Introduction
Small cell lung cancer (SCLC) is an aggressive lung
cancer disease with a dismal prognosis in advanced stages. It is
highly responsive to chemotherapy and radiotherapy but usually
recurs within a few months in patients with extensive-stage SCLC.
Recently, a first-line platinum plus etoposide-based chemotherapy
with atezolizumab improved outcomes in first-line settings
(1,2). In relapsed patients, in particular for
resistant/refractory cases (during or within three months from the
last day of upfront therapy), the progression of disease occurs
rapidly with second-line agents (3). Topotecan, a camptothecin analog, has
been demonstrated to increase overall survival (OS) compared with
the best supportive care alone and results in greater symptom
management relative to polychemotherapy regimens (3,4). The
primary toxicities of TOPO are hematologic, with most patients
experiencing grade [G]3 or 4 neutropenia, anemia, or
thrombocytopenia. Recently, a phase III study compared TOPO alone
with a combination of carboplatin/etoposide as a rechallenge
schedule in patients with sensitive relapsed SCLC (5). Although a combination did not increase
median OS, it provided a two-month benefit in progression-free
survival (PFS) and showed similar rates of severe (G3-4)
hematological toxicities. Despite platinum-based combinations may
have a role in platinum-sensitive SCLC TOPO remain one of the
referent treatment in relapsed disease. Recently it has been
compared with platinum-etoposide-based doublets and triplets so it
is reasonable to consider TOPO a pivotal comparator as second line
agent.
To update the current state of the art, we performed
a systematic review and meta-analysis of second-line studies
comparing TOPO with other agents in patients with relapsed
SCLC.
Materials and methods
Identification of trials and inclusion
criteria
Trials were identified through a comprehensive
systematic search of Pubmed, EMBASE, and The Cochrane Library from
inception, up to September 12th, 2020. All randomized clinical
trials reporting on SCLC patients that examined the efficacy of
TOPO compared with other agents or best supportive care as
second-line therapy for relapsed (sensitive or
refractory/resistant) disease and were published in the English
language were identified. The search terms used to identify studies
for the meta-analysis were: (‘small-cell lung carcinoma’[MeSH
Major Topic] OR ‘small-cell lung cancer’[All Fields] OR ‘small-cell
lung carcinoma’[All Fields] OR ‘sclc’[All Fields]) AND
(‘recurrence’[MeSH Terms] OR ‘recurrence’[All Fields] OR
‘relapse’[All Fields] OR ‘relapses’[All Fields] OR ‘relapsing’[All
Fields] OR ‘relapsed’[All Fields] OR ‘relapser’[All Fields] OR
‘relapsers’[All Fields] OR ‘previously treated’[All Fields] OR
(‘recurrance’[All Fields] OR ‘recurrence’[MeSH Terms] OR
‘recurrence’[All Fields] OR ‘recurrences’[All Fields] OR
‘recurrencies’[All Fields] OR ‘recurrency’[All Fields] OR
‘recurrent’[All Fields] OR ‘recurrently’[All Fields] OR
‘recurrents’[All Fields]) OR ‘second line’[All Fields] OR
(‘pretreat’[All Fields] OR ‘pretreated’[All Fields] OR
‘pretreating’[All Fields] OR ‘pretreatment’[All Fields] OR
‘pretreatments’[All Fields])) AND (‘topotecan’[MeSH Terms] OR
‘topotecan’[All Fields] OR (‘topotecan’[MeSH Terms] OR
‘topotecan’[All Fields])). Studies were excluded if they 1)
were comparative observational series, 2) were phase 1 trials, and
3) compared different schedules or administration routes of
TOPO.
Data extraction and risk of bias
assessment
Two review authors (A.G. and F.P.) determined
eligibility by reading the abstract of each study identified by the
search. A third author (A.L.) independently read these studies and
reached an agreement for trial inclusion. The primary outcome was
OS defined as any death that occurred from the randomization date.
The secondary outcomes were progression-free survival (PFS),
overall response rate (ORR) and severe (grade [G] 3-4) adverse
hematological events (anemia, thrombocytopenia, febrile neutropenia
[FN] and neutropenia). Type of study, number of patients, median
age, rate of PS 0-1 patients included, treatment setting, schedule
of TOPO and the experimental arm, the HR for OS and PFS for TOPO
vs. experimental arms, rate of overall response defined as the sum
of partial and complete response, and rate of G3-4 hematological
toxicities were extracted by two authors (F.P. and A.G.)
independently from each study. For each trial, we assessed the risk
of bias (‘low risk,’ ‘some concerns,’ or ‘high risk’) in the
overall effect of TOPO on the outcome and serious adverse events
using version 2 of the Cochrane Risk of Bias Assessment Tool
(6). Risk of bias assessments were
carried out independently by three of the investigators (A.G., F.P.
and A.L.), with disagreements resolved through discussion. We used
the Grading of Recommendations Assessment, Development and
Evaluation (GRADE) approach to assess the certainty of the evidence
that TOPO increased OS compared with other agents in patients with
relapsed SCLC (7).
Statistical analysis
We classified the trials according to the setting of
the intervention (refractory or sensitive disease). The
convention-sensitive disease was considered, as progression
occurred at least three months after the end of first-line,
platinum-based chemotherapy. The primary analysis was an inverse
variance-weighted fixed or random-effect meta-analysis of HRs for
OS and PFS and an inverse variance-weighted fixed or random-effect
analysis using risk ratios (RRs) for ORR and rates of toxicity. We
used Parmar's method if HRs were not reported in the study
(8). We quantified inconsistency in
associations among the trials using the I2 statistic and
derived P-values for heterogeneity using the Cochran Q statistic.
We report precise P-values. A P-value <0.05 denoted statistical
significance. A meta-regression analysis was performed to examine
the potential effect of the number of sensitive SCLC cases and
death. All analyses were conducted using RevMan statistical
software version 5.3 (Copenhagen: The Nordic Cochrane Centre, The
Cochrane Collaboration, 2014).
Results
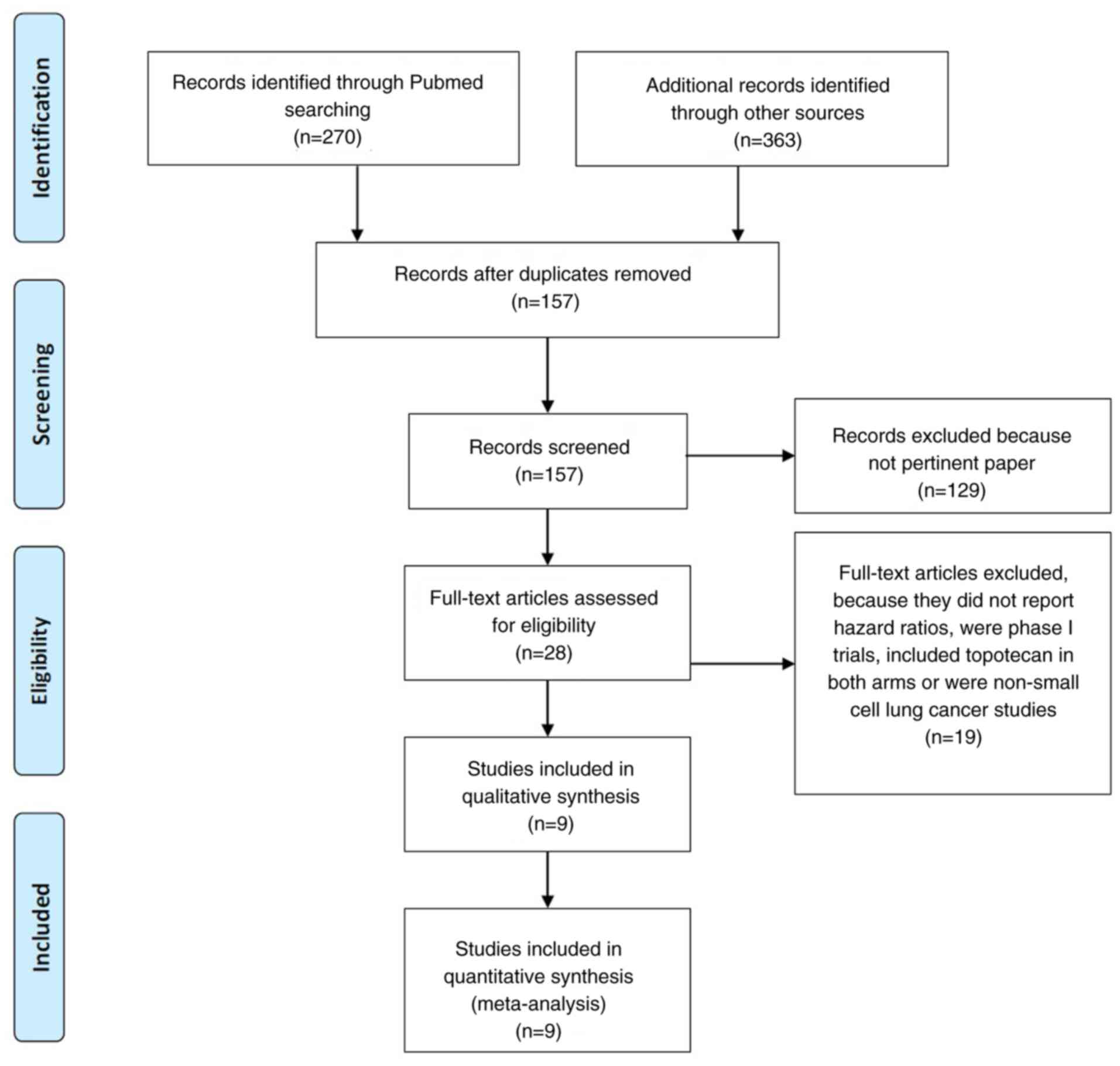
Of 633 articles that met the preliminary criteria,
we found nine eligible articles (4,5,9-14),
which included five phase 3 and four phase 2 randomized trials that
compared TOPO with other regimens (Fig.
1 and Table I). Overall, 1689
patients were included in these studies. In most of the studies,
patients had a performance status of 0 or 1. The mean or median age
was 64 years. Usually, the sensitive disease was considered when it
recurs after 90 days. Intravenous, three-weekly TOPO was used in
all studies except one where oral formulation was used. Combination
chemotherapy was the experimental arm in n=3 trials, single agent
in n=5, while best supportive care was the comparator arm in n=1
study. Sensitive disease ranged from 45 to 100% of included
patients (median, 57.3%).
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| First author/s,
year | Type of study | Patients, n | Setting (%) | PS 0-1, % | Median age,
years | Topotecan schedule,
mg/m2 | Exp arm | OS, months (Ctr vs.
Exp) | PFS, months (Ctr vs.
Exp) | ORR, % (Ctr vs.
Exp) | G3-4 anemia, % (Ctr
vs. Exp) | G3-4 thrombocy
topenia, % (Ctr vs. Exp) | FN, % (Ctr vs.
Exp) | G3-4 neutropenia, %
(Ctr vs. Exp) | Risk of bias | (Refs.) |
|---|
| Baize, 2020 | Phase 3 | 162 | Sensitive (100) | 92.5 | 64.5 | Oral 2.3 d1-5
q21 | Carboplatin +
Etoposide | - | 2.7 vs. 4.7 | 25 vs. 49 | 21 vs. 24 | 36 vs. 31 | 13 vs. 6 | 24 vs. 13 | Low | (5) |
| Chiappori, 2016 | Phase 2 | 44 | Refractory (54.6);
sensitive (45.4) | 36 | 64 | iv 1.5 d1-5 q21 | Linsitinib | 5.3 vs. 3.4 | 3 vs. 1.2 | 13.3 vs. 0 | 7.1 vs. 3.6 | 28.6 vs. 7.1 | - | 28.6 vs. NR | Uncertain | (14) |
| Evans, 2015 | Phase 2 | 179 | Refractory (49);
sensitive (51) | 99.4 | 61 | iv 1.5 d1-5 q21 | Cabazitaxel | 6.8 vs. 5.2 | 3 vs. 1.4 | 10.1 vs. 0 | 26.1 vs. 3.4 | 45.5 vs. 4.5 | 22.7 vs. 18 | 78.4 vs. 56.8 | Moderate | (13) |
| Goto, 2016 | Phase 3 | 180 | Sensitive (100) | 97.2 | 64 | iv 1 d1-5 q21 | Cisplatin + Etoposide
+ Irinotecan | 12.5 vs. 18.2 | 3.6 vs. 5.7 | 25.5 vs. 33.8 | 27 vs. 85 | 28 vs. 41 | 7 vs. 31 | 85 vs. 84 | Low | (12) |
| Inoue, 2008 | Phase 2 | 59 | Refractory (39);
sensitive (61) | 85 | 69 | iv 1 d1-5 q21 | Amrubicina | 8.4 vs. 8.4 | 2.2 vs. 3.5 | 13 vs. 38 | 30 vs. 21 | 40 vs. 28 | 3 vs. 14 | 87 vs. 93 | Moderate | (11) |
| Jotte, 2011 | Phase 2 | 76 | Sensitive (100) | 89.4 | 65.5 | iv 1.5 d1-5 q21 | Amrubicina | 7.6 vs. 9.2 | 3.3 vs. 4.5 | 15.4 vs. 44 | 30 vs. 25 | 61 vs. 39 | 9 vs. 10 | 78 vs. 71 | Moderate | (10) |
| O'Brien, 2006 | Phase 3 | 141 | Refractory (54);
sensitive (46) | 70.2 | 59.2 | Oral 2.3 d1-5
q21 | BSC | 6.4 vs. 3.4 | 4 vs. NR (TTP) | 7 vs. 0 | 25 vs. 0 | 38 vs. 0 | 3 vs. 0 | 61 vs. 0 | Low | (3) |
| Von Pawel, 1999 | Phase 3 | 211 | Sensitive (100) | 78.6 | - | iv 1.5 d1-5 q21 | Cyclophosp hamide +
Doxorubicin + Vincristine (CAV) | 6.2 vs. 6.1 | 3.3 vs. 3 (TTP) | 24.3 vs. 18.3 | 42.3 vs. 19.8 | 57.6 vs. 14.9 | - | 88.5 vs. 86.9 | Low | (4) |
| Von Pawel, 2014 | Phase 3 | 637 | Refractory (46.4);
sensitive (53.6) | 97.9 | 61.5 | iv 1.5 d1-5
q21 | Amrubicina | 7.8 vs. 7.5 | 3.5 vs. 4.1 | 16.9 vs. 31.1 | 31 vs. 16 | 54 vs. 21 | 3 vs. 10 | 54 vs. 41 | Low | (9) |
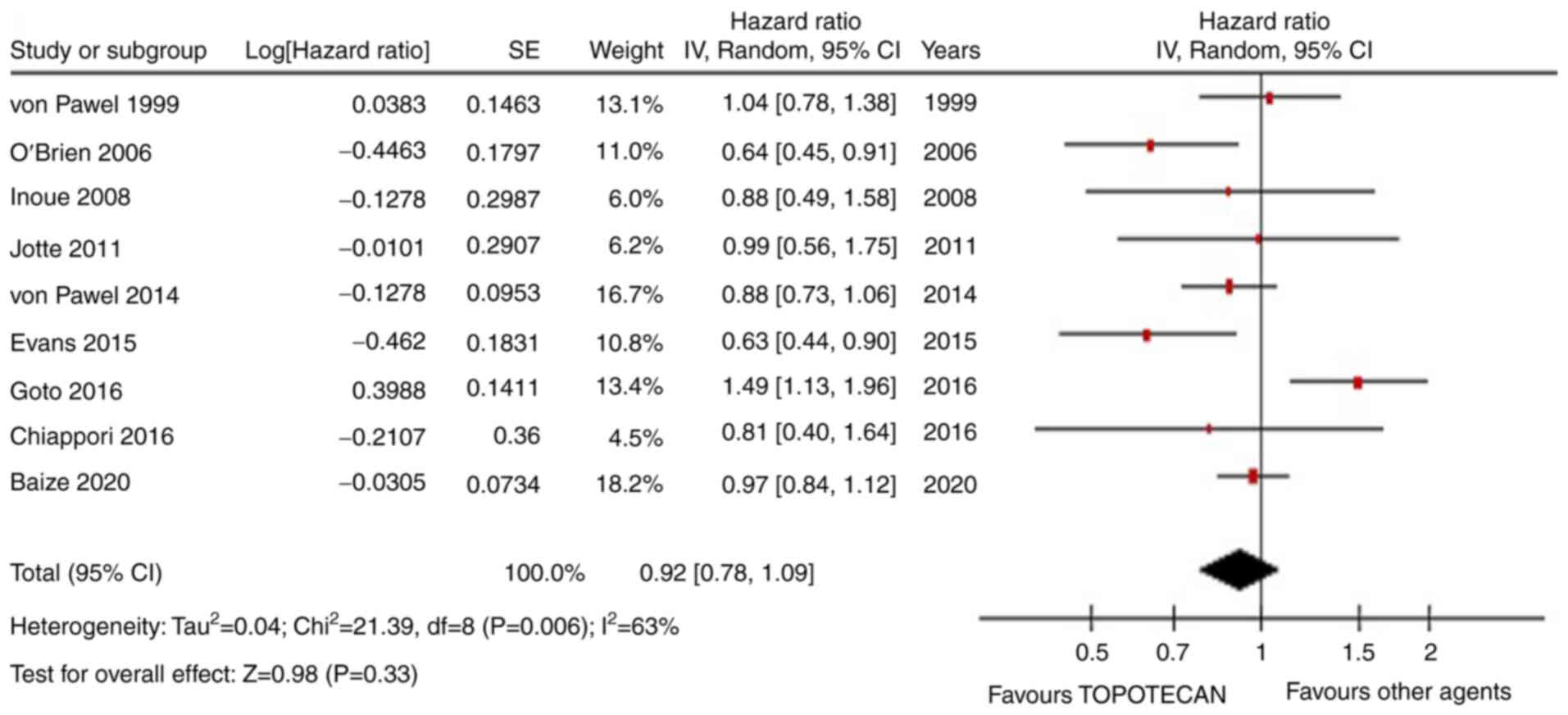
OS was not improved by TOPO with respect to other
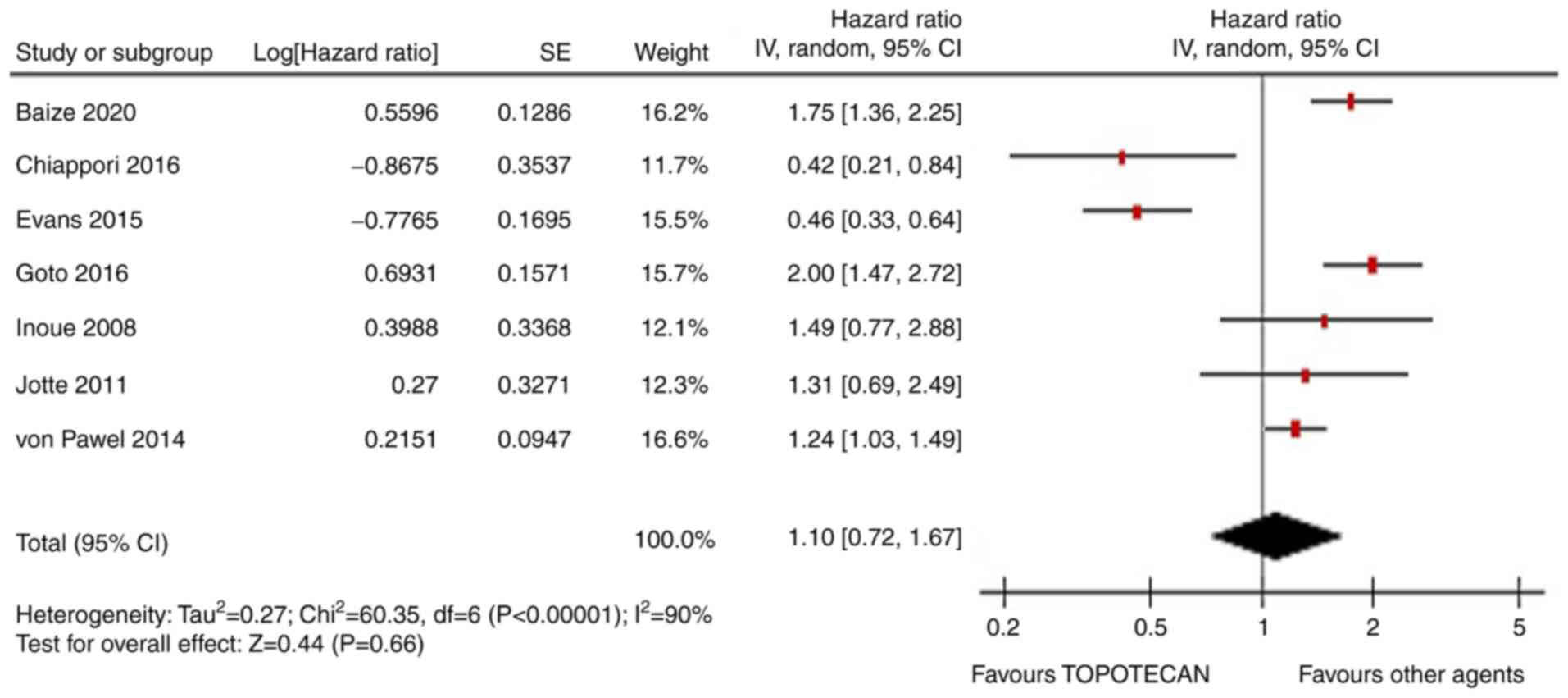
therapies (HR=0.92, 95% CI, 0.78-1.09; P=0.33; Fig. 2). Similarly, PFS was similar in the
two arms (HR=1.1, 95% CI, 0.72-1.67; P=0.66; Fig. 3). The ORR was not statistically
higher with non-TOPO agents (RR=1.53, 95% CI, 0.95-2.48; Fig. S1). In the meta-regression analysis,
the rate of sensitive SCLC patients enrolled in each trial was
significantly associated with OS (P=0.01). This means that agents
different from TOPO are better in exquisite sensitive disease. In
subgroup analysis, combination chemotherapy was associated with a
better PFS but not OS or ORR than TOPO alone (HR=1.85, 95% CI,
1.52-2.24; P<0.01). The rates of G3-4 anemia, FN and neutropenia
were similar. Instead, G3-4 thrombocytopenia was inferior in the
experimental arms (RR=0.44, 95% CI, 0.26-0.74; P<0.01) (Fig. S2, Fig.
S3, Fig. S4 and Fig. S5). In the primary analysis, both
Begg's and Egger's tests were not significant for publication bias.
There is little evidence that TOPO has a similar outcome to other
agents in a mixed population of SCLC patients. However, we can
recommend that in platinum-sensitive disease, combination agents
are probably better and similarly or even less toxic than TOPO.
Discussion
SCLC is a subtype of lung cancer burdened by high
mortality. The treatment of advanced SCLC has not changed over the
years due to the high aggressiveness and refractoriness of the
disease. Recently, two studies in a first-line setting with
chemotherapy plus anti-programmed cell death ligand-1 (PDL-1)
therapy showed an improved OS vs. chemo alone (1,2).
Most patients progressed after first-line treatment,
and the best second-line strategy remains to be elucidated.
Topotecan is the only drug approved for second-line treatment due
to the head-to-head comparison with
cyclophosphamide-doxorubicin-vincristine triplet (CAV regimen) and
a placebo-controlled trial with oral administration. However, there
is still a debate about the magnitude of its clinical benefit, and
this meta-analysis shows how the use of drugs other than TOPO could
give comparable outcomes. Based on our analysis, TOPO performs
worse than other drugs in sensitive diseases, and combination
therapy gives better response rates compared with a single agent.
TOPO, as a second-line strategy, remains controversial, with
conflicting evidence regarding its superiority in terms of
survival, toxicity and response rates. TOPO seems to give less
thrombocytopenia, while other toxicities are similar to combination
therapies across the studies.
As second-line treatments in relapsed small-cell
lung cancer are usually considered ‘palliative,’ the question
remains whether using a standard drug is still a choice to be
considered in young and fit older patients. Based on the
literature, discouraging results have come from all agents tested,
and superiority data do not support the use of TOPO over the others
in common clinical practice. Even if combination regimens have more
toxicity, the benefits are small and not durable. The rechallenge
strategy with etoposide + platinum-based chemotherapy, however, at
least in sensitive diseases, is a reasonable choice to have better
response rates and progression-free survival with manageable
toxicities (5). In this setting,
the combination chemotherapy has shown high response rates
(40-55%). For patients with refractory/resistant disease and a good
performance status, inclusion in clinical trials is the preferred
choice. Other agents as camptothecin analogues (irinotecan) have
been tested in progressive disease (Table II). Despite a relatively high
number of tumor responses in particular for combinations (range
12-52.9%) and median OS up to 10 months, neutropenia was consistent
and this agent is not currently approved for use in western
countries (15-24).
 | Table IIIrinotecan studies in pretreated
small cell lung cancer. |
Table II
Irinotecan studies in pretreated
small cell lung cancer.
| First author/s,
year | Type of study | Patients, n | Setting (%) | PS 0-1, % | Median age,
years | Schedule,
mg/m2 | OS, months | PFS, months | ORR, % | G3-4 anemia, % | G3-4 thrombocyt
openia,% | FN,% | G3-4 neutropenia,
% | (Refs.) |
|---|
| Kondo, 2018 | Phase 2 | 30 | Sensitive (40);
resistant (60) | 100 | 67 | CPT-11 100d1, 8
q21 | 10.4 | 4.1 | 43 | 13.3 | 3.3 | 6.6 | 36.7 | (15) |
| Trafalis, 2016 | Phase 2 | 32 | Resistant
(100) | 82 | 63.5 | CPT-11 175 +
bevacizumab 7.5 mg/kg q14 | 6 | 3 | 25 | 0 | 0 | 3.5 | 7.1 | (16) |
| Xenidis, 2011 | Phase 2 | 31 | Refractory
(100) | 84 | 64 | CPT-11 125 + PLD 15
d1,14 q 28 | 3.16 | 1.86 | 12.9 | 3.2 | 0 | 6.5 | 0 | (17) |
| Ramalingam,
2010 | Phase 2 | 55 | Sensitive (51);
refractory (49) | 95 | 61 | CPT-11 50 d1,8 +
PAC 75 d1,8 q21 | 7.6 and
5.5a | 3 and
2a | 19.5 | 2 | 0 | 0 | 11 | (18) |
| Chen, 2009 | Phase 2 | 40 | Sensitive
(100) | 85 | 65 | CPT-11 150 + CBDCA
AUC5 d1 q 21 | 10 | - | 50 | 15 | 23 | 3 | 55 | (19) |
| Pallis, 2009 | Randomized phase
2 | 69 | Sensitive (52 vs.
35); refractory (47 vs. 64) | 85 vs. 94 | 60 vs. 65 | CPT-11 300 d8 + GEM
1000 d1, 8 q21 vs. CPT-11 300 d1 q21 | 8.6 and
5.7a (sensitive) vs.
8.6 and 3.8 (refractory) | 4.3 and 2.5 vs. 1.7
and 1.1 (sensitive and refractory) | 23.7 vs. 0 | 5.3 vs. 3.2 | 21 vs. 9.7 | 2.6 vs. 6.4 | 23 vs. 33 | (20) |
| Ohyanagi, 2008 | Phase 2 | 30 | Sensitive (66);
refractory (34) | 65 | 90 | CPT-11 150 q21 +
GEM 1000 d1,14 q21 | 14.4 | 3 | 36.7 | 3.3 | 3.3 | 0 | 43 | (21) |
| Rocha Lima,
2007 | Phase 2 | 71 | Sensitive (50);
refractory (50) | 85 | 62 | CPT-11 100 d1,8 +
GEM 1000 d1,8 q21 | 7.1 and
3.5a | 3.1. and 1.6 | 21 | 7.5 | 31 | 4.5 | 38.5 | (22) |
| Schuette, 2005 | Phase 2 | 35 | Sensitive (57);
refractory (43) | 86 | 63 | CPT-11 100 d1,8 +
GEM 1000 d1, 8 q 21 | 5.8 | 3.4 | 17 | 8.6 | 11.4 | 0 | 5.7 | (23) |
| Ichiki, 2003 | Phase 2 | 34 | Sensitive (71);
refractory (29) | 68 | 69 | CPT-11 80 d1,8,15 +
IFO 1,5 g/m2 d1-3 q 28 | 7.2 | - | 52.9 | 29.4 | 11.8 | 0 | 52.9 | (24) |
In conclusion, TOPO is not better than other agents
in relapsed SCLC, but there is weak evidence that it is inferior to
platinum-based combinations in sensitive diseases.
Supplementary Material
Forest plot for overall response rate
of topotecan vs. other agents. M-H, Mantel Haenszel; df, degree of
freedom; 95% CI, 95% confidence interval.
Forest plot for G3-4 anemia rate of
topotecan vs. other agents. M-H, Mantel Haenszel; df, degree of
freedom; 95% CI, 95% confidence interval.
Forest plot for febrile neutropenia
rate of topotecan vs. other agents. M-H, Mantel Haenszel; df,
degree of freedom; 95% CI, 95% confidence interval.
Forest plot for G3-4 neutropenia rate
of topotecan vs. other agents. M-H, Mantel Haenszel; df, degree of
freedom; 95% CI, 95% confidence interval.
Forest plot for G3-4 thrombocytopenia
rate of topotecan vs. other agents. M-H, Mantel Haenszel; df,
degree of freedom; 95% CI, 95% confidence interval.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FP, AG and AL contributed equally to manuscript
writing. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Horn L, Mansfield AS, Szczȩsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide versus platinum-etoposide
in first-line treatment of extensive-stage small-cell lung cancer
(CASPIAN): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk
Y, Cuceviá B, Juhasz G, Thatcher N, Ross GA, Dane GC and Crofts T:
Phase III trial comparing supportive care alone with supportive
care with oral topotecan in patients with relapsed small-cell lung
cancer. J Clin Oncol. 24:5441–5447. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Von Pawel J, Schiller JH, Shepherd FA,
Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer
MC, Depierre A, et al: Topotecan versus cyclophosphamide,
doxorubicin, and vincristine for the treatment of recurrent
small-cell lung cancer. J Clin Oncol. 17:658–667. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baize N, Monnet I, Greillier L, Geier M,
Lena H, Janicot H, Vergnenegre A, Crequit J, Lamy R, Auliac JB, et
al: Carboplatin plus etoposide versus topotecan as second-line
treatment for patients with sensitive relapsed small-cell lung
cancer: An open-label, multicentre, randomised, phase 3 trial.
Lancet Oncol. 21:1224–1233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guyatt GH, Oxman AD, Vist GE, Kunz R,
Falck-Ytter Y, Alonso-Coello P and Schünemann HJ: GRADE Working
Group. GRADE: An emerging consensus on rating quality of evidence
and strength of recommendations. BMJ. 336:924–926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Von Pawel J, Jotte R, Spigel DR, O'Brien
ME, Socinski MA, Mezger J, Steins M, Bosquée L, Bubis J, Nackaerts
K, et al: Randomized phase III trial of Amrubicin versus topotecan
as second-line treatment for patients with small-cell lung cancer.
J Clin Oncol. 32:4012–4018. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jotte R, Conkling P, Reynolds C, Galsky
MD, Klein L, Fitzgibbons JF, McNally R, Renschler MF and Oliver JW:
Randomized phase II trial of single-agent amrubicin or topotecan as
second-line treatment in patients with small-cell lung cancer
sensitive to first-line platinum-based chemotherapy. J Clin Oncol.
29:287–293. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Inoue A, Sugawara S, Yamazaki K, Maemondo
M, Suzuki T, Gomi K, Takanashi S, Inoue C, Inage M, Yokouchi H, et
al: Randomized phase II trial comparing amrubicin with topotecan in
patients with previously treated small-cell lung cancer: North
Japan lung cancer study group trial 0402. J Clin Oncol.
26:5401–5406. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goto K, Ohe Y, Shibata T, Seto T,
Takahashi T, Nakagawa K, Tanaka H, Takeda K, Nishio M, Mori K, et
al: Combined chemotherapy with cisplatin, etoposide, and irinotecan
versus topotecan alone as second-line treatment for patients with
sensitive relapsed small-cell lung cancer (JCOG0605): A
multicentre, open-label, randomised phase 3 trial. Lancet Oncol.
17:1147–1157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Evans TL, Cho BC, Udud K, Fischer JR,
Shepherd FA, Martinez P, Ramlau R, Syrigos KN, Shen L, Chadjaa M
and Wolf M: Cabazitaxel versus topotecan in patients with
small-cell lung cancer with progressive disease during or after
first-line platinum-based chemotherapy. J Thorac Oncol.
10:1221–1228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chiappori AA, Otterson GA, Dowlati A,
Traynor AM, Horn L, Owonikoko TK, Ross HJ, Hann CL, Abu Hejleh T,
Nieva J, et al: A randomized phase II study of linsitinib (OSI·906)
versus topotecan in patients with relapsed small·cell lung cancer.
Oncologist. 21:1163–1164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kondo R, Watanabe S, Shoji S, Ichikawa K,
Abe T, Baba J, Tanaka J, Tsukada H, Terada M, Sato K, et al: A
phase II study of irinotecan for patients with previously treated
small-cell lung cancer. Oncology. 94:223–232. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Trafalis DT, Alifieris C, Stathopoulos GP
and Sitaras N: Phase II study of bevacizumab plus irinotecan on the
treatment of relapsed resistant small cell lung cancer. Cancer
Chemother Pharmacol. 77:713–722. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xenidis N, Vardakis N, Varthalitis I,
Giassas S, Kontopodis E, Ziras N, Gioulbasanis I, Samonis G,
Kalbakis K and Georgoulias V: Α multicenter phase II study of
pegylated liposomal doxorubicin in combination with irinotecan as
second-line treatment of patients with refractory small-cell lung
cancer. Cancer Chemother Pharmacol. 68:63–68. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramalingam SS, Foster J, Gooding W, Evans
T, Sulecki M and Belani CP: Phase 2 study of irinotecan and
paclitaxel in patients with recurrent or refractory small cell lung
cancer. Cancer. 116:1344–1349. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen G, Huynh M, Fehrenbacher L, West H,
Lara PN Jr, Yavorkovsky LL, Russin M, Goldstein D, Gandara D and
Lau D: Phase II trial of irinotecan and carboplatin for extensive
or relapsed small-cell lung cancer. J Clin Oncol. 27:1401–1404.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pallis AG, Agelidou A, Agelaki S,
Varthalitis I, Pavlakou G, Gerogianni A, Papakotoulas P, Rapti A,
Chandrinos V, Christophyllakis C and Georgoulias V: A multicenter
randomized phase II study of the irinotecan/gemcitabine doublet
versus irinotecan monotherapy in previously treated patients with
extensive stage small-cell lung cancer. Lung Cancer. 65:187–191.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ohyanagi F, Horiike A, Okano Y, Satoh Y,
Okumura S, Ishikawa Y, Nakagawa K, Horai T and Nishio M: Phase II
trial of gemcitabine and irinotecan in previously treated patients
with small-cell lung cancer. Cancer Chemother Pharmacol.
61:503–508. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rocha-Lima CM, Herndon JE II, Lee ME, Lee
ME, Atkins JN, Mauer A, Vokes E and Green MR: Cancer and Leukemia
Group B. Phase II trial of irinotecan/gemcitabine as second-line
therapy for relapsed and refractory small-cell lung cancer: Cancer
and leukemia group B study 39902. Ann Oncol. 18:331–337.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schuette W, Nagel S, Juergens S, Bork I,
Wollschlaeger B, Schaedlich S and Blankenburg T: Phase II trial of
gemcitabine/irinotecan in refractory or relapsed small-cell lung
cancer. Clin Lung Cancer. 7:133–137. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ichiki M, Rikimaru T, Gohara R, Koga T,
Kawayama T, Matunami M, Oshita Y, Kamimura T and Aizawa H: Phase II
study of irinotecan and ifosfamide in patients with advanced
non-small cell lung cancer. Oncology. 64:306–311. 2003.PubMed/NCBI View Article : Google Scholar
|