Introduction
Pathogenic mutations in the BRCA1/BRCA2 genes
increase the risk of developing breast and ovarian cancer or other
types of cancer (prostate cancer, pancreatic cancer and melanoma).
Women with BRCA1 mutations exhibit a 45-85% lifetime risk of
developing breast cancer. Other genes associated with the risk of
developing breast cancer are ATM, CDH1, CHEK2, PALB2, PTEN,
STK11, and TP53 (1-7).
The risk of developing ovarian cancer by 70 years of age is 40-50%
for BRCA1 mutation carriers and 10-20% for BRCA2
mutation carriers (8,9). Other suppressor genes and oncogenes
associated with hereditary ovarian cancers, include the mismatch
repair (MMR) genes in Lynch syndrome, the tumor suppressor gene,
TP53, in the Li-Fraumeni syndrome, genes involved in the
double-strand breaks repair system, such as CHEK2, RAD51,
BRIP1, and PALB2 (10).
The development and popularization of NGS technique
led to the detection of new genetic variations, including VUS
(variant of uncertain significance) (11). It is variant whose clinical
significance to the function or medical condition is not known. In
previous studies conducted on high-risk families, variants of
uncertain significance exhibited a frequency up to 15% for the
BRCA genes. It included the following mutations: missense
variations, small in-frame deletions, variants in regulatory
sequences and exonic or intronic variants that may affect pre-mRNA
splicing (12). The International
Agency on Cancer Research of the World Health Organization has
proposed a simple five-tier system for the clinical management of
BRCA1/BRCA2 VUSs that is not widely known to clinicians.
Class 1 and 2 variants are managed as neutral variants, whereas
class 4 and 5 as pathogenic variants. Insufficient evidence has
been reported regarding class 3 variants and therefore these are
not included in the selection process (13). The American College of Medical
Genetics and Genomics recommends the use of specific standard
terminology as follows: ‘pathogenic’, ‘likely pathogenic’,
‘uncertain significance’, ‘likely benign’ and ‘benign’. These terms
are used to describe variants identified in genes (14). Variants of uncertain significance
have also been identified in other genes such as: TP53
(15), ATM (16) or PALB2 (17). There are only few studies, which
describe histopathologic characteristics of VUS (18).
Assessing the pathogenicity of VUS in BRCA1
and BRCA2 poses a serious challenge. Functional assays
provide an important tool for classification. BRCA1/2 promote the
maintenance of genome stability via homologous recombination. Thus,
related assays are particularly relevant to cancer risk. Currently,
there is need for high-throughput assays with sufficient
sensitivity to characterize the large number of identified
variants.
The influence of BRCA1/2 VUSs on the cancer
risk and their association with the response to treatment is
uncertain. The aim of the present study was to characterized breast
cancer patients according to clinical and histopathological
factors.
Case report
The present case report describes two cases of
breast cancer patients with BRCA VUSs. All diagnosis and
treatment took place in National Research Institute of Oncology,
Gliwice Branch. Genetic counseling was performed. During the
genetic consultation, a family history was collected, the pedigree
was prepared and the medical records were reviewed. The patients
provided written informed consent regarding the use of their
biological material for clinical research (all the samples were
collected by routine laboratory analyses). The complete coding
sequence of the BRCA1 and BRCA2 genes was analyzed on
the genomic DNA material using next generation sequencing on the
Ion Torrent platform. The libraries were prepared using the
Oncomine BRCA Assay Chef Ready Kit, according to the manufacturer's
instructions (ThermoFisher Scientific). Sequencing was performed on
the Ion S5 sequencer (ThermoFisher Scientific) using the Ion 530
Chip Kit and 510&520&530 Kit-Chef. The raw data generated
during sequencing was processed using Ion Reporter v.5.6 software
(ThermoFisher Scientific). The wANNOVAR program (www.wannovar.usc.edu) was used to annotate the
detected variants from Ion Reporter. The reference sequences were
the following: NM_007294.3 (BRCA1) and NM_000059.3 (BRCA2). The
complete coding sequence of the BRCA1 and BRCA2 genes
was performed together with adjacent intron sequences. The
interpretation of the variants was carried out in accordance with
the following databases: NBCI ClinVar, dbSNP, 1000 Genomes Project,
BIC, LOVD, VarSome in the current version on the day of the
examination. Classification of variants was done according to
guidelines of American College of Medical Genetics (ACMG) (14). The presence of these variants in the
BRCA1 and BRCA2 genes was confirmed using the Sanger
method.
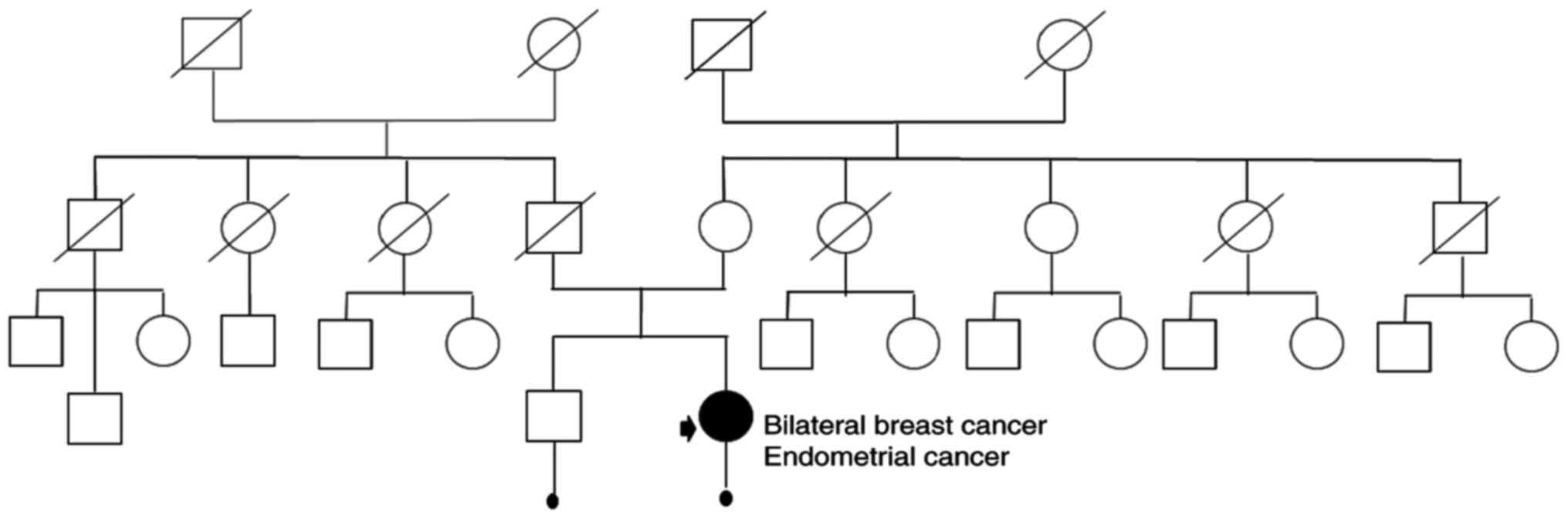
A 63-year-old woman was admitted to the Genetic
Outpatient Clinic with the diagnosis of bilateral breast cancer. At
the age of 60, she was also diagnosed with endometrial cancer
(T2N0M0). The patient was eligible for surgical treatment.
Histopathological examination indicated adenocarcinoma papillare
serosum (pT2N0Mx). Following surgery, she received
chemotherapy, radiotherapy and brachytherapy (standard therapy).
Genetic counseling was performed. There was no cancer in family
history. Genetic pedigree Fig.
1.
Two years later (at the age of 62), the patient was
diagnosed with bilateral breast cancer as determined by
mammography. One tumor was located centrally in the right breast
and exhibited the following dimensions: 2x1.5 cm. The second tumor
was situated in the upper outer quadrant of the left breast and
exhibited the following dimensions: 9x8x5 mm. A thick-needle biopsy
was performed. Histopathological examination indicated ductal
invasive carcinoma with neuroendocrine differentiation. The
biochemical and clinical characteristics of the tumors were the
following: NG2, G2, ER (+++), PR (+++), HER2 (+), Ki-67 10%,
luminal A subtype in the right breast and ductal invasive carcinoma
G2, ER (+++), PR (+++), HER2 (+), Ki-67 5% and luminal A subtype in
the left breast. Breast-conserving therapy with sentinel lymph node
biopsy and IORT was performed in the left breast. Postoperative
histopathological examination indicated the following tumor
characteristics: pT2 N1a Mx, invasive breast carcinoma with
neuroendocrine differentiation NG-2 G-2, HER2 (+), ER (+++), PR
(+++) and luminal A subtype. Metastases were demonstrated in one
lymph node. The patient was eligible for adjuvant radiotherapy and
hormonotherapy based on aromatase inhibitors. In addition,
right-sided mastectomy with right arm SNB was performed.
Genetic analysis was conducted. The presence of the
c.3454G>A (p.Asp1152Asn) mutation in the BRCA1 gene,
which is a VUS has been previously reported. The detected mutation
causes a change of the amino acid aspartic acid to the amino acid
asparagine at position 1,152 of the amino acid sequence of the
BRCA1 protein. This variant is found in the ClinVar and BRCA
Share databases, where it has the status of a VUS. In order to
determine the pathogenicity of this variant, in silico
analyses were performed using the SIFT, Mutation Taster and Align
GVGT algorithms. These algorithms indicate that the c.3454G>A
(p.Asp1152Asn) variant in the BRCA1 gene is probably not
pathogenic. Mutations in the BRCA2 gene were not detected.
Moreover, the absence of mutations in the CHEK2 (c.1100delC
and mutation c.470T>C) (GenBank NM_145862.2.), the PALB2
(c.508_509delAG and c.172_175delTTGT) and the TP53 (exons
2-11) genes were observed. The presence of the c.3454G>A
(p.Asp1152Asn) variant in the BRCA1 gene was confirmed using
the Sanger method. Genetic tests were also offered to the patient's
relatives (brother and mother). They have not yet decided to
examination. The woman has no children.
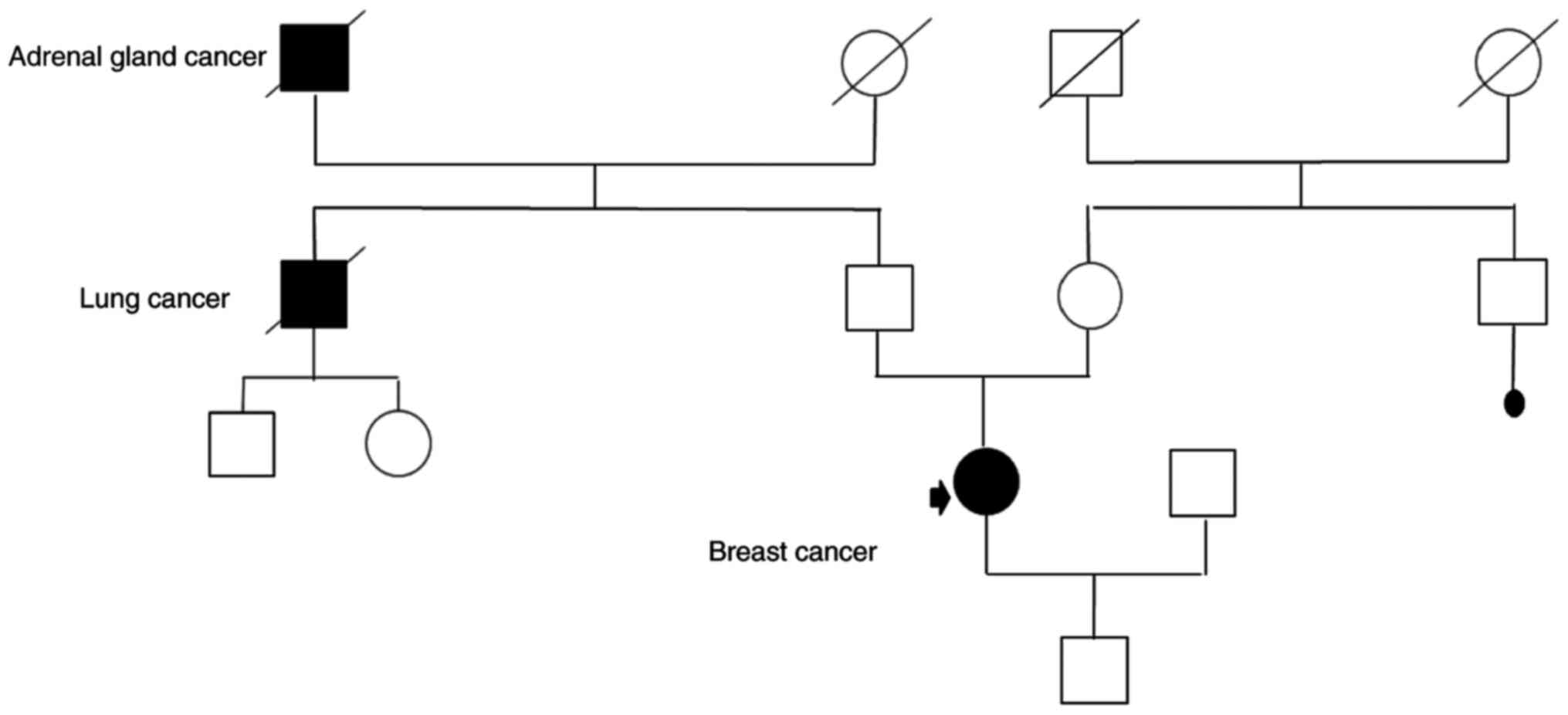
The second patient was 33-year-old woman, who was
admitted to the Genetic Outpatient Clinic with the diagnosis of
right breast cancer (cT2N1M0) and with a previous family history of
cancer (two of her relatives were diagnosed with cancer-one with
lung cancer and the second with adrenal gland cancer) (genetic
pedigree Fig. 2). At initial
diagnosis, PET testing was carried out and the data indicated the
presence of a 3 cm tumor in the right breast with involvement of
the right armpit lymph nodes. A coarse-needle biopsy was performed.
Histopathological examination demonstrated the presence of invasive
breast carcinoma, which exhibited the following characteristics:
NG-2 G-2, ER (+++), PR (+++), Ki-67 10%, HER2 (+++) and luminal B
subtype. Metastases were reported in the lymph nodes of the right
armpit. The patient was eligible for neoadjuvant chemotherapy (IV
cycles of AC regimen and 12 cycles of paclitaxel monotherapy) and
immunotherapy (pertuzumab and trastuzumab). In addition, the
patient was eligible for surgical treatment following neoadjuvant
therapy. A radical amputation of the right breast with the SNB of
the lymph nodes was performed. Postoperative histopathological
examination demonstrated a tumor with the presence of
angioinvasion, without neuroinvasion. The exact clinical and
biochemical characteristics were as follows: pT1c N0 M0, NST NG-2
G-2, Ki-67 10%, ER (+++), PR (+++), and HER2 overexpression.
Genetic analysis has shown the presence of the
c.2374T>C (p.Tyr792His) variant in the BRCA2 gene, which
is a VUS. The detected variant caused a change of tyrosine to
histidine at position 792 of the amino acid sequence of the
BRCA2 protein. This variant is found in the ClinVar and BRCA
Share databases and it is characterized as a VUS. Genetic variants
of this type may exert a diverse impact on protein function. The
presence of the c.2374T>C (p.Tyr792His) mutation in the
BRCA2 gene was confirmed using the Sanger method. The
analysis did not detect mutations in the BRCA1 gene.
Moreover, the data indicated the absence of mutations in the
PALB2 (c.508_509delAG and c.172_175delTTGT) gene. The
CHEK2 gene (c.1100delC and mutation c.470T>C) (GenBank
NM_145862.2.) was also examined. The c.470T>C mutation in
CHEK2 gene has been found. Genetic tests were also offered
to the patient's first-degree relatives. However, they have not yet
decided to examination. The patient's only son is underage (he is 5
year old now). The comparison of both patients according to
clinicopathological and molecular factors is shown in Tables I and II.
 | Table IClinicopathological characteristics of
patients. |
Table I
Clinicopathological characteristics of
patients.
| Factors | First patient with
breast cancer | Second patient with
breast cancer |
|---|
| Patient age,
years | 63 | 33 |
| Second cancer | Endometrial
cancer | No second
cancer |
| Stage of
disease | T2N1M0 | T1N0M0 |
| Histological
grade | G2 | G2 |
| ER | Positive | Positive |
| PR | Positive | Positive |
| HER2-positive | Lack of
overexpresion | Overexpression |
| Ki67, % | 10 | 10 |
| Breast cancer
subtype | Luminal A
subtype | Luminal B
HER-positive subtype |
| History of cancer
in family | No history of
cancer | Lung cancer,
adrenal gland cancer |
 | Table IIResults of molecular examination. |
Table II
Results of molecular examination.
| Genes | First patient with
breast cancer | Second patient with
breast cancer |
|---|
| BRCA1
NGS | c.3454G>A
(p.Asp1152Asn) | Not detected |
| BRCA2
NGS | Not detected | c.2374T>C
(p.Tyr792His) |
| CHEK2 | Not detected | c.470T>C
mutation |
| PALB2 | Not detected | Not detected |
| TP53 | Not detected | Not examined |
Discussion
In the present study, VUSs were detected in two
breast cancer patients with family history of cancer. In the first
case, the presence of the c.3454G>A (p.Asp1152Asn) variant was
reported in the BRCA1 gene. The detected variant causes a
change of aspartic acid to asparagine at position 1,152 of the
amino acid sequence of the BRCA1 protein. In the second case, the
presence of the c.2374T>C (p.Tyr792His) variant was detected in
the BRCA2 gene. The reported variant causes a change of
tyrosine to histidine at position 792 of the amino acid sequence of
the BRCA2 protein.
According to the previous studies VUSs should be
interpreted as non-informative and should not influence cancer
management. In that cases clinical management should be based on
personal and family history of cancer. Cancer prevention and
screening strategies should be individualized (19). The determination of VUS
pathogenicity should be based on multifactorial prediction models
including the co-segregation test for family members with known
cancers, periodic re-classification of VUSs, and other genetic
tests, in addition to the detection of the BRCA gene
mutations (20). NCCN guidelines
recommend patients with VUS to be included in the variant
reclassification programs (21).
The variant BRCA1 c.3454G>A results in the
p.Asp1152Asn (D1152N) mutation which causes a change of an Aspartic
Acid to an Asparagine (GAC>AAC) residue in the polypeptide chain
of the protein. Based on alternative nomenclature, this variant
would be defined as BRCA1 3573G>A. This variant has been
previously identified in patients with cancer or subjects with
family history of cancer (family history of breast and/or ovarian
cancer). It has also been identified in a pediatric patient with
leukemia (22-26).
BRCA1 Asp1152Asn was not observed at a significant allele frequency
in large population cohorts (27).
BRCA1 Asp1152Asn is not located in a known functional
domain. The aspartic acid at codon 1152 is moderately conserved,
and computational analyses (SIFT, PolyPhen-2) predict that this
variant is tolerated. In vitro functional analyses
demonstrate activity similar to wildtype in a homology-directed
repair assay (28). One individual
reported in BRCA Share database also carried a likely pathogenic
variant in BRCA1 c.4485_4675del/p.Ser1496GlyfsX14, further
supporting benign nature of this variant. Based on current data,
BRCA1 Asp1152Asn is a VUS as reported by multiple laboratories in
ClinVar (Variation ID: 54890).
Currently, limited information exists with regard to
the c.2374T>C (p.Tyr792His) mutation of the BRCA2 gene,
suggesting that it has uncertain classification and consequently
uncertain clinical significance. This variant causes a change of
tyrosine to histidine at position 792 of the amino acid sequence of
the BRCA2 protein. The tyrosine and histidine are amino
acids with similar properties. This amino acid position is not well
conserved in vertebrate species. In addition, this alteration is
predicted to be tolerated by in silico analysis (SIFT,
Mutation Taster). There is no functional studies concerning this
variant. In the present study, the c.2374T>C (p.Tyr792His)
mutation in the BRCA2 gene was detected in women with stage
IIB cancer according to the TNM classification and luminal B HER2
positive breast cancer subtype. The other characteristics of the
patient were a diagnosis at a younger age (33 years) and family
history of cancer (one relative with lung cancer and the second
relative with adrenal gland cancer). A diagnosis at a younger age
and cancer family history are suggestive of the pathological nature
of c.2374T>C (p.Tyr792His) variant in the BRCA2 gene. The
absence of detected mutations was noted in the BRCA1 gene
and PALB2 gene. However, the c.2374T>C (p.Tyr792His)
mutation in the BRCA2 gene coexisted with c.470T>C
mutation in CHEK2 gene. The meta-analysis demonstrates that
the CHEK2 I157T (also known as c.470T>C) variant
increases cancer risk, especially breast and colorectal cancer in
Caucasian (28).
Borg et al (23) examined a young woman with
contralateral breast cancer (CBC, n=705) and with unilateral breast
cancer (UBC, n=1,398) according to the presence of mutations and
sequence variants of unknown clinical significance (VUS). The
majority of VUSs were not associated with an increased risk of CBC
and represented neutral alleles of no or little significance in the
etiology of breast cancer (23). In
the present study, one patient had unilateral breast cancer (UBC)
and the second patient exhibited contralateral breast cancer (CBC).
Both cases presented stage IIB disease according to the TNM
classification (AJCC system, effective since January 2018).
In some studies BRCA1 mutation carriers were
characterized by ER-negative/PR-negative steroid receptor status of
breast cancer (29,30). A Meta-Analysis conducted by Chen
et al (31) has suggested
that TNBC was more common among the breast cancer patients with
BRCA1 mutation than among BRCA2 mutation carries or
non-carriers. Authors have not found information about
clinicopathological characteristics of patients with BRCA1
c.3454G>A variant. There is also no studies concerning
c.2374T>C (p.Tyr792His) variant in the BRCA2 gene. In our
study, the c.2374T>C (p.Tyr792His) mutation in the BRCA2
gene was associated with luminal B HER2 positive breast cancer
subtype. The variant BRCA1 c.3454G>A was characterized by
ductal invasive carcinoma histologic type G2, ER (+++), PR (+++),
HER2 (+), Ki-67 5% and luminal A subtype.
The limitation of this study is small number of
patients. We presented two patients carrying two VUSs. A larger
group of breast cancer patients with VUSs is required to verify the
current findings and additional data derived from literature
studies are necessary to redefine the clinical significance of VUSs
(change to benign or pathological variant). We do not have
possibility to check ATM or PTEN genes. There was no
mutation in TP53 gene in first patient. The second women
hasn't come yet to continue genetic diagnosis.
In conclusion, the c.3454G>A mutation in the
BRCA1 gene was characterized by NG-2 G-2, HER2 (+), ER
(+++), PR (+++), luminal A subtype and endometrial cancer in
history. However, the c.2374T>C (p.Tyr792His) mutation carriers
in the BRCA2 gene had breast cancer with NG-2 G-2, ER (+++),
PR (+++), Ki-67 10%, HER2 (+++), luminal B subtype, cancer in
family history, younger age at diagnosis and c.470T>C mutation
in CHEK2 gene. Patients with VUSs should be managed based on
their family history of cancer and clinicopathological
characteristics. The clinical significance of VUSs in
BRCA1/2 may change over time and may be reclassified as
‘pathogenic’ or ‘benign’ variants. It is advisable to examine the
presence of VUSs in healthy and sick family members in order to
analyze the co-existence of the variant with breast and/or ovarian
cancer. Patients with VUS should be followed up regularly with use
of individualized screening and prevention strategies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JH was responsible for study design, preparation of
the manuscript and final approval of the version to be published.
WP, MM, JPP, AZ, AFK and MOW were responsible for genetic analysis
and gave final approval of the version to be published. AFK and MOW
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The patients provided written informed consent
regarding the use of their biological material for clinical
research.
Patient consent for publication
Written informed consent was obtained from the
patients for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prokopcova J, Kleibl Z, Banwell CM and
Pohlreich P: The role of ATM in breast cancer development. Breast
Cancer Res Treat. 104:121–128. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Corso G, Montagna G, Figueiredo J, La
Vecchia C, Fumagalli Romario U, Fernandes MS, Seixas S, Roviello F,
Trovato C, Guerini-Rocco E, et al: Hereditary gastric and breast
cancer syndromes related to CDH1 germline mutation: A
multidisciplinary clinical review. Cancers (Basel).
12(1598)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cybulski C, Wokołorczyk D, Jakubowska A,
Huzarski T, Byrski T, Gronwald J, Masojć B, Deebniak T, Górski B,
Blecharz P, et al: Risk of breast cancer in women with a CHEK2
mutation with and without a family history of breast cancer. J Clin
Oncol. 29:3747–3752. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Evans MK and Longo DL: PALB2 mutations and
breast-cancer risk. N Engl J Med. 371:566–568. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tan MH, Mester JL, Ngeow J, Rybicki LA,
Orloff MS and Eng Ch: Lifetime cancer risks in individuals with
germline PTEN mutations. Clin Cancer Res. 18:400–407.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lim W, Olschwang S, Keller JJ, Westerman
AM, Menko FH, Boardman LA, Scott RJ, Trimbath J, Giardiello FM,
Gruber SB, et al: Relative frequency and morphology of cancers in
STK11 mutation carriers. Gastroenterology. 126:1788–1794.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schon K and Tischkowitz M: Clinical
implications of germline mutations in breast cancer: TP53. Breast
Cancer Res Treat. 167:417–423. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Practice bulletin No. 182 summary.
Hereditary breast and ovarian cancer syndrome. Obstet Gynecol.
130:657–659. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
King MC, Marks JH and Mandell JB: New York
Breast Cancer Study Group. Breast and ovarian cancer risks due to
inherited mutations in BRCA1 and BRCA2. Science. 302:643–646.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Toss A, Tomasello Ch, Razzaboni E, Contu
G, Grandi G, Cagnacci A, Schilder RJ and Cortesi L: Hereditary
ovarian cancer: Not only BRCA 1 and 2 genes. Biomed Res Int.
2015(341723)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheon JY, Mozersky J and Cook-Deegan R:
Variants of uncertain significance in BRCA: A harbinger of ethical
and policy issues to come? Genome Med. 6(121)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Radice P, De Summa S, Caleca L and Tommasi
S: Unclassified variants in BRCA genes: Guidelines for
interpretation. Ann Oncol. 22 (Suppl 1):i18–i23. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lindor NM, Goldgar DE, Tavtigian SV, Plon
SE and Couch FJ: BRCA1/2 sequence variants of uncertain
significance: A primer for providers to assist in discussions and
in medical management. Oncologist. 18:518–524. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Richards S, Aziz N, Bale S, Bick D, Das S,
Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al:
Standards and guidelines for the interpretation of sequence
variants: A joint consensus recommendation of the American college
of medical genetics and genomics and the associa-tion for molecular
pathology. Genet Med. 17:405–424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bittar CM, Vieira IA, Sabato CS, Andreis
TF, Alemar B, Artigalás O, Galvão HCR, Macedo GS, Palmero EI and
Ashton-Prolla P: TP53 variants of uncertain significance:
Increasing challenges in variant interpretation and genetic
counseling. Fam Cancer. 18:451–456. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han FF, Guo CL and Liu LH: The effect of
CHEK2 variant I157T on cancer susceptibility: Evidence from a
meta-analysis. DNA Cell Biol. 32:329–335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Boonen RACM, Vreeswijk MPG and van Attikum
H: Functional characterization of PALB2 variants of uncertain
significance: Toward cancer risk and therapy response prediction.
Front Mol Biosci. 7(169)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abdulrahman AA, Heintzelman RC, Corbman M
and Garcia FU: Invasive breast carcinomas with ATM gene variants of
uncertain significance share distinct histopathologic features.
Breast J. 24:291–297. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chern JY, Lee SS, Frey MK, Lee J and Blank
SV: The influence of BRCA variants of unknown significance on
cancer risk management decision-making. J Gynecol Oncol.
30(e60)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Choi MC: Clinical significance of variants
of unknown significances in BRCA genes. J Gynecol Oncol.
30(e80)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines®). Genetic/familial high-risk
assessment: Breast and ovarian. Version 3.2019, 2019.
|
|
22
|
Hansen NT, Brunak S and Altman RB:
Generating genome-scale candidate gene lists for pharmacogenomics.
Clin Pharmacol Ther. 86:183–189. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Borg Å, Haile RW, Malone KE, Capanu M,
Diep A, Törngren T, Teraoka S, Begg CB, Thomas DC, Concannon P, et
al: Characterization of BRCA1 and BRCA2 deleterious mutations and
variants of unknown clinical significance in unilateral and
bilateral breast cancer: The WECARE study. Hum Mutat.
31:E1200–E1240. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stegel V, Krajc M, Zgajnar J, Teugels E,
De Grève J, Hočevar M and Novaković S: The occurrence of germline
BRCA1 and BRCA2 sequence alterations in Slovenian population. BMC
Med Genet. 12(9)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kluska A, Balabas A, Paziewska A, Kulecka
M, Nowakowska D, Mikula M and Ostrowski J: New recurrent BRCA1/2
mutations in Polish patients with familial breast/ovarian cancer
detected by next generation sequencing. BMC Med Genomics.
8(19)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang J, Walsh MF, Wu G, Edmonson MN,
Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA, et al:
Germline mutations in predisposition genes in pediatric cancer. N
Engl J Med. 373:2336–2346. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lek M, Karczewski KJ, Minikel EV, Samocha
KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ,
Cummings BB, et al: Analysis of protein-coding genetic variation in
60,706 humans. Nature. 536:285–291. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu Ch, Xie M, Wendl MC, Wang J, McLellan
MD, Leiserson MD, Huang KL, Wyczalkowski MA, Jayasinghe R, Banerjee
T, et al: Patterns and functional implications of rare germline
variants across 12 cancer types. Nat Commun.
6(10086)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Byrski T, Gronwald J, Huzarski T,
Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wiśniowski
R, Siolek M, et al: Response to neo-adjuvant chemotherapy in women
with BRCA1-positive breast cancers. Breast Cancer Res Treat.
108:289–296. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kirk R: Surgical oncology: Cancer risk
reduction in BRCA mutation carriers. Nat Rev Clin Oncol.
7(609)2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen H, Wu J, Zhang Z, Tang Y and Li X,
Liu S, Cao S and Li X: Association between BRCA status and
triple-negative breast cancer: A meta-analysis. Front Pharmacol.
9(909)2018.PubMed/NCBI View Article : Google Scholar
|