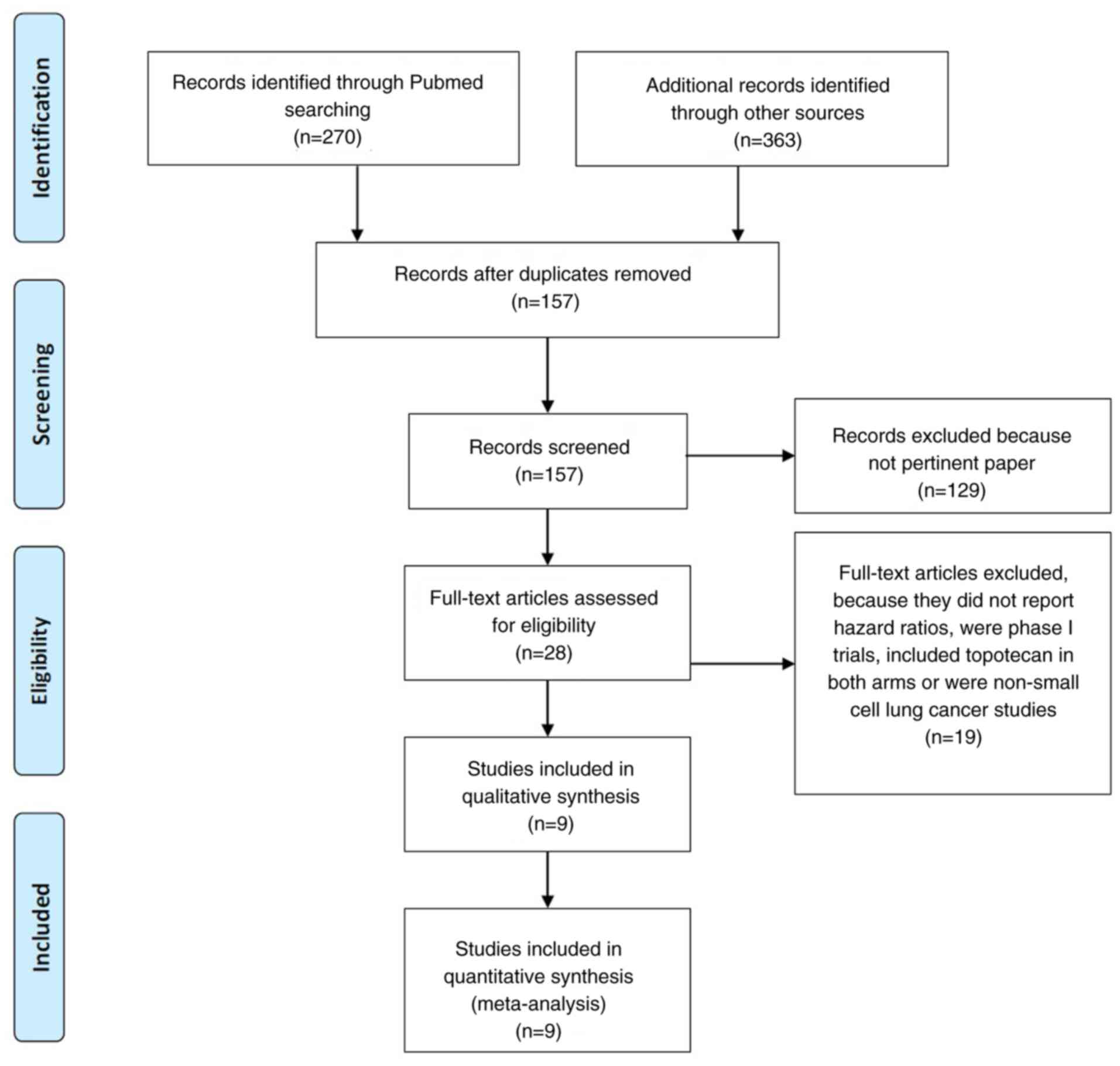
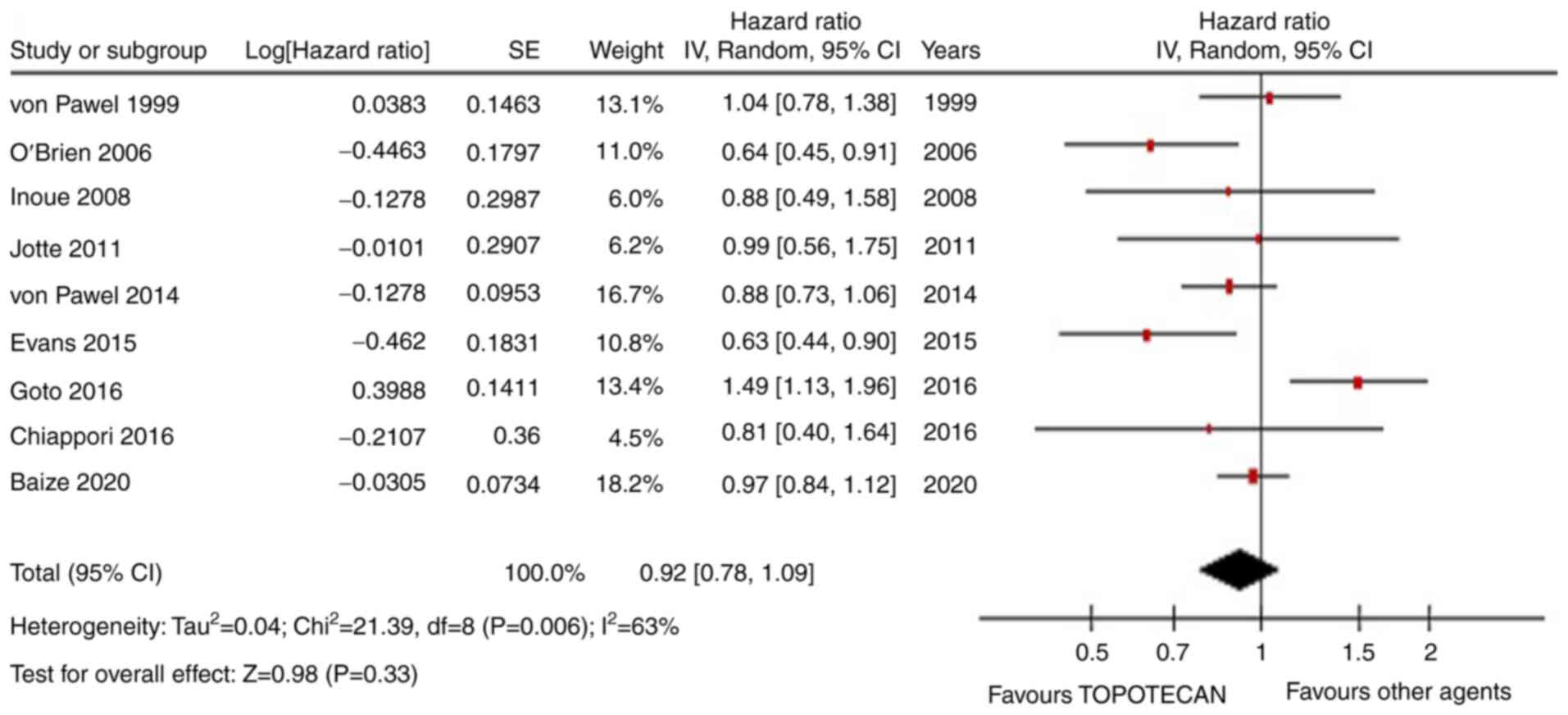
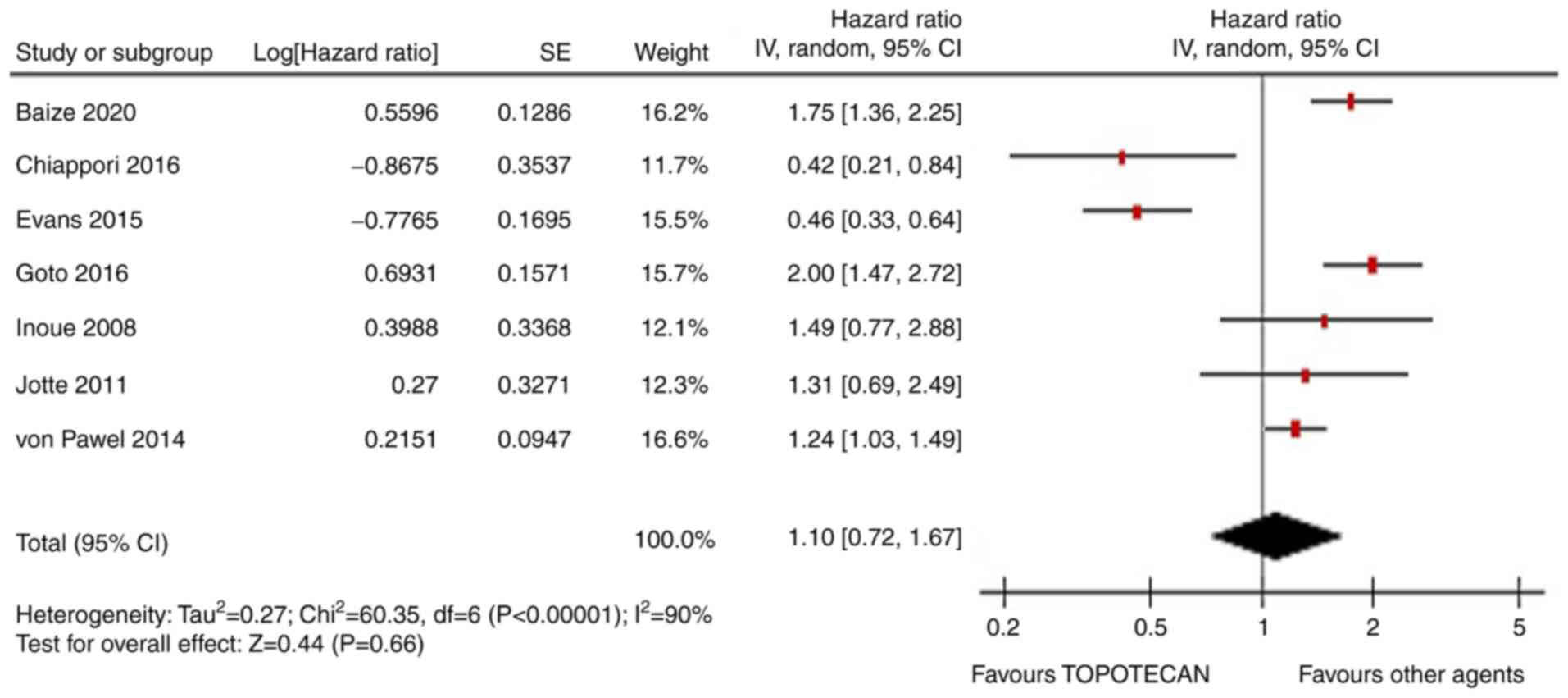
|
1
|
Horn L, Mansfield AS, Szczȩsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide versus platinum-etoposide
in first-line treatment of extensive-stage small-cell lung cancer
(CASPIAN): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk
Y, Cuceviá B, Juhasz G, Thatcher N, Ross GA, Dane GC and Crofts T:
Phase III trial comparing supportive care alone with supportive
care with oral topotecan in patients with relapsed small-cell lung
cancer. J Clin Oncol. 24:5441–5447. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Von Pawel J, Schiller JH, Shepherd FA,
Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer
MC, Depierre A, et al: Topotecan versus cyclophosphamide,
doxorubicin, and vincristine for the treatment of recurrent
small-cell lung cancer. J Clin Oncol. 17:658–667. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baize N, Monnet I, Greillier L, Geier M,
Lena H, Janicot H, Vergnenegre A, Crequit J, Lamy R, Auliac JB, et
al: Carboplatin plus etoposide versus topotecan as second-line
treatment for patients with sensitive relapsed small-cell lung
cancer: An open-label, multicentre, randomised, phase 3 trial.
Lancet Oncol. 21:1224–1233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guyatt GH, Oxman AD, Vist GE, Kunz R,
Falck-Ytter Y, Alonso-Coello P and Schünemann HJ: GRADE Working
Group. GRADE: An emerging consensus on rating quality of evidence
and strength of recommendations. BMJ. 336:924–926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Von Pawel J, Jotte R, Spigel DR, O'Brien
ME, Socinski MA, Mezger J, Steins M, Bosquée L, Bubis J, Nackaerts
K, et al: Randomized phase III trial of Amrubicin versus topotecan
as second-line treatment for patients with small-cell lung cancer.
J Clin Oncol. 32:4012–4018. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jotte R, Conkling P, Reynolds C, Galsky
MD, Klein L, Fitzgibbons JF, McNally R, Renschler MF and Oliver JW:
Randomized phase II trial of single-agent amrubicin or topotecan as
second-line treatment in patients with small-cell lung cancer
sensitive to first-line platinum-based chemotherapy. J Clin Oncol.
29:287–293. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Inoue A, Sugawara S, Yamazaki K, Maemondo
M, Suzuki T, Gomi K, Takanashi S, Inoue C, Inage M, Yokouchi H, et
al: Randomized phase II trial comparing amrubicin with topotecan in
patients with previously treated small-cell lung cancer: North
Japan lung cancer study group trial 0402. J Clin Oncol.
26:5401–5406. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goto K, Ohe Y, Shibata T, Seto T,
Takahashi T, Nakagawa K, Tanaka H, Takeda K, Nishio M, Mori K, et
al: Combined chemotherapy with cisplatin, etoposide, and irinotecan
versus topotecan alone as second-line treatment for patients with
sensitive relapsed small-cell lung cancer (JCOG0605): A
multicentre, open-label, randomised phase 3 trial. Lancet Oncol.
17:1147–1157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Evans TL, Cho BC, Udud K, Fischer JR,
Shepherd FA, Martinez P, Ramlau R, Syrigos KN, Shen L, Chadjaa M
and Wolf M: Cabazitaxel versus topotecan in patients with
small-cell lung cancer with progressive disease during or after
first-line platinum-based chemotherapy. J Thorac Oncol.
10:1221–1228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chiappori AA, Otterson GA, Dowlati A,
Traynor AM, Horn L, Owonikoko TK, Ross HJ, Hann CL, Abu Hejleh T,
Nieva J, et al: A randomized phase II study of linsitinib (OSI·906)
versus topotecan in patients with relapsed small·cell lung cancer.
Oncologist. 21:1163–1164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kondo R, Watanabe S, Shoji S, Ichikawa K,
Abe T, Baba J, Tanaka J, Tsukada H, Terada M, Sato K, et al: A
phase II study of irinotecan for patients with previously treated
small-cell lung cancer. Oncology. 94:223–232. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Trafalis DT, Alifieris C, Stathopoulos GP
and Sitaras N: Phase II study of bevacizumab plus irinotecan on the
treatment of relapsed resistant small cell lung cancer. Cancer
Chemother Pharmacol. 77:713–722. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xenidis N, Vardakis N, Varthalitis I,
Giassas S, Kontopodis E, Ziras N, Gioulbasanis I, Samonis G,
Kalbakis K and Georgoulias V: Α multicenter phase II study of
pegylated liposomal doxorubicin in combination with irinotecan as
second-line treatment of patients with refractory small-cell lung
cancer. Cancer Chemother Pharmacol. 68:63–68. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramalingam SS, Foster J, Gooding W, Evans
T, Sulecki M and Belani CP: Phase 2 study of irinotecan and
paclitaxel in patients with recurrent or refractory small cell lung
cancer. Cancer. 116:1344–1349. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen G, Huynh M, Fehrenbacher L, West H,
Lara PN Jr, Yavorkovsky LL, Russin M, Goldstein D, Gandara D and
Lau D: Phase II trial of irinotecan and carboplatin for extensive
or relapsed small-cell lung cancer. J Clin Oncol. 27:1401–1404.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pallis AG, Agelidou A, Agelaki S,
Varthalitis I, Pavlakou G, Gerogianni A, Papakotoulas P, Rapti A,
Chandrinos V, Christophyllakis C and Georgoulias V: A multicenter
randomized phase II study of the irinotecan/gemcitabine doublet
versus irinotecan monotherapy in previously treated patients with
extensive stage small-cell lung cancer. Lung Cancer. 65:187–191.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ohyanagi F, Horiike A, Okano Y, Satoh Y,
Okumura S, Ishikawa Y, Nakagawa K, Horai T and Nishio M: Phase II
trial of gemcitabine and irinotecan in previously treated patients
with small-cell lung cancer. Cancer Chemother Pharmacol.
61:503–508. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rocha-Lima CM, Herndon JE II, Lee ME, Lee
ME, Atkins JN, Mauer A, Vokes E and Green MR: Cancer and Leukemia
Group B. Phase II trial of irinotecan/gemcitabine as second-line
therapy for relapsed and refractory small-cell lung cancer: Cancer
and leukemia group B study 39902. Ann Oncol. 18:331–337.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schuette W, Nagel S, Juergens S, Bork I,
Wollschlaeger B, Schaedlich S and Blankenburg T: Phase II trial of
gemcitabine/irinotecan in refractory or relapsed small-cell lung
cancer. Clin Lung Cancer. 7:133–137. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ichiki M, Rikimaru T, Gohara R, Koga T,
Kawayama T, Matunami M, Oshita Y, Kamimura T and Aizawa H: Phase II
study of irinotecan and ifosfamide in patients with advanced
non-small cell lung cancer. Oncology. 64:306–311. 2003.PubMed/NCBI View Article : Google Scholar
|