Introduction
Diagnosis of renal cell carcinoma in cases with
polycystic kidney diseases may be difficult and often delayed
despite the use of contrast-enhanced computed tomography (CT) and
magnetic resonance imaging (MRI) (1). The presence of multiple and irregular
sized cysts with haemorrhage and pain and maybe infection play a
role in rendering early diagnosis of renal cell carcinoma.
Autosomal dominant polycystic kidney disease is the most common
autosomal dominant hereditary renal disease, which frequently leads
to end-stage renal disease, necessitating dialysis during or after
the sixth decade of life. The estimated prevalence of ADPKD is 1 in
1,000-2,500 individuals (2).
Case presentation
A 72-year-old patient, height 180 cm, weight 81 kg,
BMI 25 (normal weight), with end-stage renal disease due to
autosomal polycystic kidney disease (ADPKD) received a living
kidney graft. No history of long-standing analgesics. The patient
has a history of nicotine abuse (25 cigarettes per day). The family
history of the patient revealed that the father, one sister and the
grandparents of the patient have not suffered from any tumours or
kidney diseases. The mother and another sister have suffered from
polycystic kidney disease. The patient underwent haemodialysis from
2006 until the date of transplantation. After that, he received a
transplanted kidney in the right iliac fossa in November 2010. No
coronary angiography and no renal functions before transplantation.
Preoperatively he suffered from renal anaemia, hypertension,
ischaemic heart disease and multiple severe arterial and arteriolar
atherosclerosis. Concomitantly, he suffered from arachnoid cyst,
multiple aneurysms in the carotid artery and in the cerebral
vessels. He had also multiple liver cysts and colonic
diverticulosis. He had developed renal hyperparathyreoidismus in
2009. He received parathyroidectomy with reimplantation in the neck
muscle (sternocleidomastoideus muscle). There is no nephrectomy of
the suffered native kidney because there were no medical issues. 8
years after transplantation he developed squamous cell carcinoma in
the skin of the face. Recently in November 2020, he has developed
sustained right renal pain and pressure symptoms.
In the clinical examination of the patient, there
were bad general conditions with reduced weight and renal pain. The
ultrasonography showed polycystic kidneys at both sides. There was
a suspected cystic lesion in the right kidney. The transplanted
right kidney was normal. The computer tomography showed
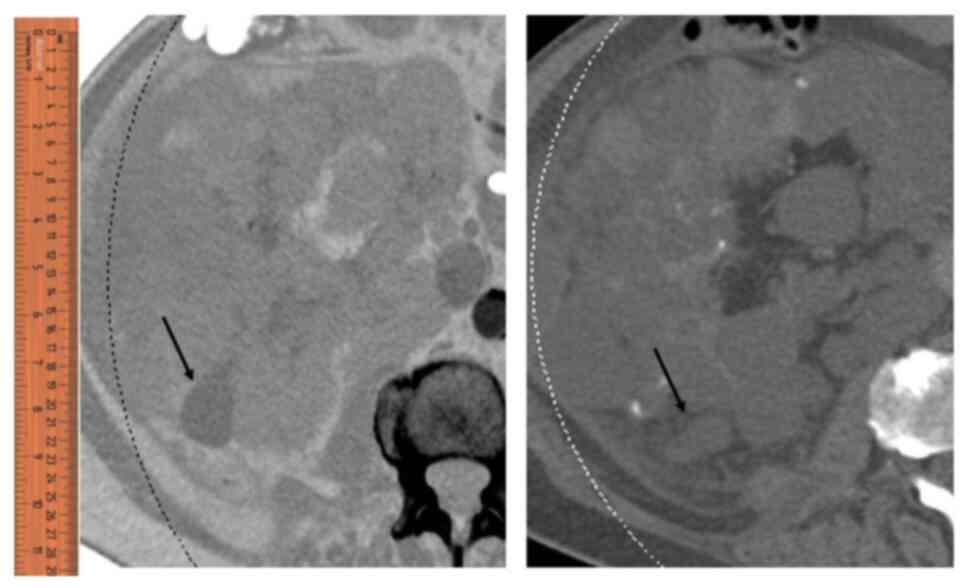
haemorrhagic renal cyst in the right kidney, which was 10 cm in
diameter (Fig. 1). Preoperatively,
there are no available Computer tomography. The examination of the
urine showed excessive RBCs. The laboratory results showed normal
renal functions of the transplanted kidney. At this point, there
was a clinical indication of nephrectomy in the right side for the
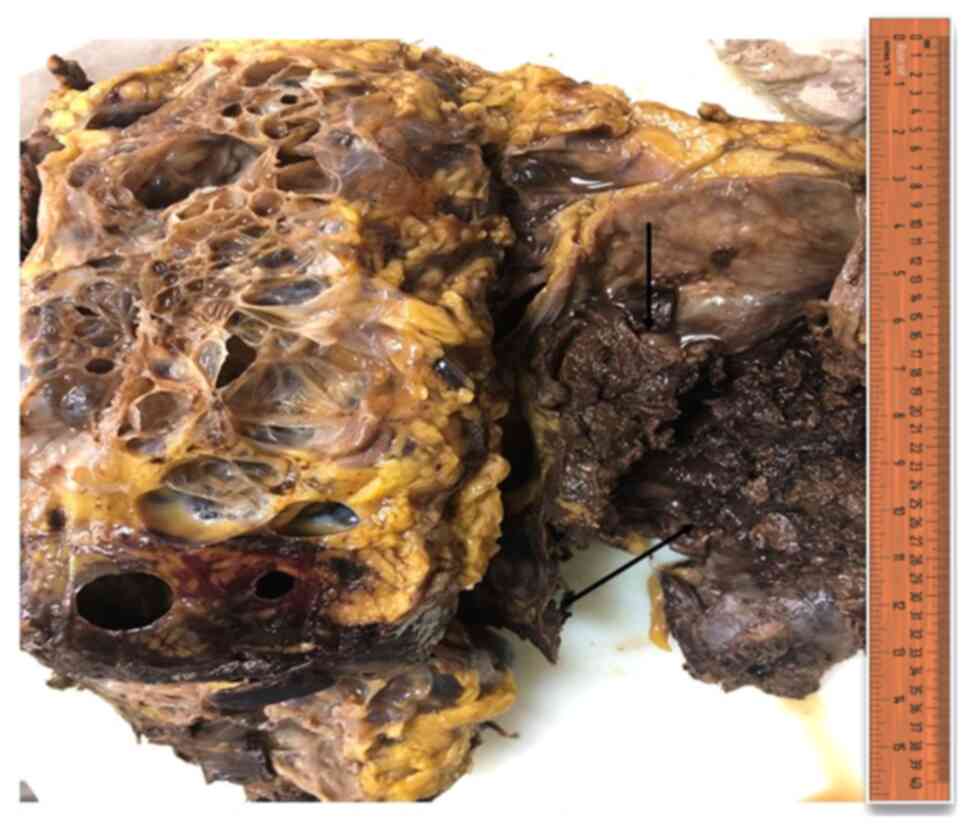
polycystic native kidney. In the gross pathology, there were
multiple cysts with thin rim of kidney tissue in-between the cysts.
One cyst in the lower pole was large, ~20 cm in diameter with solid
area of ~4 cm in diameter (Fig. 2).
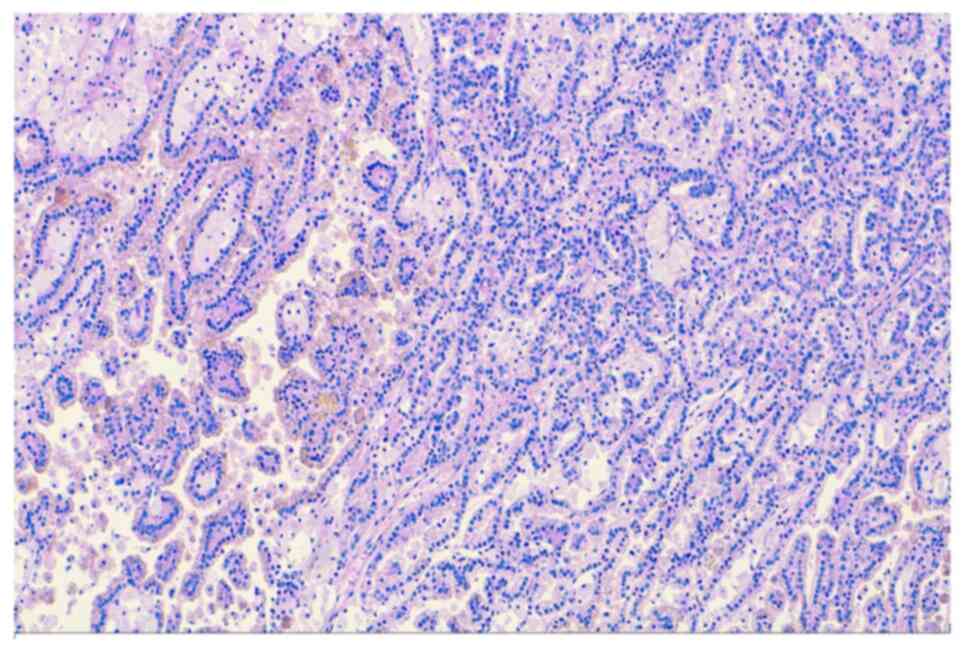
The microscopic examination showed a papillary renal cell carcinoma
(PRCC-type 1) of the right polycystic kidney, ~4 cm in diameter,
which incorporated in the large cyst (Fig. 3). After completing microscopic
examination, there is only stage pT1a (Fig. 4) without metastasis and with
complete resection (R0). PRCC has traditionally been subdivided
into two types. Type 1 carcinomas have papillae covered by cells
with nuclei arranged in a single layer, however, Type 2 carcinomas
have nuclear pseudostratification, often high nuclear grade with
abundant eosinophilic cytoplasm (1,2).
Postoperatively, there were no signs of metastasis
in the body. The transplanted kidney is well functioning.
Discussion
Almost half of the patients with ADPKD will develop
end-stage renal disease (3). There
are accepted indications of nephrectomy in ADPKD cases, such as
recurrent pyelonephritis, cyst haemorrhage requiring repeated
transfusions, pain refractory to medical management and massively
enlarged kidneys that cause pressure symptoms of the organs in the
true pelvis or mechanical pressure with reduced blood supply of the
transplanted kidney (4,5). There is overall increased tendency of
malignancy after transplantation (6). A total of 5% of all malignancies after
transplantations are kidney tumours, twice the amount of that in
the general population (7). After
kidney transplantation, there is detectable increased risk
(15-fold) of developing renal cell carcinoma (RCC) (8,9). Among
these tumours, there is increased incidence (40%) to develop
papillary renal cell carcinoma after transplantation compared to
prevalence of only 10-15% in the general population (10,11).
This is obviously because of the wide and expanded use of
ultrasound and computer tomography in the hospitals (12).
A particular attention should be given for patients
with ADPKD after transplantation, to exclude early tumours.
Previous studies (Table I) have
revealed that organ-confined RCCs, especially those smaller than 4
cm in diameter (pT1a), could be completely cured by partial or
total nephrectomy but RCCs more enlarged than 7 cm in diameter
(pT2) require an adequate tumour-free waiting period after surgery
(13,14).
 | Table IPrevious case reports and works from
1993-2016. |
Table I
Previous case reports and works from
1993-2016.
| Case report | Year | Age, years | Sex | Maximum size, cm | Pathological
subtype | (Refs.) |
|---|
| Sulser et
al | 1993 | 37 | M | 4 | PRCC | (23) |
| Keith et
al | 1994 | 31 | M | 7 | CCC | (1) |
| | | 44 | F | 6 | CCC | |
| | | 62 | F | N.A. | Sarcomatoid RCC | |
| Gatalica et
al | 1994 | 44 | M | N.A. | PRCC | (24) |
| Soderdahl et
al | 1997 | 63 | M | 4.5 (multifocal) | CCC, PRCC | (25) |
| Jürgensen et
al | 1999 | 72 | F | N.A. | CCC | (26) |
| Hama et
al | 2005 | 66 | F | N.A | CCC | (27) |
| Lang et
al | 2005 | 47 | M | 4.8 | N.A. | (28) |
| Kato et
al | 2007 | 56 | M | N.A | CCC, PRCC | (29) |
| Chang et
al | 2007 | 58 | M | 8 (bilateral) | CCC, PRCC | (30) |
| Chang et
al | 2009 | 58 | F | 4 | CCC | (31) |
| Zeile et
al | 2011 | 47 | M | 3.6 | CCC | (32) |
| Misumi et
al | 2012 | 57 | M | 1 | PRCC type 2 | (33) |
| Na et
al | 2012 | 45 | M | 6 (multifocal) | CCC, PRCC | (34) |
| Konosu-Fukaya et
al | 2013 | 58 | M | 7 | PRCC type 1 | (35) |
| | | 32 | M | 1 | PRCC type 1 | |
| Ito et
al | 2014 | 67 | F | 3 | CCC | (36) |
| Zhang et
al | 2016 | 73 | F | 3.4 | PRCC type 2 | (37) |
| Nezu et
al | 2016 | 47 | F | 3.6 | CCC | (38) |
Not only is there a risk of developing RCC in the
native kidney but also in the transplanted kidney as we already
presented in a previous work (15).
We have supposed, that there are many sustained risk factors in
these patients such as smoking, obesity and abuse of analgesics as
well as prolonged use of high-dosage immunosuppressive therapy
after transplantation which enhance the formation of tumours
(6,16).
Tumours under 4 cm are difficult to be detected in
the native polycystic kidney and there is important need of
screening and close follow-up of these patients with urine
analysis, ultrasonography and computer tomography. With persistent
symptoms such as side pain or haematuria, a nephrectomy should be
performed. There is also an elevated risk of late-onset kidney
cancer (17,18) and also in studies that included
recipients of other transplanted organs (19,20),
which is not well understood.
In conclusion, this case and our previously
published cases (15) demonstrate
from the clinical point of view the importance and urgent
development of a screening method including clinical examination,
urine analysis, ultrasonography (21,22)
and computer tomography in short intervals for detecting and
monitoring not only the transplanted kidneys but also to exclude
the malignancy of both native and transplanted kidneys as well as
to early detect the malignant tumours to enhance better outcome
without metastasis. From the histopathological point of view, there
is the need to deal macroscopically with the surgically removed
kidney with care and insisting on dissecting the kidney in thin
sections (5-10 mm thick) to detect small tumours within the heavy
large tissue of a polycystic kidney, which is normally above 4 kg
weight.
Acknowledgements
We would like to thank Miss Sandra Minns (Institute
for Pathology and Cytology, Schüttorf, Germany) for proofreading
the manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MA performed diagnosis and assisted in the
collection of data, writing and publishing. MP assisted in the
collection of clinical data and coordination. AT performed
diagnosis and sampling. OAB assisted in the coordination and
collection of clinical data. OB assisted in diagnosis of the case,
and proof-read and wrote the manuscript. All authors have read and
approved the final manuscript. MA and OB confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Keith DS, Torres VE, King BF, Zincki H and
Farrow GM: Renal cell carcinoma in autosomal dominant polycystic
kidney disease. J Am Soc Nephrol. 4:1661–1669. 1994.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cornec-Le Gall E, Alam A and Perrone RD:
Autosomal dominant polycystic kidney disease. Lancet. 393:919–935.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Headhunt B and Elbe JN: Papillary renal
cell carcinoma: A clinicopathologic and immunohistochemical study
of 105 tumors. Mod Pathol. 10:537–544. 1997.PubMed/NCBI
|
|
4
|
Delahunt B, Elbe JN, Mc Credie MR,
Bthwaite PB, Stewart JH and Bilous AM: Morphologic typing of
papillary renal cell carcinoma: Comparison of growth kinetics and
patient survival in 66 cases. Hum Pathol. 32:590–595.
2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Parfrey PS, Bear JC, Morgan J, Cramer BC,
McManamon PJ, Gault MH, Churchill DN, Singh M, Hewitt R, Somlo S,
et al: The diagnosis and prognosis of autosomal dominant polycystic
kidney disease. N Engl J Med. 323:1085–1090. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sulikowski T, Tejchman K, Zietek Z,
Rózański J, Domański L, Kamiński M, Sieńko J, Romanowski M, Nowacki
M, Pabisiak K, et al: Experience with autosomal dominant polycystic
kidney disease in patients before and after renal transplantation:
A 7-year observation. Transplant Proc. 41:177–180. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rayner BL, Cassidy MJ, Jacobsen JE, Pascoe
MD, Pontin AR and van Zyl Smit R: Is preliminary binephrectomy
necessary in patients with autosomal dominant polycystic kidney
disease undergoing renal transplantation? Clin Nephrol. 34:122–124.
1990.PubMed/NCBI
|
|
8
|
Morath C, Mueller M, Goldschmidt H,
Schwenger V, Opelz G and Zeier M: Malignancy in renal
transplantation. J Am Soc Nephrol. 15:1582–1588. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Penn I: Primary kidney tumours before and
after renal transplantation. Transplantation. 59:480–485.
1995.PubMed/NCBI
|
|
10
|
Kasiske BL, Snyder JJ, Gilbertson DT and
Wang C: Cancer after kidney transplantation in the United States.
Am J Transplant. 4:905–913. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suson KD, Sausville JE, Sener A and Phelan
MW: Native nephrectomy for renal cell carcinoma in transplant
recipients. Transplantation. 91:1376–1379. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schwarz A, Vatandaslar S, Merkel S and
Haller H: Renal cell carcinoma in transplant recipients with
acquired cystic kidney disease. Clin J Am Soc Nephrol. 2:750–756.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hoshida Y, Tsukuma H, Yasunaga Y, Xu N,
Fujita MQ, Satoh T, Ichikawa Y, Kurihara K, Imanishi M, Matsuno T
and Aozasa K: Cancer risk after renal transplantation in Japan. Int
J Cancer. 71:517–520. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mihara S, Kuroda K, Yoshioka R and Koyama
W: Early detection of renal cell carcinoma by ultrasonographic
screening-based on the results of 13 years screening in Japan.
Ultrasound Med Biol. 25:1033–1039. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Patard JJ, Shvarts O, Lam JS, Pantuck AJ,
Kim HL, Ficarra V, Cindolo L, Han KR, De La Taille A, Tostain J, et
al: Safety and efficacy of partial nephrectomy for all T1 tumors
based on an international multicenter experience. J Urol.
171:2181–2185, 2435. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Thompson RH, Leibovich BC, Cheville JC,
Webster WS, Lohse CM, Kwon ED, Frank I, Zincke H and Blute ML: Is
renal sinus fat invasion the same as perinephric fat invasion for
pT3a renal cell carcinoma? J Urol. 174:1218–1221. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gerth HU, Pohlen M, Thoennissen NH,
Suwelack B, Pavenstädt HJ, Störkel S, Abbas M, Spieker T and
Thölking G: Two papillary renal cell carcinomas of different origin
following renal transplantation (Case report). Oncol Lett. 4:80–82.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Engels EA, Pfeiffer RM, Fraumeni JF,
Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly
AR, Clarke CA, et al: Spectrum of cancer risk among US solid organ
transplant recipients. JAMA. 306:1891–1901. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alexander MP, Farag YM, Mittal BV, Rennke
HG, Tullius SG and Singh AK: De novo multifocal renal cell
carcinoma in the renal allograft. Kidney Int. 75:111–114.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Karami S, Yanik EL, Moore LE, Pfeiffer RM,
Copeland G, Gonsalves L, Hernandez BY, Lynch CF, Pawlish K and
Engels EA: Risk of renal cell carcinoma among kidney transplant
recipients in the United States. Am J Transplant. 16:3479–3489.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Collett D, Mumford L, Banner NR, Neuberger
J and Watson C: Comparison of the incidence of malignancy in
recipients of different types of organ: A UK Registry audit. Am J
Transplant. 10:1889–1896. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bennett WM, Simonich EL, Garre AM, McEvoy
KM, Farinola MA and Batiuk TD: Renal cell carcinoma in renal
transplantation: The case for surveillance. Transplant Proc.
49:1779–1782. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sulser T, Fehr JL, Hailemariam S, Briner J
and Hauri D: Papillary renal cell carcinoma associated with
autosomal dominant polycystic kidney disease. Urol Int. 51:164–166.
1993.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gatalica Z, Schwarting R and Petersen RO:
Renal cell carcinoma in the presence of adult polycystic kidney
disease. Urology. 43:102–105. 1994.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Soderdahl DW, Thrasher JB and Hansberry
KL: Bilateral renal cell carcinoma in autosomal dominant polycystic
kidney disease. A case report and literature review. Am J Nephrol.
17:96–99. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jürgensen JS, Müller V, Kettritz U,
Woywodt A, Göbel U and Luft FC: A malignant ‘incidentaloma’ in a
patient with autosomal dominant polycystic kidney disease. Nephrol
Dial Transplant. 14:490–492. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hama Y, Kaji T, Ito K, Hayakawa M, Tobe M
and Kosuda S: Erythropoietin-producing renal cell carcinoma arising
from autosomal dominant polycystic kidney disease. Br J Radiol.
78:269–271. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lang EK and Davis R: Autosomal dominant
polycystic disease with renal cell carcinoma. J Urol.
173(987)2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kato T, Takahashi Y, Nakane K, Yokoi S,
Ehara H, Shinoda I and Deguchi T: Bilateral renal cell carcinoma
associated with polycystic kidney disease: Case report and
literature review. Hinyokika Kiyo. 53:117–119. 2007.PubMed/NCBI(In Japanese).
|
|
30
|
Chang YL, Chung HJ and Chen KK: Bilateral
renal cell carcinoma in a patient with autosomal dominant
polycystic kidney disease. J Chin Med Assoc. 70:403–405.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chang MY, Chen YM, Chen YC, Tian YC, Fang
JT and Yang CW: Concurrent renal cell carcinoma and central nervous
system lymphoma in a patient with autosomal dominant polycystic
kidney disease. Med Princ Pract. 18:486–489. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zeile M, Andreou D, Poellinger A, Tunn PU
and Dudeck O: Identification of the primary tumour with the help of
diffusion-weighted MRI in a patient with autosomal dominant
polycystic kidney disease and metastatic renal cell carcinoma. Br J
Radiol. 84:e142–e145. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Misumi T, Ide K, Onoe T, Banshodani M,
Tazawa H, Teraoka Y, Hotta R, Yamashita M, Tashiro H and Ohdan H:
Incidental renal cell carcinoma presenting in a renal transplant
recipient with autosomal dominant polycystic kidney disease: A case
report. J Med Case Rep. 6(154)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Na KY, Kim HS, Park YK, Chang SG and Kim
YW: Multifocal renal cell carcinoma of different histological
subtypes in autosomal dominant polycystic kidney disease. Korean J
Pathol. 46:382–386. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Konosu-Fukaya S, Nakamura Y, Fujishima F,
Kasajima A, Takahashi Y, Joh K, Ikeda Y, Ioritani N, Watanabe M and
Sasano H: Bilateral papillary renal cell carcinoma and
angiomyolipoma in the patients with autosomal dominant polycystic
kidney disease: Case report of two cases and literature review. Pol
J Pathol. 64:303–307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ito K and Asano T, Tominaga S, Yoshii H,
Sawazaki H and Asano T: Erythropoietin production in renal cell
carcinoma and renal cysts in autosomal dominant polycystic kidney
disease in a chronic dialysis patient with polycythemia: A case
report. Oncol Lett. 8:2032–2036. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang W, Tan AY, Blumenfeld J, Liu G,
Michaeel A, Zhang T, Robinson BD, Salvatore SP, Kapur S, Donahue S,
et al: Papillary renal cell carcinoma with a somatic mutation in
MET in a patient with autosomal dominant polycystic kidney disease.
Cancer Genet. 209:11–20. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nezu K, Sakai T, Kuromoto A, Kanno H, Sato
M, Numahata K and Hoshi S: A case of autosomal dominant polycystic
kidney disease (ADPKD) with metastases from bilateral small renal
cell carcinoma. Hinyokika Kiyo. 62:313–316. 2016.PubMed/NCBI(In Japanese).
|