Introduction
Platinum-based antineoplastic drugs, such as
cisplatin, are reported to be effective against several malignant
tumors (1); however, they
frequently induce nausea and vomiting (2). Chemotherapy-induced nausea and
vomiting (CINV) can cause anorexia and weakness (3,4) and is
one of the most unpleasant subjective symptoms experienced by
patients (5). CINV significantly
reduces the quality of life of patients (3) and adversely affects treatment
continuation for the underlying disease (6,7).
Therefore, measures to alleviate CINV are crucial for the effective
and safe administration of cancer chemotherapy.
Several guidelines (2,8-10)
have consistently recommended a three-drug combination therapy,
consisting of serotonin 5-HT3 receptor antagonists,
selective neurokinin-1 (NK1) receptor antagonists, and
corticosteroids as antiemetics for combating anticancer agents with
a high emetic risk (≥90%; cisplatin), as well as those with
moderate emetic risk (30-90%; carboplatin) (2).
Patients with metastatic or advanced urothelial
carcinoma are treated with gemcitabine plus cisplatin (GC) therapy,
in which a single dose of cisplatin is administered in combination
with gemcitabine as the standard of care (11,12).
In contrast, patients with reduced kidney function are prescribed
GC split therapy (split-dose of cisplatin) (13,14) or
GCa therapy (administration of carboplatin instead of cisplatin)
(15,16). To our knowledge, the incidence of
CINV and the efficacy of chemotherapy for these alternate treatment
regimens compared to standard GC therapy in patients with reduced
renal function has not been investigated previously.
To elucidate which regimen presented the lowest risk
of CINV without compromising effectiveness, in this study, we
compared the prevalence of gastrointestinal symptoms, use of
antiemetics, therapeutic responses, and survival rates in patients
receiving GC therapy, GC split therapy, or GCa therapy for
urothelial carcinoma.
Materials and methods
Patients and therapeutic regimens
Patients who were treated with (a) GC therapy, (b)
GC split therapy, or (c) GCa therapy (see below and Table I) at the urology ward of Nagoya
University Hospital between March 1, 2011, and March 31, 2017, were
retrospectively evaluated. For each therapy, the following
scenarios were excluded from the analysis: patients who
discontinued the use of platinum preparations (cisplatin or
carboplatin), cases in which nausea and vomiting during the 10-day
observation period could not be clarified, and cases in which the
use of opioids rendered it challenging to determine whether nausea
and vomiting were induced by chemotherapy.
 | Table ITreatment schedules for GC, GC split
and GCa therapy. |
Table I
Treatment schedules for GC, GC split
and GCa therapy.
| |
Treatment
day, administration method |
|---|
| Regimen | Emetic risk | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 15 |
|---|
| GC therapy | | | | | | | | | | | | |
|
Gemcitabine
(1,000 mg/m2) | Low | iv | | | | | | | iv | | | iv |
|
Cisplatin
(70 mg/m2) | High | | iv | | | | | | | | | |
|
Serotonin
5-HT3 receptor antagonist | | (iv) | iv | | | | | | (iv) | | | |
|
Dexamethasone | | iv | iv | iv | iv | | | | iv | | | iv |
|
Aprepitant | | | po | po | po | (po) | (po) | | | | | |
|
Dopamine
D2 receptor antagonist | | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | |
| GC split
therapy | | | | | | | | | | | | |
|
Gemcitabine
(1,000 mg/m2) | Low | iv | | | | | | | iv | | | |
|
Cisplatin
(35 mg/m2) | High | iv | | | | | | | iv | | | |
|
Serotonin
5-HT3 receptor antagonist | | iv | | | | | | | iv | | | |
|
Dexamethasone | | iv | iv | iv | | | | | iv | iv | iv | |
|
Aprepitant | | po | po | po | (po) | (po) | | | | | | |
|
Dopamine
D2 receptor antagonist | | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | |
| GCa therapy | | | | | | | | | | | | |
|
Gemcitabine
(750-1,000 mg/m2) | Low | iv | | | | | | | iv | | | iv |
|
Carboplatin
(AUC=5 mg·min/ml) | Moderate | | iv | | | | | | | | | |
|
Serotonin
5-HT3 receptor antagonist | | (iv) | iv | | | | | | (iv) | | | |
|
Dexamethasone | | iv | iv | | | | | | iv | | | iv |
|
Aprepitant | | (po) | (po) | (po) | (po) | (po) | | | | | | |
|
Dopamine
D2 receptor antagonist | | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | (iv/po) | |
(a) GC therapy
The GC therapy regimen was administered for 28 days
(4-week interval), with cisplatin (70 mg/m2)
administered on treatment day 2 and gemcitabine (1,000
mg/m2) administered on treatment days 1, 8, and 15
(Table I). On the day of cisplatin
administration, patients were pre-administered serotonin
5-HT3 receptor antagonists (ramosetron, granisetron, or
palonosetron), a corticosteroid (dexamethasone), and a selective
NK1 receptor antagonist (aprepitant). Aprepitant was administered
on treatment days 2 (125 mg; before cisplatin administration), 3
(80 mg), and 4 (80 mg).
(b) GC split therapy
For GC split therapy, the treatment regimen was
administered for 21 days (3-week interval), with cisplatin (35
mg/m2) and gemcitabine (1,000 mg/m2)
administered on treatment days 1 and 8 (Table I). On the days of cisplatin
administration, patients were pre-administered serotonin
5-HT3 receptor antagonist and dexamethasone. Aprepitant
was administered on treatment days 1 (125 mg; before administration
of cisplatin), 2 (80 mg), and 3 (80 mg).
(c) GCa therapy
GCa treatment was performed for 28 days (4-week
interval) and consisted of carboplatin [area under the blood
concentration-time curve (AUC)=5 mg·min/ml] administered on
treatment day 2 and gemcitabine (750-1,000 mg/m2)
administered on treatment days 1, 8, and 15 (Table I). On the day of carboplatin
administration, patients were pre-administered with a serotonin
5-HT3 receptor antagonist and dexamethasone.
Assessment
In this study, a 10-day (treatment days 1 to 10)
observation period was employed from the start date of each course
(GC therapy, GC split therapy, or GCa therapy). The following
parameters were investigated: (i) number of days with nausea and
vomiting, (ii) prevalence of nausea and vomiting on each treatment
day, (iii) administration of a serotonin 5-HT3 receptor
antagonist, (iv) total and additional use of antiemetics (average
use count, total dose, and use date of serotonin 5-HT3
receptor antagonists, aprepitant, dopamine D2 receptor
antagonists), (v) therapeutic response [complete response (CR),
partial response (PR), stable disease (SD), or progressive disease
(PD)] based on the new response evaluation criteria in solid tumors
(RECIST v.1.1) (17), and (vi) the
duration of patient survival from the start date of each course of
treatment to the date of the final follow-up observation or date of
death.
Statistical analysis
Fisher's exact test was used to analyze categorical
data related to sex, rate of incidence of nausea and vomiting
events on each treatment day, use rate of each serotonin
5-HT3 receptor antagonist, use rate of additional
antiemetics and prevalence of patients with each therapeutic
response. The Shapiro-Wilk test was used to determine normality,
and Leven's test was used to assess the equality of variances. The
Kruskal-Wallis test was used to compare median values of age,
estimated glomerular filtration rate (eGFR), creatinine clearance
(Ccr) and the number of days with nausea/vomiting per course among
three groups, followed by post-hoc testing using the unpaired
Mann-Whitney U test, with a Bonferroni-adjusted alpha level. The
Cramér-von Mises test was used to assess survival rates.
Statistical significance was set at P<0.05. Data
analysis, power analysis, and sample size calculations were
performed using the R statistical software (version 3.6.2; R
Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
In total, 67 patients (48 men and 19 women) were
included in this study. There were 43 patients (27 men, 16 women)
in the GC therapy group, 9 patients (8 men, 1 woman) in the GC
split therapy group, and 15 patients (13 men, 2 women) in the GCa
group. There were no statistically significant differences in age
or sex among the groups. However, eGFR and Ccr levels were
significantly lower in the GC split and GCa therapy groups than in
the GC therapy group (P<0.01), and the level of Ccr was also
significantly lower in the GCa therapy group than in the GC split
therapy group (P<0.05; Table
II).
 | Table IIPatient characteristics. |
Table II
Patient characteristics.
| Characteristic | GC therapy | GC split
therapy | GCa therapy |
|---|
| Median age, years
(range) | 67.0 (36-80) | 67.0 (49-76) | 73.0 (57-84) |
| Sex, n (%) | | | |
|
Men | 27 (62.8) | 8 (88.9) | 13 (86.7) |
|
Women | 16 (37.2) | 1 (11.1) | 2 (13.3) |
| Number of
chemotherapy courses | 72 | 23 | 19 |
| Median eGFR,
ml/min/1.73 m2 (range) | 61.8
(36.9-162.5) | 38.4
(31.8-50.6)a | 37.1
(15.8-64.5)a |
| Median Ccr, ml/min
(range) | 57.6
(31.5-153.8) | 46.0
(34.5-62.3)a | 41.2
(18.1-70.6)a,b |
Number of days with nausea/vomiting
per course
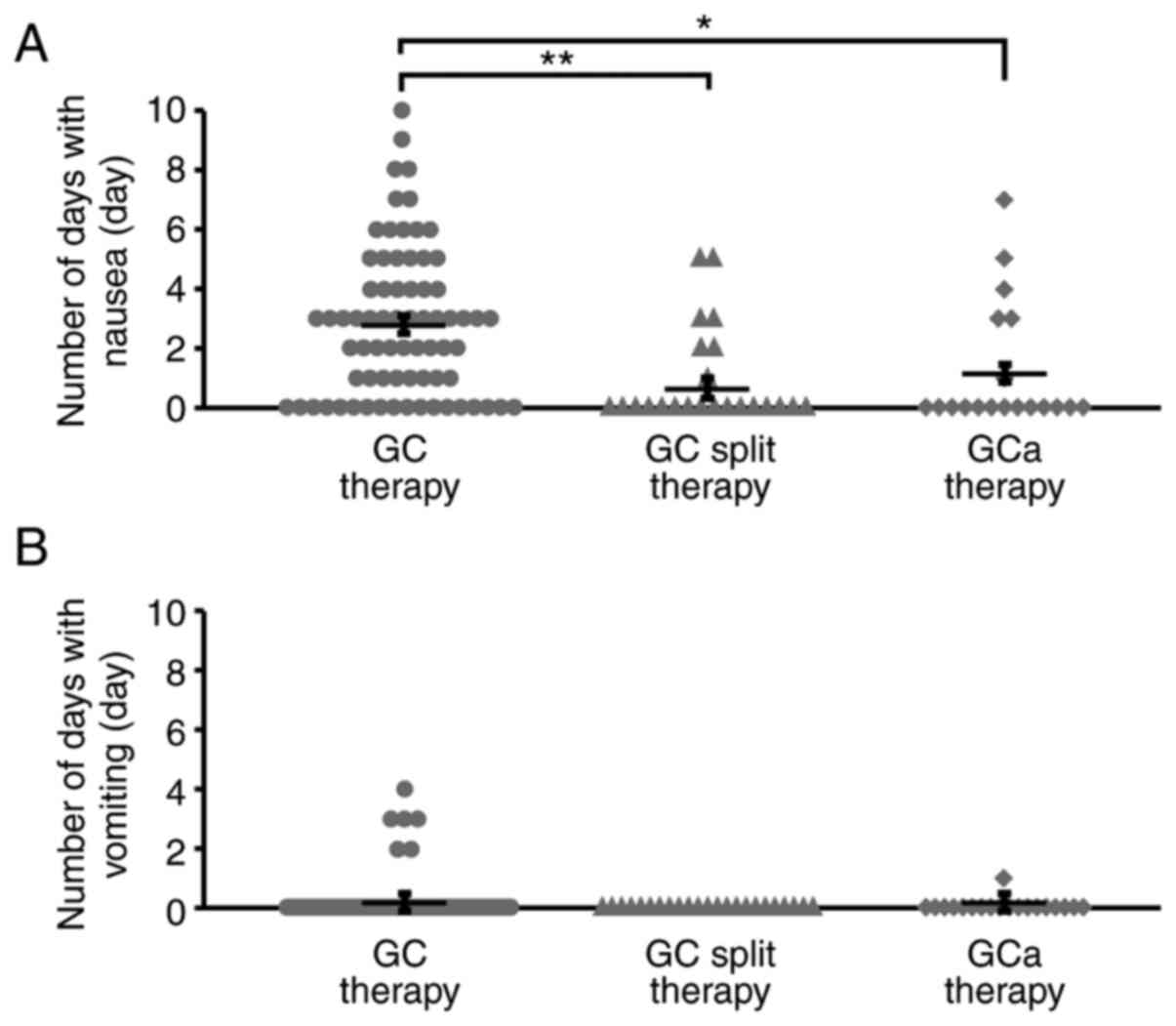
The number of days with nausea/vomiting per course ±
standard deviation in the GC, GC split, and GCa therapy groups is
shown in Fig. 1. We found that the
number of days with nausea per course was significantly lower in
the GC split and GCa therapy groups than in the GC therapy group
(P<0.01, P<0.05, respectively; Fig. 1A). Although several patients
experienced vomiting during the observation period in the GC and
GCa therapy groups, there were no patients with vomiting episodes
in the GC split therapy group (Fig.
1B).
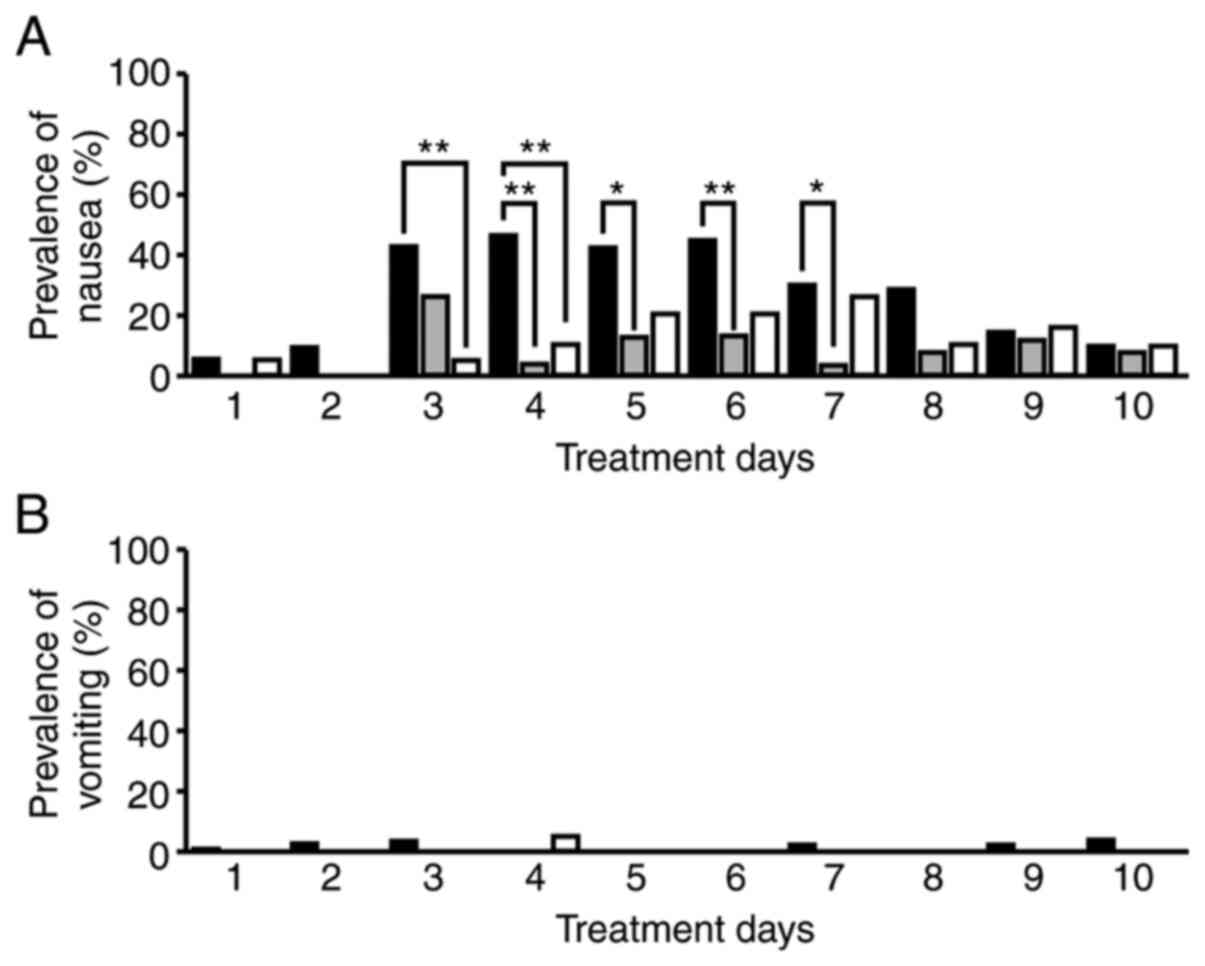
Prevalence of nausea and vomiting on
each treatment day
The prevalence of nausea on treatment days 4 to 7
was significantly lower in the GC split therapy group than in the
GC therapy group (treatment days 4 and 6: P<0.01, treatment days
5 and 7: P<0.05; Fig. 2A). On
treatment days 3 and 4, prevalence of nausea/vomiting was
significantly lower in the GCa therapy group than in the GC therapy
group (P<0.01; Fig. 2A). No
differences in the prevalence of vomiting were observed among the
treatment groups (P<0.05; Fig.
2B).
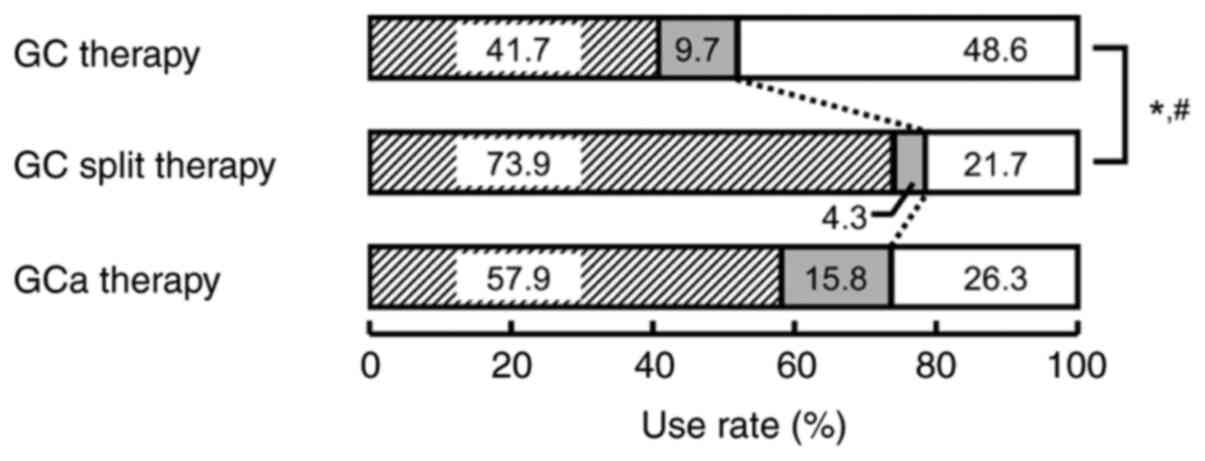
Use rate of each serotonin
5-HT3 receptor antagonist
The use rates of serotonin 5-HT3 receptor
antagonists (ramosetron, granisetron, or palonosetron) in each
therapy group are shown in Fig. 3.
A significant difference was observed in the use rate between the
GC split therapy and the GC therapy groups (P<0.05; Fig. 3). In particular, a second-generation
serotonin 5-HT3 receptor antagonist (palonosetron) was
significantly lower in the GC split therapy group (P<0.05;
Fig. 3). No differences were
observed between the GCa and GC therapy groups or the GC split
therapy group.
Total and additional use of
antiemetics
The total and additional use of antiemetics is
summarized in Table III. The
total dose of antiemetics during the observation period was
significantly lower in the GC split therapy group than in the GC
and GCa therapy groups (P<0.01). The metoclopramide dose was
significantly lower in the GC split therapy group than in the GC
therapy group (P<0.05). Aprepitant dose was significantly lower
in the GCa therapy group than in the GC and GC split therapy groups
(P<0.01).
 | Table IIIAntiemetics. |
Table III
Antiemetics.
| A, Total use of
antiemetics (included in regimen and additional use). |
|---|
| Antiemetics | | Regimen | Na | Mean count ± SD
(range) | Mean dose,
mg/course ± SD (range) |
|---|
| Granisetron | | GC | 7 | 1.14±0.38
(1-2) | 2.00±1.00
(1-3) |
| | | GC split | 1 | 1.00±0.00(1) | 3.00±0.00(3) |
| | | GCa | 3 | 1.00±0.00(1) | 3.00±0.00(3) |
| Palonosetron | | GC | 35 | 1.00±0.00(1) | 0.75±0.00
(0.75) |
| | | GC split | 5 | 1.00±0.00(1) | 0.75±0.00
(0.75) |
| | | GCa | 5 | 1.00±0.00(1) | 0.75±0.00
(0.75) |
| Ramosetron | | GC | 30 | 2.23±0.94
(1-3) | 0.67±0.28
(0.3-0.9) |
| | | GC split | 17 | 1.00±0.00(1) | 0.30±0.00
(0.3)d |
| | | GCa | 11 | 2.55±0.82
(1-3) | 0.74±0.28
(0.3-0.9)e |
| Domperidone | | GC | 72 | 0.15±0.82
(0-6) | 3.61±22.35
(0-180) |
| Metoclopramide | | GC | 72 | 4.04±8.96
(0-31) | 20.97±45.48
(0-160) |
| | | GC split | 23 | 0.06±0.24
(0-1) | 0.29±1.21
(0-5)c |
| | | GCa | 19 | 2.16±4.89
(0-15) | 11.05±24.47
(0-75) |
| Aprepitant | | GC | 72 | 3.57±0.90
(3-5) | 330.56±72.09
(285-445) |
| | | GC split | 23 | 3.35±0.49
(3-4) | 313.24±39.41
(285-365) |
| | | GCa | 19 | 0.95±1.43
(0-3) | 90.00±136.11
(0-285)d,e |
| B, Additional use
of antiemetics (not included in regimen). |
| Antiemetics | Regimen | Nb | Mean count ± SD
(range) | Mean dose,
mg/course ± SD (range) | Day administered
(N) |
| Granisetron | GC | 1 | 1.00±0.00(1) | 1.00±0.00(1) | Day 1(1) |
| |
| Ramosetron | GC | 20 | 1.85±0.37
(1-2) | 0.56±0.11
(0.3-0.6) | Day 1(20), day
8(17) |
| | GCa | 9 | 1.89±0.33
(1-2) | 0.57±0.10
(0.3-0.6) | Day 1(9), day
8(8) |
| Domperidone | GC | 3 | 3.67±2.08
(2-6) | 86.67±83.27
(20-180) | Day 3(1), day 4(2),
day 5(3), day 6(2), day 7(1) |
| Metoclopramide | GC | 29 | 10.03±11.88
(1-30) | 52.07±59.72
(5-150) | Day 1(7), day 2(8),
day 3(12), day 4(13), day 5(15), day 6(16), day 7(15), day 8(15),
day 9(10), day 10(9) |
| | GC split | 1 | 1.00±0.00(1) | 5.00±0.00(5) | Day 9(1) |
| | GCa | 4 | 10.25±5.74
(2-15) | 52.50±26.30
(15-75) | Day 3(1), day 4(1),
day 5(2), day 6(3), day 7(3), day 8(2), day 9(3), day 10(2) |
| Aprepitant | GC | 21 | 1.95±0.22
(1-2) | 156.19±17.46
(80-160) | Day 5(21), day
6(20) |
| | GC split | 9 | 1.00±0.00(1) |
80.00±0.00(80)d | Day 4(9) |
| | GCa | 6 | 3.00±0.00(3) |
285.00±0.00(285)d,e | Day 2(6), day 3(6),
day 4(6) |
The use rates of additional antiemetics in the GC,
GC split, and GCa therapy groups were 70.8% (51/72 courses), 39.1%
(9/23 courses), and 78.9% (15/19 courses), respectively. The use
rate was significantly lower in the GC split therapy group than in
the GC and GCa therapy groups (P<0.05). Considering the dose of
additional antiemetics during the observation period, aprepitant
dose was significantly lower in the GC split therapy group than in
the GC therapy group (P<0.01), whereas it was significantly
higher in the GCa therapy group than in the GC and GC split therapy
groups (P<0.01; Table
III).
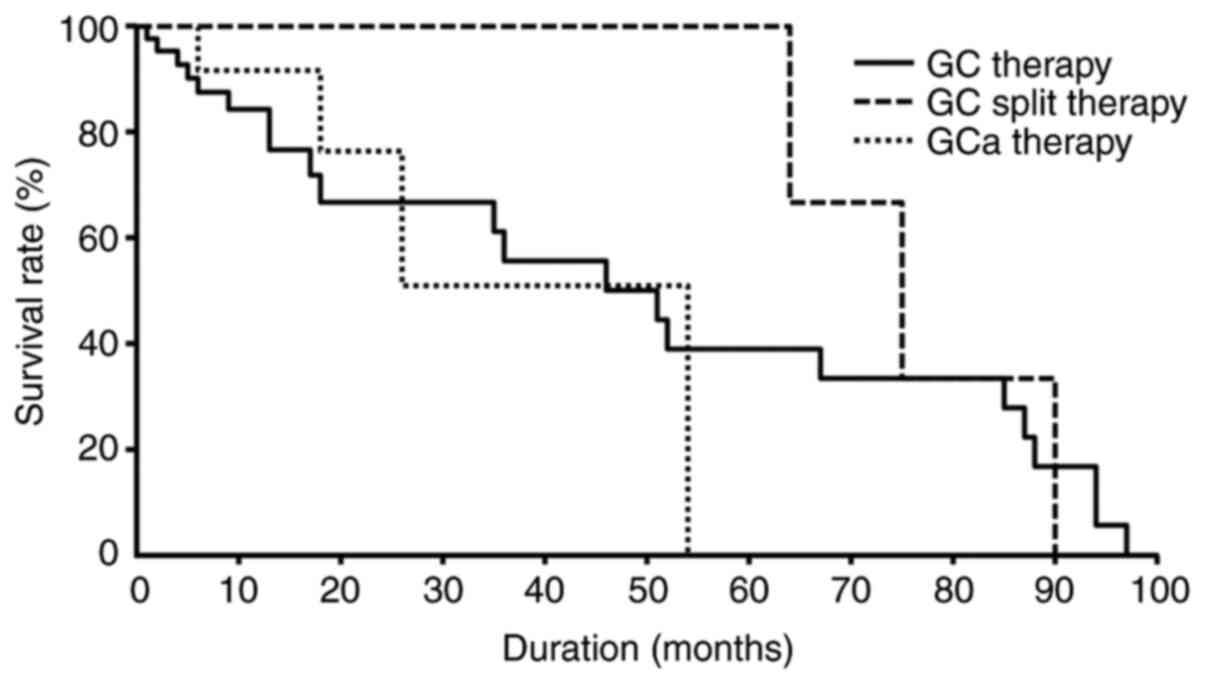
Therapeutic response and survival
rates
No differences were observed in the proportion of
each therapeutic response in the GC therapy group [CR: 0% (0/34
patients), PR: 8.8% (3/34 patients), SD: 50.0% (17/34), PD: 41.2%
(14/34 patients)], GC split therapy group [CR: 0% (0/4 patients),
PR: 25.0% (1/4 patients), SD: 75.0% (3/4 patients), PD: 0.0% (0/4
patients)], and GCa therapy group [CR: 0% (0/6 patients), PR: 16.7%
(1/6 patients), SD: 66.7% (4/6 patients), PD: 16.7% (1/6 patients)]
(P>0.05). No differences in the duration of survival or survival
rates were observed among the treatment groups (P>0.05; Fig. 4).
Power analysis and sample size
calculation
This study had a power of 0.42-0.59 to detect a
medium effect (18) and could not
obtain the estimated sample size (n=53-108).
Discussion
This study compared the prevalence of
gastrointestinal symptoms, use of antiemetics, therapeutic
responses, and survival rates in patients receiving GC therapy, GC
split therapy, or GCa therapy for urothelial carcinoma. Although
CINV in the acute phase (within the first 24 h after chemotherapy)
was well-controlled in all therapy groups, patients receiving GC
therapy showed a higher incidence of vomiting in the delayed phase
(>24 h after chemotherapy) than the other groups. In the GC
split therapy group, there were no vomiting episodes, and the
number of days with nausea per course and the prevalence of nausea
on treatment days 4 to 7 were significantly lower than those
observed in the GC therapy group. This suggests that low-dose
cisplatin administration can suppress the emergence of delayed
nausea. In the GCa therapy group, the number of days with nausea
per course was significantly lower, and the prevalence of nausea on
treatment days 3 and 4 was significantly lower than that observed
in the GC therapy group. These results can be explained by the fact
that carboplatin is a platinum-based antineoplastic drug with
moderate emetic risk (2).
A second-generation serotonin 5-HT3
receptor antagonist, palonosetron, is effective against both the
acute and delayed phases of CINV (19). Among the serotonin 5-HT3
receptor antagonists used in this survey, the use rate of
palonosetron in the GC split and GCa therapy groups was lower than
that in the GC therapy group. This could be attributed to the fewer
number of days with nausea per course, as well as the lower
prevalence of nausea on each treatment day in both the GC split and
GCa therapy groups than in the GC therapy group. Therefore, our
findings suggest that both GC split therapy and GCa therapy can
reduce the prevalence of acute and delayed CINV, regardless of
palonosetron administration. The GC split therapy group presented a
significantly lower administration rate and aprepitant dose of
additional antiemetics than the GC therapy and GCa therapy groups.
This could be attributed to several factors. First, GC split
therapy has a lower risk of CINV than GC or GCa therapy. Second,
all patients receiving GC therapy and GC split therapy, who were
administered cisplatin (a high emetic risk drug), were also
administered a three-drug combination antiemetic therapy according
to the treatment schedules. Third, patients receiving GCa therapy,
using carboplatin, were additionally administered aprepitant, which
was not listed in the treatment schedule. When we compared the need
for antiemetic use during the observation period, the aprepitant
dose was significantly lower in the GCa therapy group than in the
GC and GC split therapy groups. Moreover, the frequency of
additional use of serotonin 5-HT3 receptor antagonist
was lower in aprepitant users (16.7%) than in non-users (61.5%).
GCa therapy can reduce both early and late CINV with the minimum
requirement of aprepitant use, and aprepitant use may result in
additional antiemetic effects. Furthermore, although the treatment
duration for the GC split therapy was shorter (21 days) than that
for a single course of GC therapy (28 days), CINV events were
adequately controlled with the three-drug antiemetic therapy.
Therefore, if the dose of cisplatin administered per course was the
same, split-dose treatments may be more effective in mitigating
CINV onset than single-dose treatments.
Cisplatin-based treatment regimens are used as
first-line therapies for metastatic or advanced urothelial
carcinoma; however, the use of cisplatin has been associated with
renal dysfunction, deterioration of the general condition, and
medical complications in 40-50% of patients (20). Carboplatin or split-dose
administration of cisplatin is employed as an alternative platinum
formulation in patients presenting with these adverse events
(13-16).
In this study, we found that eGFR and Ccr levels were significantly
lower in patients receiving GC split therapy and GCa therapy than
in those receiving GC therapy, suggesting that GC split therapy and
GCa therapy can be prioritized in patients with reduced kidney
function. It has been suggested that adverse events are less common
in regimens that use carboplatin instead of cisplatin (21), although the therapeutic efficacy of
cisplatin is significantly inferior to that of carboplatin
(22). However, only a few reports
have presented direct comparisons between GC, GC split, and GCa
therapies (16,23). Results from our preliminary survey
indicated that there were no significant differences in the
therapeutic effects and survival rates among the three groups.
Therefore, the selection of GC split therapy or GCa therapy,
instead of GC therapy, may reduce CINV incidence without adversely
affecting the therapeutic effects and survival rates in patients
with urothelial carcinoma.
This study was limited by its retrospective design
based on electronic medical records and a small sample size
compared to the estimated ideal sample size (n=53-108), which may
induce biases owing to insufficient statistical power (0.42-0.59 to
detect a medium effect). Additionally, it is possible that the
patients' memories and medical staff records were inaccurate,
resulting in recall bias. As there was probably some information
that was not recorded in the medical records, it should be noted
that the occurrence of nausea/vomiting may have been
underestimated. Furthermore, we did not examine the relationship
between patient-related factors (e.g., age, sex, and history of
alcohol intake) or genetic risk factors (6,24,25)
and the incidence of CINV. Therefore, these results should be
interpreted with caution.
In conclusion, GC split therapy and GCa therapy were
superior to GC therapy in reducing the incidence of CINV in
patients with urothelial carcinoma. In particular, GCa therapy is
likely to be superior to GC and GC split therapy because
carboplatin can be administered regardless of renal function and
has few side effects such as nausea and vomiting.
Although further investigations on the therapeutic
effects and other adverse events are required, split-dose cisplatin
administration or the use of carboplatin instead of cisplatin may
be useful in patients who experience CINV without compromising
treatment effectiveness.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from The Encouragement of
Scientific Research, Promoted Research Center Subsidy by Meijo
University Research Institute.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AY, MM, KY and YN conceived and designed the current
study. AY, YS, MI, MN, ATG, HH, NT and MM acquired the data. AY,
YS, MI, MN, ATG and YN analyzed and interpreted the data. AY, YS
and MI performed statistical analysis. AY and YS drafted the
manuscript. AY and YN supervised the study and confirmed the
authenticity of all the raw data. YN critically revised the
manuscript for important intellectual content. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This survey was conducted in accordance with the
Declaration of Helsinki and the Ethical Guidelines for Medical and
Health Research Involving Human Subjects (26). This study was approved by the Ethics
Committee of Nagoya University Graduate School of Medicine
(approval no. 2016-0539-3), and written informed consent was waived
because of the retrospective design. To protect personal
information, all data obtained were handled after
anonymization.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Razvi Y, Chan S, McFarlane T, McKenzie E,
Zaki P, DeAngelis C, Pidduck W, Bushehri A, Chow E and Jerzak KJ:
ASCO, NCCN, MASCC/ESMO: A comparison of antiemetic guidelines for
the treatment of chemotherapy-induced nausea and vomiting in adult
patients. Support Care Cancer. 27:87–95. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bloechl-Daum B, Deuson RR, Mavros P,
Hansen M and Herrstedt J: Delayed nausea and vomiting continue to
reduce patients' quality of life after highly and moderately
emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol.
24:4472–4478. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Navari RM: Management of
chemotherapy-induced nausea and vomiting: Focus on newer agents and
new uses for older agents. Drugs. 73:249–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Coates A, Abraham S, Kaye SB, Sowerbutts
T, Frewin C, Fox RM and Tattersall MH: On the receiving end-patient
perception of the side-effects of cancer chemotherapy. Eur J Cancer
Clin Oncol. 19:203–208. 1983.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hesketh PJ: Chemotherapy-induced nausea
and vomiting. N Engl J Med. 358:2482–2494. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Van Laar ES, Desai JM and Jatoi A:
Professional educational needs for chemotherapy-induced nausea and
vomiting (CINV): Multinational survey results from 2388 health care
providers. Support Care Cancer. 23:151–157. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hesketh PJ, Kris MG, Basch E, Bohlke K,
Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina
SB, et al: Antiemetics: American society of clinical oncology
clinical practice guideline update. J Clin Oncol. 35:3240–3261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Berger MJ, Ettinger DS, Aston J, Barbour
S, Bergsbaken J, Bierman PJ, Brandt D, Dolan DE, Ellis G, Kim EJ,
et al: NCCN guidelines insights: Antiemesis, version 2.2017. J Natl
Compr Canc Netw. 15:883–893. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Roila F, Molassiotis A, Herrstedt J, Aapro
M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer
P, et al: 2016 MASCC and ESMO guideline update for the prevention
of chemotherapy- and radiotherapy-induced nausea and vomiting and
of nausea and vomiting in advanced cancer patients. Ann Oncol. 27
(Suppl 5):v119–v133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bellmunt J, von der Maase H, Mead GM,
Skoneczna I, De Santis M, Daugaard G, Boehle A, Chevreau C,
Paz-Ares L, Laufman LR, et al: Randomized phase III study comparing
paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in
patients with locally advanced or metastatic urothelial cancer
without prior systemic therapy: EORTC intergroup study 30987. J
Clin Oncol. 30:1107–1113. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hussain SA, Stocken DD, Riley P, Palmer
DH, Peake DR, Geh JI, Spooner D and James ND: A phase I/II study of
gemcitabine and fractionated cisplatin in an outpatient setting
using a 21-day schedule in patients with advanced and metastatic
bladder cancer. Br J Cancer. 91:844–849. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim YR, Lee JL, You D, Jeong IG, Song C,
Hong B, Hong JH and Ahn H: Gemcitabine plus split-dose cisplatin
could be a promising alternative to gemcitabine plus carboplatin
for cisplatin-unfit patients with advanced urothelial carcinoma.
Cancer Chemother Pharmacol. 76:141–153. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Santis M, Bellmunt J, Mead G, Kerst JM,
Leahy M, Maroto P, Gil T, Marreaud S, Daugaard G, Skoneczna I, et
al: Randomized phase II/III trial assessing gemcitabine/carboplatin
and methotrexate/carboplatin/vinblastine in patients with advanced
urothelial cancer who are unfit for cisplatin-based chemotherapy:
EORTC study 30986. J Clin Oncol. 30:191–199. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Einstein DJ and Sonpavde G: Treatment
approaches for cisplatin-ineligible patients with invasive bladder
cancer. Curr Treat Options Oncol. 20(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cohen J: A power primer. Psychol Bull.
112:155–159. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Saito M, Aogi K, Sekine I, Yoshizawa H,
Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T and Mitsuhashi S:
Palonosetron plus dexamethasone versus granisetron plus
dexamethasone for prevention of nausea and vomiting during
chemotherapy: A double-blind, double-dummy, randomised, comparative
phase III trial. Lancet Oncol. 10:115–124. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Morales-Barrera R, Bellmunt J, Suárez C,
Valverde C, Guix M, Serrano C, Gallén M and Carles J: Cisplatin and
gemcitabine administered every two weeks in patients with locally
advanced or metastatic urothelial carcinoma and impaired renal
function. Eur J Cancer. 48:1816–1821. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sakaeda T, Kadoyama K and Okuno Y: Adverse
event profiles of platinum agents: Data mining of the public
version of the FDA adverse event reporting system, AERS, and
reproducibility of clinical observations. Int J Med Sci. 8:487–491.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
de Vos FY and de Wit R: Choosing
chemotherapy in patients with advanced urothelial cell cancer who
are unfit to receive cisplatin-based chemotherapy. Ther Adv Med
Oncol. 2:381–388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dogliotti L, Carteni G, Siena S, Bertetto
O, Martoni A, Bono A, Amadori D, Onat H and Marini L: Gemcitabine
plus cisplatin versus gemcitabine plus carboplatin as first-line
chemotherapy in advanced transitional cell carcinoma of the
urothelium: Results of a randomized phase 2 trial. Eur Urol.
52:134–141. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sekine I, Segawa Y, Kubota K and Saeki T:
Risk factors of chemotherapy-induced nausea and vomiting: Index for
personalized antiemetic prophylaxis. Cancer Sci. 104:711–717.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mukoyama N, Yoshimi A, Goto A, Kotani H,
Ishikawa K, Miyazaki N, Miyazaki M, Yamada K, Kikkawa F, Hasegawa
Y, et al: An analysis of behavioral and genetic risk factors for
chemotherapy-induced nausea and vomiting in Japanese subjects. Biol
Pharm Bull. 39:1852–1858. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ministry of Education, Culture Sports,
Science and Technology/Ministry of Health, Labor and Welfare: The
ethical guidelines for medical and health research involving human
subjects. https://www.mhlw.go.jp/content/000757566.pdf. Accessed
March 23, 2021. (In Japanese).
|