| HER2 | F616La | 1.46 | 11 |
| ErbB4 | N280I | 24.41 | |
| GATA1 | S12P | 1.17 | |
| GNAS | T386P | 1.82 | |
| KIT | M1R | 2.58 | |
| KIT | A621S | 21.79 | |
| MSH3 | K281fs | 1.73 | |
| TP53 | G154V | 33.57 | |
| VEGFA | L139R | 1.08 | |
The time to progression (TTP) of the patient was 6
months. The disease started to progress slowly, after six cycles of
pyrotinib therapy. CT scans performed in April 2019 revealed new
lesions in the liver, and the CEA level increased to 40 ng/ml. The
regimen was switched to afatinib (40 mg/day). After 1 month, the
disease rapidly progressed with a marked increase in CEA (639
ng/ml) and CA125 (1,255 U/ml; cutoff value <35 U/ml) levels. A
CT scan revealed slight progression in the lesions in the chest,
but the liver lesions markedly progressed. A liver biopsy was
performed in June 2019. NGS was performed using the same method as
before. The results revealed HER2 amplification (gene copy number:
23), and more gene mutations (Table
II). No ErbB4 mutations were identified. The tumor mutation
burden increased to 26.66/Mb. Programmed death-ligand 1 (SP142) was
negative as detected by IHC. As the results showed high copy number
of HER2, high TMB, and high rates of chemotherapy-resistant gene
mutations [such as KEAP1(9) and
NOTCH1(10)], pyrotinib (480
mg/day) was continued, along with anlotinib (12 mg/day, days 1-14,
every 3 weeks) and nivolumab (3 mg/kg, once every 2 weeks). The
disease remained stable until December 2019, and the patient
achieved another 6 months of TTP. The patient eventually succumbed
to the disease in July 2020. The overall survival was 28 months.
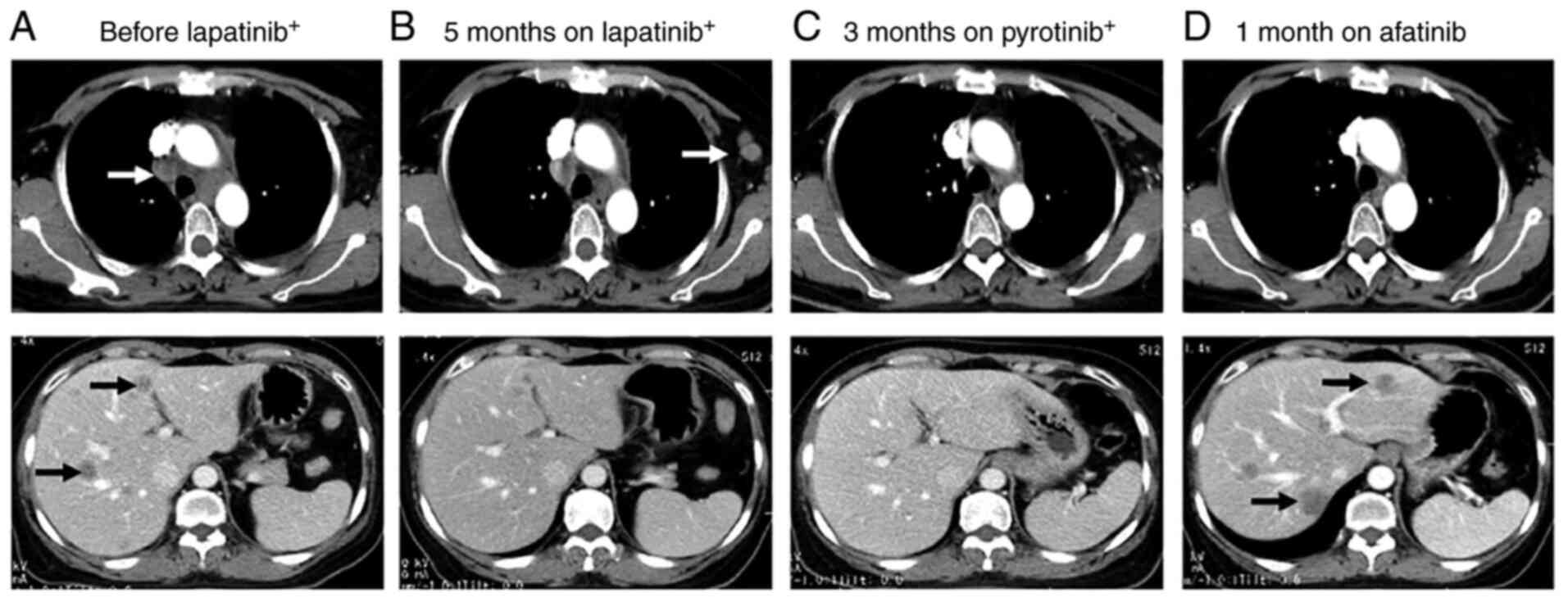
The changes in CT images and CEA levels over the course of
treatment are shown in Figs. 1 and
2. The treatment regimens and
responses are summarized in Table
SI.
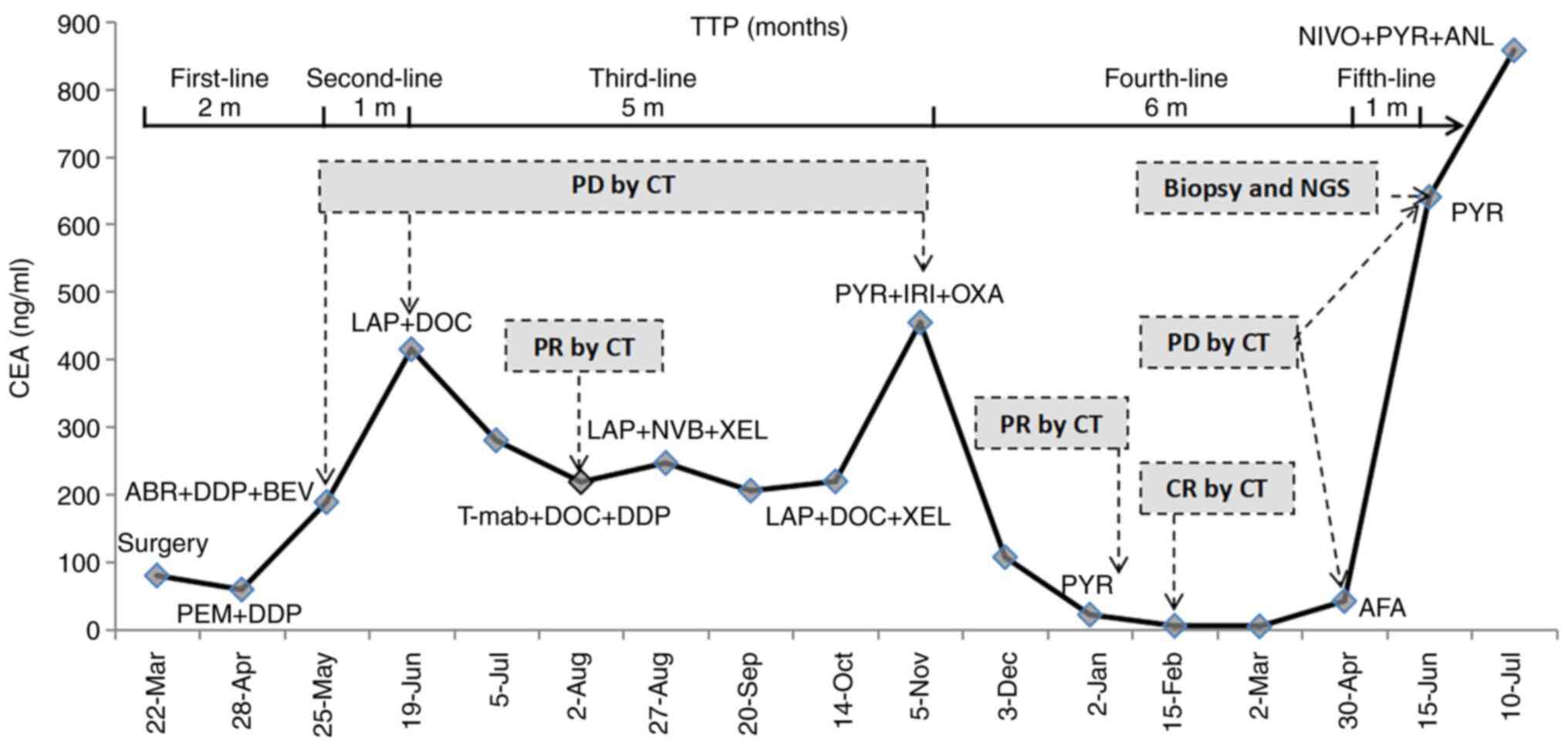 | Figure 2Changes of CEA levels over time. PEM,
pemetrexed; DDP, cisplatin; ABR, abraxane; LAP, lapatinib; DOC,
docetaxel; T-mab, trastuzumab; NVB, navelbine; XEL, xeloda; PYR,
pyrotinib; IRI, irinotecan; OXA, oxaliplatin; AFA, afatinib; NIVO,
nivolumab; ANL, anlotinib; TTP, time to progression; PD,
progressive disease; CR, complete response; PR, partial response;
CEA, carcinoembryonic antigen. |
 | Table IIGene mutational profile of lung
adenocarcinoma by next-generation sequencing of DNA from biopsy
specimen after disease progression. |
Table II
Gene mutational profile of lung
adenocarcinoma by next-generation sequencing of DNA from biopsy
specimen after disease progression.
| Genes | Mutation sites | Frequency, % | Amplification (copy
no.) |
|---|
| HER2 | | | 23.69 |
| PIGF | | | 3.6 |
| ACAD5B | G360A | 12.61 | |
| AXIN1 | T332S | 39.39 | |
| ARID1A | P65Rfs*36 | 72.87 | |
| CD79A | W34R | 25.52 | |
| CDKN2A | 151-1G>T | 50.78 | |
| CREBBP | D539G | 19.31 | |
| FAT1 | G1318 | 24.81 | |
| FLT3 | W105C | 23.4 | |
| IRF4 | G314V | 47.34 | |
| KIT | A621S | 36.94 | |
| KEAP1 | R234P | 75.63 | |
| KMT2A | G2409V | 71.49 | |
| NOTCH1 | P2199S | 44.87 | |
| PIK3CA | E218Tfs*7 | 13.17 | |
| PRKCI | I124L | 14.52 | |
| PRKCI | Y125C | 14.61 | |
| RPTOR | M280V | 35.67 | |
| TP53 | G154V | 67.9 | |
| WT1 | G216R | 57.51 | |
Discussion
Pyrotinib, a pan-ErbB blocker, has shown activity
against EGFR (IC50, 5.6±3.9 nM), HER2 (IC50,
8.1±2.3 nM) and ErbB4(11). By
covalently binding with ATP-binding sites of intracellular kinase
regions, pyrotinib inhibits the formation of homologous/heterodimer
and auto-phosphorylation of HER family members, thus blocking the
activation of the RAS/RAF/MEK/MAPK and PI3K/AKT signaling pathways
and restricting tumor development (11). Based on the results of a phase II
trial, the drug was conditionally approved in August 2018 in China
for combination with capecitabine in patients with HER2-positive
advanced or metastatic breast cancer previously treated with
anthracyclines or taxane-based chemotherapy (12); however, it has not yet been
approved by the US Food and Drug Administration. Second-line
therapy with pyrotinib was found to be superior to lapatinib in
patients with breast cancer previously treated with trastuzumab
[progression-free survival (PFS): 18.1 vs. 5.6 months,
respectively], probably due to the fact that pyrotinib targets more
signaling molecules compared with lapatinib, which only acts on
EGFR and HER2 (13,14). However, to the best of our
knowledge, whether pyrotinib is effective in patients with lung
cancer with HER2 amplification has not been reported to date.
Experience from breast cancer has shown that some patients who were
treated with trastuzumab or lapatinib developed primary resistance
to anti-HER2 therapy. Therefore, clinically, in patients with
breast cancer, both trastuzumab and lapatinib are administered in
combination with chemotherapy in most cases, except for trastuzumab
monotherapy in the adjuvant setting. The present case had HER2
amplification with ErbB4 mutation. The patient also harbored a
F616L mutation, which is not a classic activating mutation and is
unlikely to be responsible for the responsiveness to HER2-targeted
therapy. The patient was treated with four different HER2
inhibitors and at least five chemotherapeutic regimens. However,
chemotherapy was ineffective during the first- and second-line
treatment. The patient achieved PR with lapatinib, but developed
primary resistance to trastuzumab. However, pyrotinib was
effective, leading to CR, particularly after resistance to
lapatinib. Afatinib, which is also a pan-ErbB (EGFR/HER2/ErbB4)
inhibitor, has been reported to be effective in a case of bladder
cancer with HER2 amplification (15). After progression, the patient in
the present case was switched to afatinib. However, the disease
rapidly progressed, indicating that afatinib may not be suitable
for patients resistant to pyrotinib. Diarrhea is the most common
adverse effect observed with tyrosine kinase inhibitors targeting
EGFR/HER2. The incidence of diarrhea in patients treated with
pyrotinib plus capecitabine in breast cancer was 96.9%, mainly
grade 1-2, with 15.4% of patients developing grade 3 diarrhea
(14). The main adverse effect
experienced by our patient was also diarrhea, which indicates that
the adverse effects of pyrotinib in patients with lung cancer may
be similar to those in patients with breast cancer.
HER2 amplification has been implicated as an
oncogenic driver in lung cancers by The Cancer Genome Atlas
(16). Although therapy targeting
HER2 amplification or protein overexpression in lung cancer has
long been explored, it is far less effective compared with breast
cancer, and less effective compared with lung cancer with HER2
mutation. The objective response rate (ORR) of trastuzumab plus
chemotherapy in lung cancer with HER2 amplification has been
reported to be lower compared with that of chemotherapy alone
(17,18). Other new agents, including
trastuzumab plus pertuzumab [ORR: 12.5% (2/16)] (19) and trastuzumab emtansine [ORR: 0
(0/8); 20% (4/20)] (20,21) exhibited low efficiency in patients
with HER2 amplification or overexpression [IHC (3+), FISH (+), or
NGS (copy number increased)]. The pan-HER blocker dacomitinib was
also found to be ineffective in lung cancer with HER2 amplification
(0/4) (22). The use of other
tyrosine kinase inhibitors, including lapatinib, niratinib,
afatinib and pyrotinib, have not been reported in NSCLC. Anti-HER2
therapy targeting HER2 amplification in lung cancer has been
characterized by low efficacy, for which there are possibly two
main reasons: First, the HER2 signaling pathway in lung cancer is
more complicated compared that in breast cancer. As HER2 does not
have a known endogenous ligand for its extracellular domain, it
activates downstream signaling pathways by forming heterodimers
with other ErbB family receptors (23). Other ErbB family members (for
example, ErbB4 mutation in this case) affect HER2 signaling, and
targeting a single signaling molecule is not sufficient in lung
cancer with HER2 amplification. Second, concurrent gene mutations
in lung cancer contribute to the poor efficacy of HER2 therapy. In
addition to HER2 amplification, the present case had a TP53
mutation in the early stage of therapy and a KEAP1 mutation in the
resistance stage. Concomitant TP53 or KEAP1 mutations have been
reported in patients with EGFR mutations (24-26).
The PFS of patients with these mutations receiving EGFR tyrosine
kinase inhibitors was significantly shorter compared with that of
patients without mutations. It was hypothesized that TP53 mutation
contributed to the shorter TTP in our patient in the first- and
subsequent second-line anti-HER2 therapy. Thus, HER2 amplification
or overexpression as a single parameter is an insufficient
predictive biomarker in NSCLC.
There are currently no reports on the resistance
mechanism of pan-ErbB blockers in HER2-amplified NSCLC. The
resistance mechanism of lapatinib and niratinib in HER2-amplified
breast cancer has been reported to be associated with bypass
activation, such as HER2 mutations and mutations in other ErbB
family genes (27). In the present
case, the inferred resistance mechanisms are as follows: The first
is the insufficient ability to inhibit the HER2 signaling pathway,
as the copy number of HER2 was higher than before and there was a
mutation of PIK3CA. The second is bypass activation, which includes
mutations in CDKN2A, Notch1 and KEAP1. However, it does not appear
to be associated with mutations in the ErbB family gene.
Our current case suggests that the development of
novel drugs may offer new and effective therapeutic regimens for
lung cancer with HER2 amplification. Clinical trials or studies
should be conducted to investigate the efficacy of novel
therapeutic regimens for lung cancer with HER2 amplification.
Supplementary Material
Immunohistochemistry of surgical
specimen showing HER2 overexpression (3+); magnification, x20.
Treatment regimen summary.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to protection of
patient privacy and biosecurity reasons, but are available from the
corresponding author on reasonable request.
Authors' contributions
KG and XY made the diagnosis and wrote the
manuscript. YY, HH and XK created the tables and figures. KG and YY
treated and followed up the patient, and they have also seen and
can confirm the authenticity of all the raw data. All the authors
have read, carefully revised and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The present case report was approved by the Ethics
Committee of Daping Hospital, Army Medical University, Chongqing,
China [no. 2019(116)]. Written informed consent was obtained from
the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case details and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri
S, Lau C, Zaidinski M, Paik PK, Zakowski MF, Kris MG and Ladanyi M:
Prevalence, clinicopathologic associations, and molecular spectrum
of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas.
Clin Cancer Res. 18:4910–4918. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pillai RN, Behera M, Berry LD, Rossi MR,
Kris MG, Johnson BE, Bunn PA, Ramalingam SS and Khuri FR: HER2
mutations in lung adenocarcinomas: A report from the lung cancer
mutation consortium. Cancer. 123:4099–4105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li BT, Ross DS, Aisner DL, Chaft JE, Hsu
M, Kako SL, Kris MG, Varella-Garcia M and Arcila ME: HER2
amplification and HER2 mutation are distinct molecular targets in
lung cancers. J Thorac Oncol. 11:414–419. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stephens P, Hunter C, Bignell G, Edkins S,
Davies H, Teague J, Stevens C, O'Meara S, Smith R, Parker A, et al:
Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature.
431:525–526. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Robichaux JP, Elamin YY, Vijayan RSK,
Nilsson MB, Hu L, He J, Zhang F, Pisegna M, Poteete A, Sun H, et
al: Pan-cancer landscape and analysis of ERBB2 mutations identifies
poziotinib as a clinically active inhibitor and enhancer of T-DM1
activity. Cancer Cell. 36:444–57.e7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Y, Jiang T, Qin Z, Jiang J, Wang Q,
Yang S, Rivard C, Gao G, Ng TL, Tu MM, et al: HER2 exon20
insertions in non-small-cell lung cancer are sensitive to the
irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib.
Ann Oncol. 30:447–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peters S, Curioni-Fontecedro A, Nechushtan
H, Shih JY, Liao WY, Gautschi O, Spataro V, Unk M, Yang JCH,
Lorence RM, et al: Activity of afatinib in heavily pretreated
patients with ERBB2 mutation-positive advanced NSCLC: Findings from
a global named patient use program. J Thorac Oncol. 13:1897–1905.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oh DY and Bang YJ: HER2-targeted
therapies-a role beyond breast cancer. Nat Rev Clin Oncol.
17:33–48. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jeong Y, Hellyer JA, Stehr H, Hoang NT,
Niu X, Das M, Padda SK, Ramchandran K, Neal JW, Wakelee H and Diehn
M: Role of KEAP1/NFE2L2 mutations in the chemotherapeutic response
of patients with non-small cell lung cancer. Clin Cancer Res.
26:274–281. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang LZ, Lei CC, Zhao YP, Sun HW, Yu QH,
Yang EJ and Zhan X: MicroRNA-34c-3p target inhibiting NOTCH1
suppresses chemosensitivity and metastasis of non-small cell lung
cancer. J Int Med Res. 48(300060520904847)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J,
Luo Y, Xing P, Lan B, Li M, et al: Phase I study and biomarker
analysis of pyrotinib, novel irreversible Pan-ErbB receptor
tyrosine kinase inhibitor, in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Clin Oncol.
35:3105–3112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Blair HA: Pyrotinib: First global
approval. Drugs. 78:1751–1755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gourd E: Pyrotinib versus lapatinib in
HER2-positive breast cancer. Lancet Oncol. 20(e562)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu
Y, Li H, Yu S, Feng J, Wang S, et al: Pyrotinib or lapatinib
combined with capecitabine in HER2-positive metastatic breast
cancer with prior taxanes, anthracyclines, and/or trastuzumab: A
randomized, phase II study. J Clin Oncol. 37:2610–2619.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Choudhury NJ, Campanile A, Antic T, Yap
KL, Fitzpatrick CA, Wade JL III, Karrison T, Stadler WM, Nakamura Y
and O'Donnell PH: Afatinib activity in platinum-refractory
metastatic urothelial carcinoma in patients with ERBB alterations.
J Clin Oncol. 34:2165–2171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cancer Genome Atlas Research Network.
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Langer CJ, Stephenson P, Thor A, Vangel M
and Johnson DH: Eastern Cooperative Oncology Group Study 2598.
Trastuzumab in the treatment of advanced non-small-cell lung
cancer: is there a role? focus on eastern cooperative oncology
group study 2598. J Clin Oncol. 22:1180–1187. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gatzemeier U, Groth G, Butts C, Van
Zandwijk N, Shepherd F, Ardizzoni A, Barton C, Ghahramani P and
Hirsh V: Randomized phase II trial of gemcitabine-cisplatin with or
without trastuzumab in HER2-positive non-small-cell lung cancer.
Ann Oncol. 15:19–27. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hainsworth JD, Meric-Bernstam F, Swanton
C, Hurwitz H, Spigel DR, Sweeney C, Burris H, Bose R, Yoo B, Stein
A, et al: Targeted therapy for advanced solid tumors on the basis
of molecular profiles: Results from mypathway, an open-label, phase
IIa multiple basket study. J Clin Oncol. 36:536–542.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hotta K, Aoe K, Kozuki T, Ohashi K,
Ninomiya K, Ichihara E, Kubo T, Ninomiya T, Chikamori K, Harada D,
et al: A phase II study of trastuzumab emtansine in HER2-positive
non-small cell lung cancer. J Thorac Oncol. 13:273–279.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peters S, Stahel R, Bubendorf L, Bonomi P,
Villegas A, Kowalski DM, Baik CS, Isla D, De Castro Carpeno J,
Garrido P, et al: Trastuzumab emtansine (T-DM1) in patients with
previously treated HER2-overexpressing metastatic non-small cell
lung cancer: Efficacy, safety, and biomarkers. Clin Cancer Res.
25:64–72. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kris MG, Camidge DR, Giaccone G, Hida T,
Li BT, O'Connell J, Taylor I, Zhang H, Arcila ME, Goldberg Z and
Jänne PA: Targeting HER2 aberrations as actionable drivers in lung
cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor
dacomitinib in patients with HER2-mutant or amplified tumors. Ann
Oncol. 26:1421–1427. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roskoski R Jr: Small molecule inhibitors
targeting the EGFR/ErbB family of protein-tyrosine kinases in human
cancers. Pharmacol Res. 139:395–411. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Labbé C, Cabanero M, Korpanty GJ, Tomasini
P, Doherty MK, Mascaux C, Jao K, Pitcher B, Wang R, Pintilie M, et
al: Prognostic and predictive effects of TP53 co-mutation in
patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung
Cancer. 111:23–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hou H, Qin K, Liang Y, Zhang C, Liu D,
Jiang H, Liu K, Zhu J, Lv H, Li T and Zhang X: Concurrent TP53
mutations predict poor outcomes of EGFR-TKI treatments in Chinese
patients with advanced NSCLC. Cancer Manag Res. 11:5665–5675.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hellyer JA, Stehr H, Das M, Padda SK,
Ramchandran K, Neal JW, Diehn M and Wakelee HA: Impact of
KEAP1/NFE2L2/CUL3 mutations on duration of response to EGFR
tyrosine kinase inhibitors in EGFR mutated non-small cell lung
cancer. Lung Cancer. 134:42–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xuhong JC, Qi XW, Zhang Y and Jiang J:
Mechanism, safety and efficacy of three tyrosine kinase inhibitors
lapatinib, neratinib and pyrotinib in HER2-positive breast cancer.
Am J Cancer Res. 9:2103–2119. 2019.PubMed/NCBI
|