Introduction
Renal cell carcinoma accounts for approximately 2-3%
of adult cancers, and the rate of its incidental diagnosis is
increasing with the increased use of imaging methods. Numerous
diagnostic, predictive and prognostic biomarkers have been studied
for the diagnosis, treatment planning, and follow-up. Especially
the differentiation of eosinophilic kidney tumors has become more
challenging with newly defined tumors and the need for different
immunohistochemical and molecular-genetic studies for differential
diagnosis has increased day by day (1,2). The
marker thought to help in the differentiation of these tumors is
TRPM4 (transient receptor potential cation channel subfamily M4), a
protein localized on the cell membrane.
In mammals, there are 28 TRP (transient receptor
potential) channels, which are cation-selective and located on the
cell membrane. Although this protein superfamily has 6 subgroups,
one of the most important is TRP that contains Melastatin (3). TRPM (transient receptor potential
cation channel subfamily M) has eight subgroups, most of which are
permeable to bivalent cations, while TRPM4 and TRPM5 are channels
that are impermeable to calcium (Ca+2) but only
permeable to monovalent cations. When the amount of cytosolic ATP
decreases and Ca+2 level increases, the TRPM4 channel is
activated (4) and indirectly help
regulate intracellular Ca+2 through the regulation of
cell membrane potential. These ion channels and Ca+2 are
responsible for cell proliferation, apoptosis, and differentiation
to maintain cell homeostasis (5).
The expression of TRPM4 has been evaluated in cancer of several
organs, especially the prostate and colon (6,7).
However, there is no clear data regarding the place of the kidney
in the differential diagnosis of eosinophilic tumors.
The aim of our study was to analyze the staining
pattern of TRPM4 antibody in common eosinophilic kidney tumors such
as eosinophilic clear cell renal cell carcinoma (CCRCC),
Chromophobe Renal Cell Carcinoma (ChRCC), Renal Oncocytoma (RO) and
Papillary Renal Cell Carcinoma Type 2 (PRCC2), and to determine
whether it can be a diagnostic marker.
Materials and methods
Patient
The study included a total of 112 patients,
including 97 patients diagnosed with eosinophilic CCRCC, ChRCC,
P2RCC, RO and 15 patients with P1RCC from the partial and radical
nephrectomy specimens studied at Bezmialem Vakif University Faculty
of Medicine between January 2014 and 2019. This study is a
retrospective study made of paraffin blocks. The presence of TRPM4
could also be demonstrated by Western Blot analysis; however,
Western Blot analysis could not be performed in this study as there
were no fresh tissues of the cases. The study was approved by the
ethics committee board of Bezmialem University.
Immunohistochemistry
In the present study, hematoxylin and eosin
(H&E) stained preparations were re-evaluated, and block
selection was made for immunohistochemical staining. Two-micron
thick slides were taken from the paraffin blocks prepared from
formalin-fixed specimens obtained from the primary tumor. On these
slides, TRPM4 (monoclonal mouse antibody; Novus Biologicals, USA
Clone OTI10H5) immunohistochemistry was performed by being diluted
at a ratio of 1/100 in 2 h of incubation in Ventana Benchmark Ultra
and Bench Mark XT devices.
Scoring of immunoreactivity
The extent of TRPM4 staining was evaluated
semi-quantitatively and interpreted as 0, negative; 1, focal
positive (1-10%); and 2, diffuse positive staining (more than 10%).
Staining patterns were evaluated as membranous-cytoplasmic and
basolateral. Staining patterns and their extent were compared with
tumor types, demographic data, and cytokeratin 7 (CK7) staining
status.
Statistical analysis
The statistical analysis was evaluated using the IBM
SPSS 22.0 statistical software package. The distribution of
categorical variables was evaluated with the Chi-square test.
Descriptive statistics were expressed as standard deviation,
frequency, and percentage. A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Patient data and histopathological
parameters
Of the 112 patients included in the study, 33
(29.5%) had eosinophilic CCRCC, 35 (31.3%) had eosinophilic ChRCC,
8 (7.1%) had P2RCC, 21 (18.8%) had RO, and 15 (13.4%) had P1RCC. Of
the patients, 46 (41.1%) were female and 66 (58.9%) were male, with
a mean age of 57.8 years. The clinical and pathological parameters
of the patients are summarized in Table
I.
 | Table IClinicopathological data of
patients. |
Table I
Clinicopathological data of
patients.
| Variable | CCRCC (n=33) | ChRCC (n=35) | RO (n=21) | P2RCC (n=8) |
|---|
| Age, years (mean ±
SD) | 58.97±12.5 | 56.43±11.6 | 57.14±13.38 | 59.13±12.69 |
| Sex, n (%) | | | | |
|
Male | 21 (63.6) | 15 (42.9) | 10 (47.6) | 6 (75.0) |
|
Female | 12 (36.4) | 20 (57.1) | 11 (52.4) | 2 (25.0) |
| Tumor size, cm (mean
± SD) | 6.03±2.98 | 5.85±0.85 | 4.51±2.98 | 6.81±2.25 |
| Tumour location, n
(%) | | | | |
|
Left | 12 (36.4) | 19 (54.3) | 11 (52.4) | 3 (37.5) |
|
Right | 21 (63.6) | 16 (45.7) | 10 (47.6) | 5 (62.5) |
| pT, n (%) | | | | |
|
pT1 | 16 (48.5) | 15 (42.9) | | 3 (37.5) |
|
pT2 | 5 (15.2) | 13 (37.1) | | 2 (25.0) |
|
pT3 | 11 (33.3) | 6 (17.1) | | 2 (2.5) |
|
pT4 | 1 (3.0) | 1 (2.9) | | 1 (12.5) |
TRPM4 staining rates and distributions
in CCRCC, ChRCC, RO and P2RCC tumors
The staining ratios of the tumors with TRPM4 are
presented in Table II. Among the
97 eosinophilic tumors, 2 (4.9%) of the 41 patients with diffuse
staining for TRPM4 were CCRCC, 15 (36.6%) were ChRCC, 20 (48.8%)
were RO, and 4 (9.8%) were P2RCC. While a significant result was
obtained between the RO and ChRCC and CCRCC and P2RCC patients
(P=0.043), there was no significant result between the RO and ChRCC
patients (P=0.065).
 | Table IITransient Receptor Potential
Melastatin 4 staining rates in eosinophilic kidney tumors. |
Table II
Transient Receptor Potential
Melastatin 4 staining rates in eosinophilic kidney tumors.
| Tumor numbers | Negative, n (%) | Focal positive, n
(%) | Diffuse positive, n
(%) |
|---|
| CCRCC (n=33) | 31 (93.9) | 0 (0.0) | 2 (6.1) |
| ChRCC (n=35) | 8 (22.9) | 12 (34.3) | 15 (42.9) |
| RO (n=21) | 0 (0.0) | 1 (4.8) | 20 (95.2) |
| P2RCC (n=8) | 1 (4.9) | 3 (37.5) | 4 (50.0) |
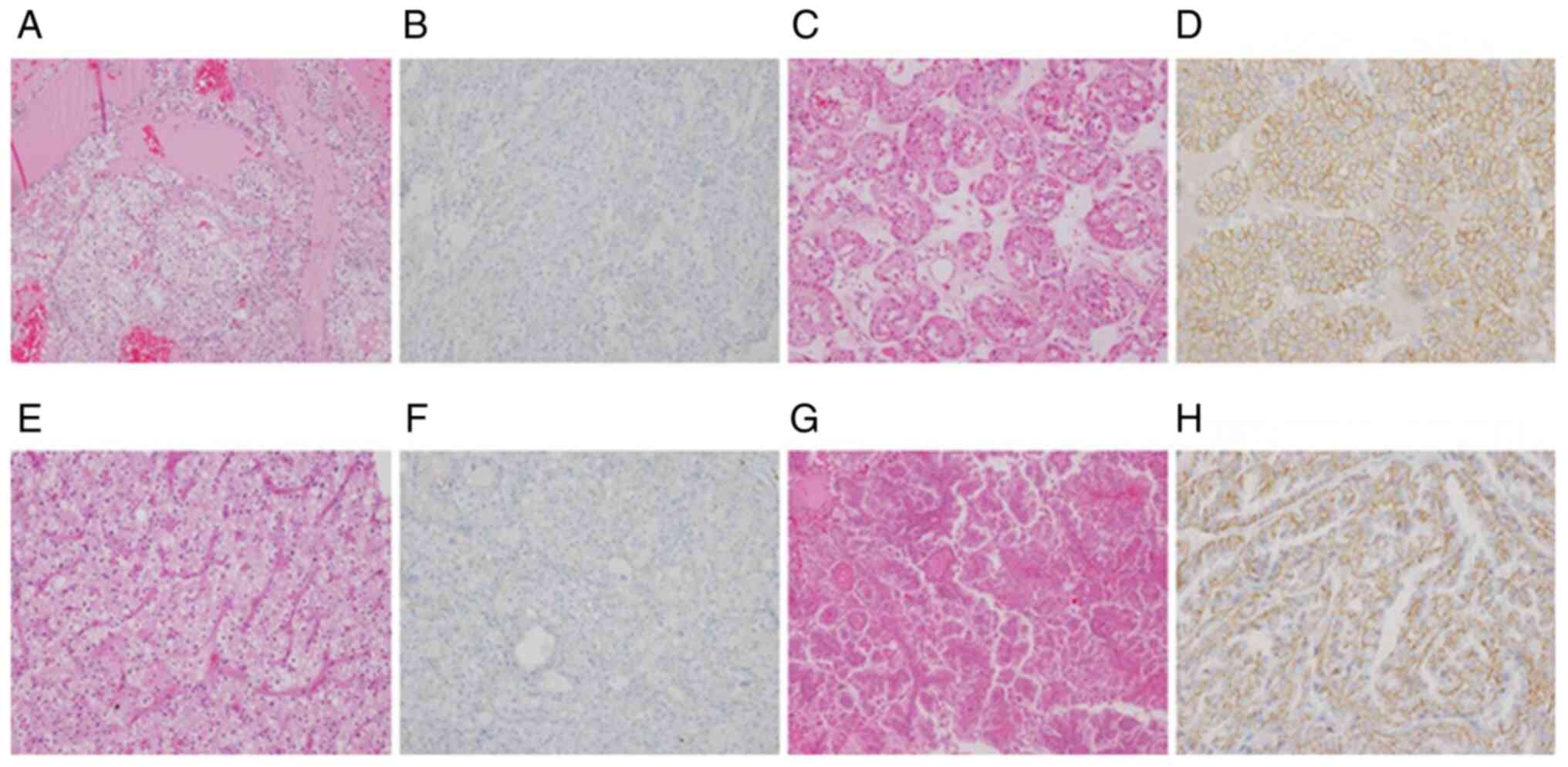
While TRPM4 staining was diffuse membranous in the
collecting ducts of the healthy kidney, different staining patterns
were observed in the oncocytoma and eosinophilic RCC subtypes.
Staining was observed in 57 of the patients. Membranous staining
was observed in 50 patients and basolateral staining in 7 patients.
Of the patients with membranous staining, 26 (52%) had ChRCC, 21
(42%) had RO, 3 (6%) had P2RCC. Membranous staining was not
observed in any of the CCRCC patients, and there was a
statistically significant difference between the RO and ChRCC
patients (P<0.005). There were 2 (28.6%) CCRCC, 1 (14.3%) ChRCC,
and 4 (57.1%) P2RCC patients with basolateral staining. In terms of
P1RCC, 1 (6.7%) patient had negative staining, 3 (20%) patients had
focal, and 11 (73.3%) patients had diffuse staining. Of the P1RCC
patients, 11 (73.3%) had basolateral and 3 (20%) had membranous
staining (Fig. 1).
TRPM4 staining (negative, focal, diffuse staining)
of the patients were compared with CK7 immunohistochemistry, which
is used for the diagnosis and differentiation of tumors. CK7 and
TRPM4 staining by the diagnoses of the tumors are summarized in
Fig. 2. In CCRCC, focal staining
was observed in 5 (15%) cases with CK7 and diffuse staining in 2
(4.9%) cases with TRPM4. In RO, focal staining was observed in 13
(61.9%) cases with CK7, diffuse staining in 1 (4.7%) case, diffuse
staining in 20 (86.9%) cases with TRPM4, and focal staining in 1
(8.6%) case. ChRCC had diffuse staining in 34 (97.1%) cases with
CK7, focal staining in 1 (2.8%) case, diffuse staining in 15
(42.8%) cases with TRPM4, and focal staining in 12 (34.2%) cases.
In P2RCC, diffuse staining was observed in 3 (37.5%) cases with CK7
and focal staining in 3 (37.5%) cases, while diffuse staining was
observed in 4 (50%) cases with TRPM4 and focal staining in 3
(37.5%) cases.
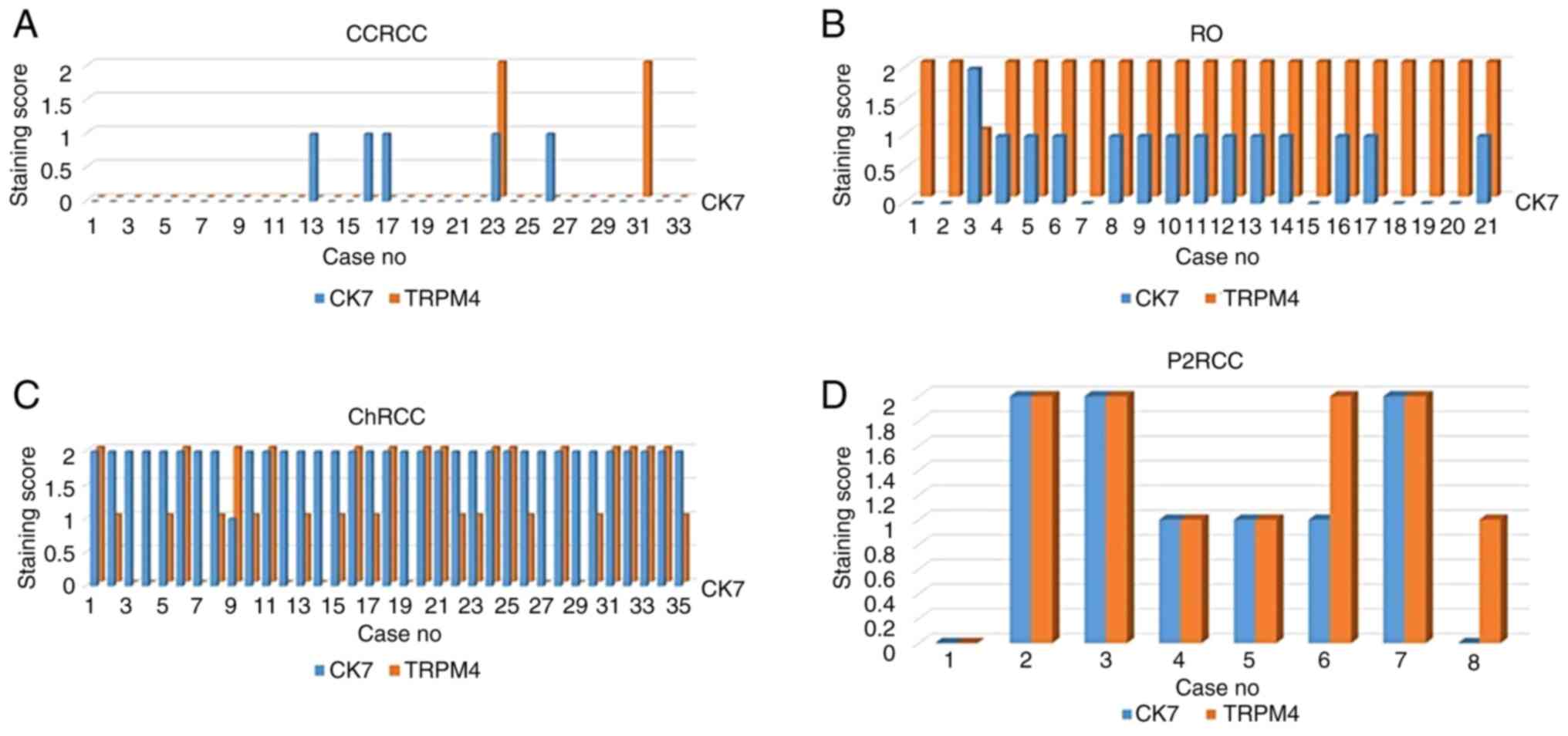 | Figure 2Comparison of TRPM4 and CK7 staining
in patients with CCRCC, RO, ChRCC and P2RCC. (A) Comparison of
TRPM4 and CK7 staining in patients with CCRCC. (B) Comparison of
TRPM4 and CK7 staining in patients with RO. (C) Comparison of TRPM4
and CK7 staining in patients with ChRCC. (D) Comparison of TRPM4
and CK7 staining in patients with P2RCC. Y-axis, Prevalence of
staining; 0, negative; 1, focal positive; and 2, diffuse positive.
X-axis: Number of cases stained with CK7 and TRPM4
immunohistochemical staining TRPM4, transient receptor potential
cation channel subfamily M member 4; CK7, cytokeratin 7; CCRCC,
clear cell renal cell carcinoma; RO, renal oncocytoma; ChRCC,
chromophobe renal cell carcinoma; P2RCC, papillary renal cell
carcinoma type 2. |
Discussion
Ca+2 dependent signaling pathways are
associated with proliferation, migration, invasion, metastasis, and
apoptosis of tumor cells (8,9). TRPM4
is a monovalent nonselective cation channel activated by decreased
ATP level and increased Ca+2 in case of hypoxia, the
membrane is depolarized, and the voltage-dependent calcium channel
is blocked by the Na+ current, decreasing the permeation
of Ca+2 (10).
The expression of TRPM4 has been studied in multiple
sclerosis (11), subarachnoid
haemorrhage (12), and cerebral
infarction (13), but there are
very few studies on the relationship between the tumor and
TRPM4(14). It has recently been
evaluated in prostate (15),
bladder (16), colorectal cancer
(17), cervical cancer (18), large B-cell lymphoma (19), and liver cancer (20). It has been determined that it is
higher in the tumor in the prostate compared to healthy tissues,
and its expression increases (21,15) in
the transition from prostatic intraepithelial neoplasia to
prostatic carcinoma, and high levels of TRPM4 are associated with
recurrence after prostatectomy (22). It has been found to be highly
expressed in cervical cancer and large B-cell lymphoma compared to
reactive tissues (18,19). No difference has been found in the
expression of TRPM4 between carcinoma of the bladder and healthy
tissue (16). In our study, diffuse
membranous staining was detected in healthy tissue and oncocytoma,
while loss of expression was observed in CCRCC and different rates
of staining were observed in ChRCC and P2RCC. In addition to
healthy tissue, its staining in RO and ChRCCs suggests that it may
be related to the same origin.
Over the past two decades, there have been many
morphological, immunohistochemical and prognostic innovations in
renal tumors (23). Among tumors
with eosinophilic cytoplasm, there are new entities such as
SDH-deficient RC, eosinophilic solid and cystic RCC, Warthin-like
papillary RCC, and low grade oncocytic tumor in addition to ChRCC,
RO, P2RCC, eosinophilic CCRCC (2,23).
There are a limited number of cases regarding these tumors, and
studies are ongoing in terms of both diagnosis and prognosis. It is
important to differentiate the oncocytoma, which accounts for
approximately 10% of renal tumors, from malignant tumors,
especially in needle biopsies. In addition to morphological
findings, CK7 is the most important marker in differentiating
ChRCC, another CD117-positive eosinophilic tumor (2,24).
While the staining pattern of CK7 is negative or focal positive in
RO, it is usually diffuse positive in ChRCC. In our study, the
staining of TRPM4 expression was diffuse positive in oncocytoma,
unlike CK7. But negative, focal positive and diffuse positive
stainings were detected in ChRCC. Besides morphological findings in
the differentiation between eosinophilic CCRCC and ChRCC, the use
of stains such as CK7, CD117 and Carbonic Anhydrase IV along with
TRPM4 will be supportive in the differentiation.
Papillary RCC has an incidence rate of 15% and
consists of type 1 with amphophilic cytoplasm and type 2 with
eosinophilic cytoplasm (25). TRPM4
generally showed diffuse basolateral staining in P2RCC, and the
staining pattern was completely different from other eosinophilic
tumors included in the study. Among papillary RCCs, P2RCC has worse
prognosis than P1RCC, and it is important to differentiate between
them. Diffuse positive basolateral staining was usually seen for
TRPM4 in both of them. Although TRPM4 is not a marker that can be
used to differentiate between P1RCC and P2RCC, it is interesting
that basolateral staining is dominant in papillary structuring.
There are publications in the literature showing an
increase in the expression of TRPM4 in prostate, cervix, and large
cell lymphoma. In these studies, there are arguments that
suppression of TRPM4 can prevent tumor growth and metastasis
(26). Wong and Hussain (27) also found TRPM4 to be associated with
poor prognostic parameters in breast carcinoma. However, in this
study, different expressions of TRPM4 were observed in malignant
tumors of the kidney, while diffuse strong expression was
remarkable in healthy tissue and oncocytoma, a benign tumor. In
this study, TRPM4 was studied only for diagnostic purposes,
although no interpretation could be made regarding the treatment or
prognosis in RCCs, it is thought that TRPM4 can be used together
with other immunohistochemical markers in eosinophilic renal
tumors.
In this study, it was concluded that TRPM4 was
useful in the differentiation of eosinophilic kidney tumors. The
extent of staining along with CK7 may be helpful in the
differentiation between common oncocytoma and chromophobe RCC, and
for the diagnosis of papillary RCC, it can be helpful in cases of
difficulty with different staining patterns. In this respect, its
reliability can be increased to a more significant level with other
markers and large series.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GÇ, PY and NŞ conceived and designed the study. GÇ,
NŞ, and BD acquired the data. GÇ and ZG analyzed and interpreted
the data. GÇ, NŞ and ZG confirm the authenticity of the raw data.
GÇ and NŞ wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Bezmialem Vakif University Hospital (Istanbul, Turkey;
approval no. 11-202), and written informed consent was obtained
from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iczkowski KA and Czaja RC: Eosinophilic
kidney tumors: Old and new. Arch Pathol Lab Med. 143:1455–1463.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kryvenko ON, Jorda M, Argani P and Epstein
JI: Diagnostic approach to eosinophilic renal neoplasms. Arch
Pathol Lab Med. 138:1531–1541. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guinamard R, Sallé L and Simard C: The
non-selective monovalent cationic channels TRPM4 and TRPM5. Adv Exp
Med Biol. 704:147–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vennekens R and Nilius B: Insights into
TRPM4 function, regulation and physiological role. Handb Exp
Pharmacol. 179:269–285. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Prevarskaya N, Skryma R, Bidaux G,
Flourakis M and Shuba Y: Ion channels in death and differentiation
of prostate cancer cells. Cell Death Differ. 14:1295–1304.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kappel S, Stokłosa P, Hauert B,
Ross-Kaschitza D, Borgström A, Baur R, Galván JA, Zlobec I and
Peinelt C: TRPM4 is highly expressed in human colorectal tumor buds
and contributes to proliferation, cell cycle, and invasion of
colorectal cancer cells. Mol Oncol. 13:2393–2405. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Holzmann C, Kappel S, Kilch T, Jochum MM,
Urban SK, Jung V, Stöckle M, Rother K, Greiner M and Peinelt C:
Transient receptor potential melastatin 4 channel contributes to
migration of androgen-insensitive prostate cancer cells.
Oncotarget. 6:41783–41793. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gao Y and Liao P: TRPM4 channel and
cancer. Cancer Lett. 454:66–69. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shapovalov G, Ritaine A, Skryma R and
Prevarskaya N: Role of TRP ion channels in cancer and
tumorigenesis. Semin Immunopathol. 38:357–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Monteith GR, McAndrew D, Faddy HM and
Roberts-Thomson SJ: Calcium and cancer: Targeting Ca2+
transport. Nat Rev Cancer. 7:519–530. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Schattling B, Steinbach K, Thies E, Kruse
M, Menigoz A, Ufer F, Flockerzi V, Brück W, Pongs O, Vennekens R,
et al: TRPM4 cation channel mediates axonal and neuronal
degeneration in experimental autoimmune encephalomyelitis and
multiple sclerosis. Nat Med. 18:1805–1811. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Tosun C, Kurland DB, Mehta R, Castellani
RJ, deJong JL, Kwon MS, Woo SK, Gerzanich V and Simard JM:
Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and
cognitive impairment in subarachnoid hemorrhage. Stroke.
44:3522–3528. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mehta RI, Tosun C, Ivanova S, Tsymbalyuk
N, Famakin BM, Kwon MS, Castellan RJ, Gerzanich V and Simard JM:
Sur1-Trpm4 cation channel expression in human cerebral infarcts. J
Neuropathol Exp Neurol. 74:835–849. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qin F, Lao L, Huang M, Tan H, Jin X, Ma X
and Zeng J: Evaluation of the TRPM protein family as potential
biomarkers for various types of human cancer using public database
analyses. Exp Ther Med. 20:770–785. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Holzmann C, Kappel S, Kilch T, Jochum MM,
Urban SK, Jung V, Stöckle M, Rother K, Griner M and Peinelt C:
Transient receptor potential melastatin 4 channel contributes to
migration of androgen-insensitive prostate cancer cells.
Oncotarget. 8:41783–41793. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ceylan GG, Önalan EE, Kuloğlu T, Aydoğ G,
Keleş İ, Tonyal Ş and Ceylan C: Potential role of
melastatin-related transient receptor potential cation channel
subfamily M gene expression in the pathogenesis of urinary bladder
cancer. Oncol Lett. 12:5235–5239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sozucan Y, Kalender ME, Sari I, Suner A,
Oztuzcu S, Arman K, Yumrutas O, Bozgeyik I, Cengiz B, Igci YZ, et
al: TRP genes family expression in colorectal cancer. Exp Oncol.
37:208–212. 2015.PubMed/NCBI
|
|
18
|
Narayan G, Bourdon V, Chaganti S,
Arias-Pulido H, Nandula SV, Rao PH, Gissmann L, Dürst M, Schneider
A, Pothuri B, et al: Gene dosage alterations revealed by cDNA
microarray analysis in cervical cancer: Identification of candidate
amplified and overexpressed genes. Genes Chromosomes Cancer.
40:373–384. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Loo SK, Ch'ng ES, Md Salleh MS, Banham AH,
Pedersen LM, Møller MB, Green TM and Wong KK: TRPM4 expression is
associated with activated B cell subtype and poor survival in
diffuse large B cell lymphoma. Histopathology. 71:98–111.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen J, Luan Y, Yu R, Zhang Z, Zhang J and
Wang W: Transient receptor potential (TRP) channels, promising
potential diagnostic and therapeutic tools for cancer. Biosci
Trends. 8:1–10. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Ashida S, Nakagawa H, Katagiri T, Furihata
M, Liizumi M, Anazawa Y, Tsunoda T, Takata R, Kasahara K, Miki T,
et al: Molecular features of the transition from prostatic
intraepithelial neoplasia (PIN) to prostate cancer: Genome-wide
gene-expression profiles of prostate cancers and PINs. Cancer Res.
64:5963–5972. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Berg KD, Soldini D, Jung M, Dietrich D,
Stephan C, Jung K, Dietel M, Vainer B and Kristiansen G: TRPM4
protein expression in prostate cancer: A novel tissue biomarker
associated with risk of biochemical recurrence following radical
prostatectomy. Virchows Arch. 468:345–355. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Trpkov K and Hes O: New and emerging renal
entities: A perspective post-WHO 2016 classification.
Histopathology. 74:31–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu L, Qian J, Singh H, Meiers I, Zhou X
and Bostwick DG: Immunohistochemical analysis of chromophobe renal
cell carcinoma, renal oncocytoma, and clear cell carcinoma: An
optimal and practical panel for differential diagnosis. Arch Pathol
Lab Med. 131:1290–1297. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Delahunt B and Eble JN: Papillary renal
cell carcinoma: A clinicopathologic and mmunohistochemical study of
105 tumors. Mod Pathol. 10:537–544. 1997.PubMed/NCBI
|
|
26
|
Hong X and Yu JJ: MicroRNA-150 suppresses
epithelial mesenchymal transition, invasion and metastasis in
prostate cancer through the TRPM4-mediated β-catenin signaling
pathway. Am J Physiol Cell Physiol. 316:C463–C480. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wong KK and Hussain FA: TRPM4 is
overexpressed in breast cancer associated with estrogen response
and epithelial-mesenchymal transition gene sets. PLoS One.
15(e0233884)2020.PubMed/NCBI View Article : Google Scholar
|