Introduction
Given that bladder cancer (BC) is the most frequent
form of genitourinary tract cancer and taking into account the
serious nature of the presenting symptoms, treatment is urgent, due
to the high recurrence and treatment-related morbidity associated
with the disease (1). Smoking is
the main risk factor for BC, accounting for 50-65% of new cases in
men and 20-30% in women (2).
Occupational exposure to formaldehydes, polycyclic aromatic
hydrocarbons and other solvents is also considered to be a
significant risk factor (3). A
third identified risk factor is the application of a vesical
catheter which, over a long period of time, can lead to chronic
irritation of the vesical mucosa. Untreated vesical lithiasis and
cystitis, as a result of Schistosoma hematobium infection,
have been found to be causally linked to the onset of bladder
squamous cell carcinoma. Urothelial carcinoma (UC), sometimes
referred to as transitional cell carcinoma, is an occurring form of
BC. However, UC has a propensity for disparate differentiation and
presentation of morphological variants, including the sarcomatoid
subtype. This variant has historically been given different names,
such as sarcomatoid carcinoma (SaC), pseudosarcoma, malignant mixed
mesodermal/Müllerian tumor, metaplastic carcinoma and spindle cell
carcinoma. Accounting for ~0.3% of all primary urinary BC cases
(1), SaC has similar
epidemiological characteristics to those of UC, although they
differ in terms of behavior and prognosis (4). As a rare malignant neoplasm of the
urinary bladder, SaC is a high-grade biphasic neoplasm. Bladder SaC
comprises two different components, one malignant epithelial
component and one sarcomatoid component of mesenchymal origin. The
latter component often manifests itself as a high-grade spindle
cell neoplasm, while the former component may manifest itself as
UC, squamous cell carcinoma, adenocarcinoma, small cell carcinoma
or overlying carcinoma in situ. It frequently occurs in
older patients, normally those in their sixth or seventh decade of
life, and is three times more common in men than in women (5). A survey by the Mayo Clinic conducted
between 1936 and 1995 showed that its frequency was higher in men
aged 72 years, who had a history of smoking (6). The precise etiology of SaC has not yet
been established, but radiation therapy and intravesical
cyclophosphamide chemotherapy have been reported to enhance the
risk of SaC and smoking has been identified as a major risk factor
(5). In addition, the definite
histogenesis of SaC is disputed. According to some studies, SaC is
the result of the ability of undifferentiated, totipotential
neoplastic cells to form multiple pathways of terminal
differentiation in histologically recognized mesenchymal and
epithelial elements. This view is based on the immunoreactivity of
epithelial markers [cytokeratin and epithelial membrane antigen
(EMA)] in mesenchymal areas. Other studies have suggested that SaC
results from the ‘collision’ of two distinct malignant tumors that
are mutually invasive. A third explanation is the possible
occurrence of metaplasia of malignant epithelial elements into
sarcomatoid elements. This theory is backed by the evidence of
positive staining for keratins (AE1/AE3) in the epithelial and
mesenchymal components (7,8). The presentation of SaC is similar to
that of urothelial carcinoma, with symptoms such as gross
hematuria, dysuria, pollakiuria and urinary infection.
Macroscopically, these tumors present as large exophytic polyps
(5-7 cm in diameter on average) with areas of necrosis and
ulceration (7). However, the
histopathological diagnosis of SaC can be challenging and it may be
preferable for it to be combined with immunohistochemistry.
Evidence in support of histological diagnosis includes H&E and
immunohistochemical staining results [positive staining for
cytokeratins in the epithelial component and vimentin, desmin
HHF-35, smooth muscle actin (SMA) or S100 calcium binding protein B
in the mesenchymal component, respectively] (7,9,10).
This positive staining can differentiate this variant from other
types of sarcoma (11). In effect,
differential diagnosis of SaC with primary sarcomas, primary
carcinomas with stromal metaplasia, carcinomas with
pseudosarcomatous stroma, sarcomas associated with
pseudoepitheliomatous hyperplasia, teratomas and prostate
carcinomas with extension into the bladder is recommended.
Considering that the incidence of divergent differentiation in
cystectomy specimens is as high as 33%, the fourth edition of the
World Health Organization (WHO) classification of tumors of the
urothelial tract provided a contemporary review of the morphology
of urothelial neoplasms, including SaC, within the group of
invasive urothelial tumors (12).
For this reason, according to the fourth edition of the 2016 WHO
Classification of Tumors of the urinary Systema and Male Genital
Organs, it is recommended that pathologists report the divergent
histologies in their report (12).
The clinical results of SaC are poorer than those of typical UC,
usually including liver, lung and distant lymph node metastases and
a postdiagnosis survival of 6 months. In effect, SaC is frequently
diagnosed at an advanced local stage exhibiting nodal or distant
metastasis. This rules out treatment approaches such as
transurethral resection of bladder tumor or partial cystectomy; for
all these reasons, the identification of this histological variant
is clinically crucial and may require a multimodal approach
(radical cystectomy plus adjuvant chemotherapy) for its treatment
(5,13). The present study reported a rare
example of bladder SaC in a 48-year-old woman without the commonly
recognized risk factors of the variant.
Case report
A 48-year-old woman was admitted to our Urology
Department after undergoing a transurethral bladder resection at
another hospital. Histopathology of the resected Transurethral
Resection of Bladder Tumor chips revealed a pT2 high-grade tumor.
The physical examination and biochemical parameter investigation
came back normal. The patient was not a smoker but had an almost
20-year history of benzodiazepine (BZD) abuse originally prescribed
for treating anxiety and depression. In particular, the patient was
receiving alprazolam at a dose of 1 mg twice daily in combination
with a selective serotonin reuptake inhibitor (SSRI). However, she
reported that, depending on the severity of her depression, she
often exceeded the dosage recommended by the specialist.
Furthermore, upon analyzing her clinical history, it emerged that
the patient had undergone sleep treatment in adolescence. She had
no family history of bladder neoplasms; furthermore, no additional
urological and gynecological symptoms or diseases were identified.
The computed tomography (CT) scan of the chest, abdomen and pelvis
revealed that a huge mass was occupying almost the entire bladder
without metastatic lesions. The mass exhibited enhancement upon the
intravenous administration of contrast material. After signing
written informed consent, the patient underwent radical cystectomy
with bilateral extended pelvic lymphadenectomy, with neobladder
reconstruction performed for urinary diversion. The most effective
surgical technique for muscle-invasive BC is radical cystectomy
which, in female patients, includes the removal of the uterus and
parts of the ventral vagina, thus resulting in sterility (14). The patient underwent a total
cystectomy with lymph node dissection, a total abdominal
hysterectomy, as well as a bilateral salpingo-oophorectomy. During
the surgery, ureters were exposed and split as close as possible to
the urinary bladder; the urinary bladder was then resected and the
uterus and both ovaries were removed. The urethra was carefully
mobilized and exposed for subsequent neobladder anastomosis.
Intraoperative pathology sections of the urethra and both ureters
showed that they were tumor-free. Lymphatic tissue around the
external iliac and internal iliac vessels and obturator nerve was
dissected. The terminal ileum was identified and 45 cm of the ileum
was isolated for the reconstruction of an orthotopic neobladder.
The ureters were placed underneath the bowel in the orthotopic
position. The neobladder remained intact, showing no leaks when
filled with 180 ml sodium chloride. A total of two ureteral stents
and an indwelling catheter were placed intraoperatively. The
follow-up was uneventful. The surgical specimen revealed an
ulcerated polypoid mass with a 6,5 cm diameter, which involved
almost the entire bladder; this mass presented a histopathological
pattern of a high-grade muscle-invasive tumor with heterologous
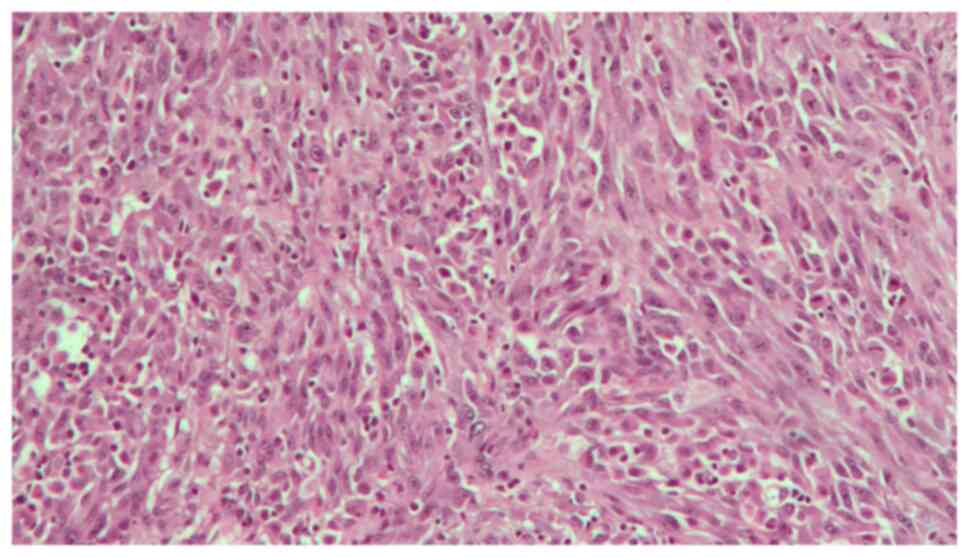
sarcomatoid elements, which consisted of elongated spindled cells
(Fig. 1). Immunohistochemistry
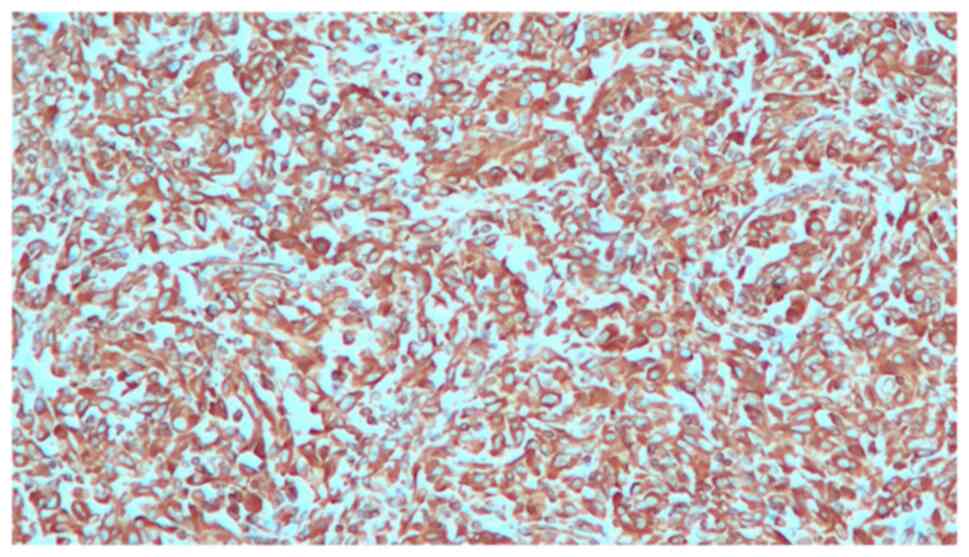
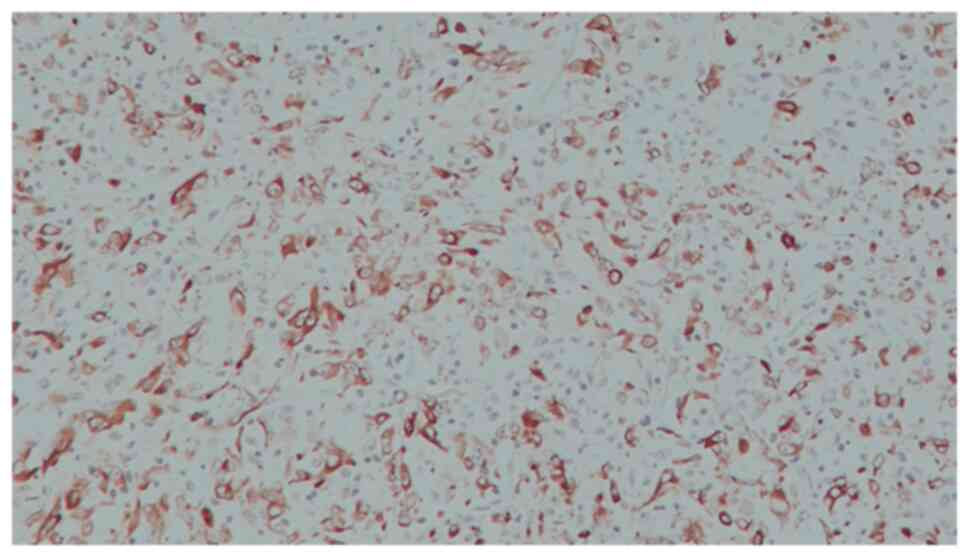
showed that these elements were positive for GATA binding protein
3, vimentin (Fig. 2), cytokeratin
AE1/AE3 (Fig. 3) and EMA. The
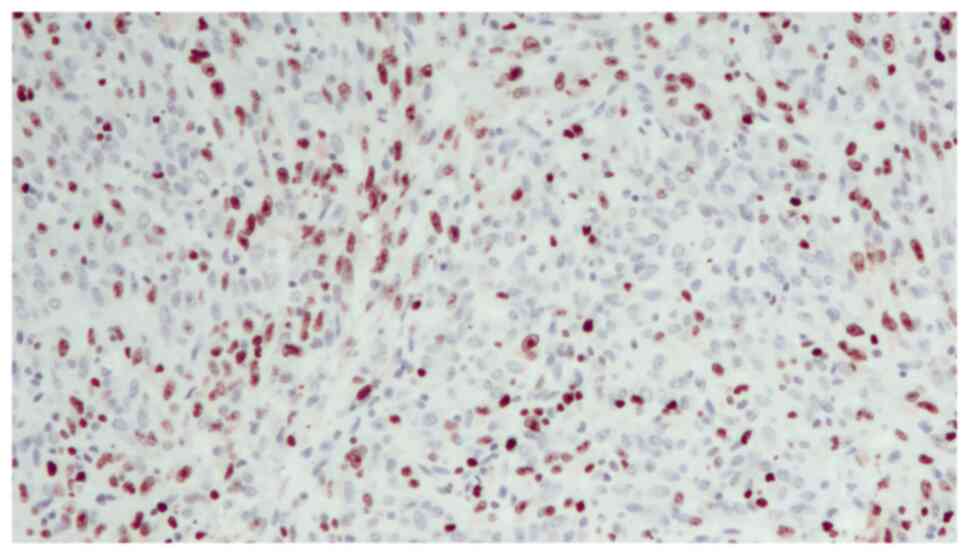
diagnosis reached was that of SaC. In addition, the proliferation
index, which was assessed with anti-Ki67 staining, was high (60%),
indicating the malignant nature of the lesion (Fig. 4). The TNM pathological stage was
pT3a N2 M0 (G3, R0) (15).
Therefore, adjuvant chemotherapy was deemed necessary. At the
6-month follow-up, the patient was alive and in complete remission,
according to CT scans. Urodynamic testing and post-void residual
urine investigation results showed that normal bladder function had
been preserved. Table I summarizes
the clinical and histopathological features of the patient and the
treatment data.
 | Table IClinical and histopathological
features and treatment data. |
Table I
Clinical and histopathological
features and treatment data.
| Clinicopathological
features | Patient |
|---|
| Age, years | 48 |
| Sex | Female |
| Usual risk factor
(smoking, occupational exposure to solvents and bladder chronic
irritation) | None |
| Past history | Benzodiazepine abuse
(for 20 years) |
| Tumour-stage and
grade after endoscopic bladder resection | T2G3 |
| Tumour-stage and
grade after radical treatment (radical cystectomy with lymph node
dissection) | T3aN2M0 (G3) |
| State of surgical
margins (negative or positive) | Negative (R0) |
| Urinary diversion
type | Orthotopic
neobladder |
| Adjuvant treatment
(yes or not) | Yes (adjuvant
chemotherapy) |
| Bladder cancer
subtype | Sarcomatoid bladder
cancer (positivity for GATA3, Vimentin, Cytokeratin AE1/AE2 and
epithelial membrane antigen. |
| Proliferation index
(anti-Ki67) | 60% |
Discussion
UC is one of the most common types of urinary BC,
accounting for ~90% of tumors of this anatomic region. UC is known
for its propensity towards disparate differentiation and
presentation of morphological variants. The most frequent variant
is the squamous variant, followed by the glandular variant
(4). As bladder SaC is a rare and
aggressive subtype of BC (16),
there is a lack of data regarding its clinical outcomes and no
effective treatments for the disease. Following a search of The
Surveillance, Epidemiology and End Results database between 1988
and 2001, only 301 reported bladder SaC cases were identified out
of 182,283 BC cases. This represented a mere 0.16% of all BC cases,
highlighting the rarity of this condition. However, despite its
rarity, this variant is a much more aggressive form of BC.
The patient in the present study and her parents did
not have a history of radiation therapy, cyclophosphamide exposure
or smoking. However, the patient had an ~20-year history of BZD
abuse, originally prescribed for treating anxiety and depression.
In particular, after having been prescribed alprazolam at a dosage
of 1 mg twice daily in combination with an SSRI by a specialist,
the patient reported that she often exceeded the recommended dose,
depending on the severity of her depression. BZDs have been
prescribed on a global scale for ~50 years, with a consumption rate
of 10-42% in the elderly population. An association has been
identified between the use of BZDs and a risk of cancer, but
further research is required; to date, most studies have focused on
cancer in animals exposed to BZDs (17,18).
Some of these studies have reported an association between
carcinoma risks and the use of BZDs, more specifically between
clonazepam and thyroid cancer (19), diazepam and breast cancer (18) and oxazepam and liver cancer
(20). Iqbal et al (21) identified clonazepam, lorazepam,
alprazolam, bromazepam, zolpidem and zopiclone as unsafe BZDs
following exposure duration experiments. In particular, the study
pointed to the high risk of cancer (15%) associated with the use of
clonazepam (21). However, the
researchers admitted that this could possibly be an aggregated
effect resulting from the combination of BZDs with long-term
polypharmacy or metabolic drug use (22). The studies listed the increased risk
for the development of various types of cancer as a result of BZDs
exposure: Overall cancer risk increased by 21%, brain cancer by
98%, colorectal cancer by 25%, lung cancer by 10%, cancer of the
esophagus by 59%, prostate cancer by 36%, BC by 39%, liver cancer
by 18%, pancreatic cancer by 41% and other types of cancer by 27%
(21). These findings have
important implications for BZDs users, particularly considering the
frequency of their prescriptions. The findings of Iqbal et
al were supported by the findings of other studies, such as
those of Rosenberg et al (23), Kripke and Langer (24) and Cronin-Fenton et al
(25). BC is the sixth most common
type of cancer worldwide, with its incidence rates being
considerably high in Taiwan, where BZD usage has been associated
with the risk of cancer (21).
Studies by Kao et al (26)
and Coogan et al (27) found
that BZD use in males was particularly linked to a risk of prostate
cancer.
Despite the rarity of this variant and the
difficulty in reaching a diagnosis, SaC merits specific
investigations, due to its aggressive nature and the poor prognosis
associated with the disease (28).
The best predictor of survival for SaC is the pathological stage at
which it is diagnosed (28).
Favorable prognostic characteristics include negative surgical
margins and metastatic disease absence at first presentation. At
the 2-year stage, the mortality rate is as high as 70%. In the
majority of patients, mortality is often caused by local disease or
by lymph node, lung, pleura, brain, liver or bone metastasis.
Survival prospects are even poorer for sarcomatoid variants than
for organ-confined and metastatic UC. Due to the relative rarity of
the disease and, consequently, the absence of findings from
randomized controlled trials, the experts' opinions with regards to
the most effective treatment are divided. Evidence suggests that
the overall survival rate is higher following radical cystectomy
with pelvic lymphadenectomy (14,29,30).
For this reason, it is considered the principal approach; however,
patients may develop local recurrence following surgery (16). In an attempt to prevent recurrence
and distance metastasis, certain adjuvant treatment approaches,
such as radiotherapy or chemotherapy, are often recommended.
In conclusion, SaC is a rare variant of BC, whose
early detection and accurate diagnosis are key to attaining
satisfactory treatment outcomes and a favorable prognosis for
patients. Histopathology and immunohistochemistry are crucial to
achieving a timely diagnosis and management of this condition. The
present study reported a case of bladder SaC in a 48-year-old
patient, thereby raising awareness for the incidence of this
malignancy in a younger population than usually recorded. The
present case was noteworthy due to the age of the patient and the
total absence of the usual risk factors for SaC. With regards to
the causal factors of this disease, several studies cited herein
have identified a large number of chemical compounds believed to be
carcinogenic. The majority of them are aromatic amines and benzene
derivatives; for that reason, there is a very high likelihood that
long-term BZD abuse played a fundamental role in tumor development
in the reported case.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RB and NS confirm the authenticity of all the raw
data. RB made substantial contributions to conception and design of
this case report and to acquisition of data and was the major
contributor to the writing of the manuscript. MS, NS and CM
analyzed and interpreted the patient data regarding the urological
disease. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Signed informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lucon AM and Falci R Jr: Câncer de Bexiga.
Vol II. Lopes AC (ed). Tratado de ClínicaMédica, São Paulo,
pp2923-2930, 2006.
|
|
2
|
Freedman ND, Silverman DT, Hollenbeck AR,
Schatzkin A and Abnet CC: Association between smoking and risk of
bladder cancer among men and women. JAMA. 306:737–745.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A:
EAU guidelines on muscle-invasive and metastatic bladder cancer:
Summary of the 2013 guidelines. Eur Urol. 65:778–792.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eble JN, Sauter G, Sesterhenn I and
Epstein JI (eds): Pathology and Genetics of Tumors of the Urinary
System and Male Genital Organs. IARC Press, Lyon, pp99-133,
2004.
|
|
5
|
Wang J, Gillaspie C, Kunadharaju R, Talmon
GA and Enke C: Sarcomatoid urothelial carcinoma: A single cancer
center experience. World J Oncol. 2:175–180. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Silva CB, Alves MC, Ribeiro JC, Garcia P
and Santos AR: Carcinoma Sarcomatóide da Bexiga. Acta Urológica.
23:61–64. 2006.
|
|
8
|
Venyo AK and Titi S: Sarcomatoid variant
of urothelial carcinoma (carcinosarcoma, spindle cell carcinoma): A
review of the literature. ISRN Urol. 2014(794563)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J and Rouprêt M: EAU guidelines on
non-muscle-invasive urothelial carcinoma of the bladder: Update
2013. Eur Urol. 64:639–653. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sung MT, Wang M, MacLennan GT, Eble JN,
Tan PH, Lopez-Beltran A, Montironi R, Harris JJ, Kuhar M and Cheng
L: Histogenesis of sarcomatoid urothelial carcinoma of the urinary
bladder: Evidence for a common clonal origin with divergent
differentiation. J Pathol. 211:420–430. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mallik AU, Rahman MZ and Sarker MMR:
Sarcomatoid carcinoma of urinary bladder-a case report. Banglajol.
4:28–29. 2010.
|
|
14
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Liss, New York, NY, 2010.
|
|
16
|
Wright JL, Black PC, Brown GA, Porter MP,
Kamat AM, Dinney CP and Lin DW: Differences in survival among
patients with sarcomatoid carcinoma, carcinosarcoma and urothelial
carcinoma of the bladder. J Urol. 178:2302–2306. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stopper H, Körber C, Spencer DL, Kirchner
S, Caspary WJ and Schiffmann D: An investigation of micronucleus
and mutation induction by oxazepam in mammalian cells. Mutagenesis.
8:449–455. 1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karmali RA, Volkman A, Muse P and Louis
TM: The influence of diazepam administration in rats bearing the
R3230AC mammary carcinoma. Prostaglandins Med. 3:193–198.
1979.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miyawaki I, Moriyasu M, Funabashi H,
Yasuba M and Matsuoka N: Mechanism of clobazam-induced thyroidal
oncogenesis in male rats. Toxicol Lett. 145:291–301.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Iida M, Anna CH, Hartis J, Bruno M,
Wetmore B, Dubin JR, Sieber S, Bennett L, Cunningham ML, Paules RS,
et al: Changes in global gene and protein expression during early
mouse liver carcinogenesis induced by non-genotoxic model
carcinogens oxazepam and Wyeth-14,643. Carcinogenesis. 24:757–770.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Iqbal U, Nguyen PA, Syed-Abdul S, Yang HC,
Huang CW, Jian WS, Hsu MH, Yen Y and Li YJ: Is long-term use of
benzodiazepine a risk for cancer? Medicine (Baltimore).
94(e483)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu W, Tamim H, Shapiro S, Stang MR and
Collet JP: Use of antidepressants and risk of colorectal cancer: A
nested case-control study. Lancet Oncol. 7:301–308. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rosenberg L, Palmer JR, Zauber AG,
Warshauer ME, Strom BL, Harlap S and Shapiro S: Relation of
benzodiazepine use to the risk of selected cancers: Breast, large
bowel, malignant melanoma, lung, endometrium, ovary, non-Hodgkin's
lymphoma, testis, Hodgkin's disease, thyroid, and liver. Am J
Epidemiol. 141:1153–1160. 1995.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kripke DF and Langer RD: Evidence for
harm, comment on ‘Use of benzodiazepines or benzodiazepine related
drugs and the risk of cancer: A population-based case-control
study’. Br J Clin Pharmacol. 78:186–187. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cronin-Fenton DP, Riis AH, Lash TL, Dalton
SO, Friis S, Robertson D and Sørensen HT: Antidepressant use and
colorectal cancer risk: A Danish population-based case-control
study. Br J Cancer. 104:188–192. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kao CH, Sun LM, Su KP, Chang SN, Sung FC,
Muo CH and Liang JA: Benzodiazepine use possibly increases cancer
risk: A population-based retrospective cohort study in Taiwan. J
Clin Psychiatry. 73:e555–e560. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Coogan-PF Rosenberg L, Palmer JR, Strom
BL, Stolley PD, Zauber AG and Shapiro S: Risk of ovarian cancer
according to use of antidepressants, phenothiazines, and
benzodiazepines (United States). Cancer Causes Control. 11:839–845.
2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lopez-Beltran A, Pacelli A, Rothenberg HJ,
Wollan PC, Zincke H, Blute ML and Bostwick DG: Carcinosarcoma and
sarcomatoid carcinoma of the bladder: Clinicopathological study of
41 cases. J Urol. 159:1497–1503. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bansal A, Kumar N and Sharma SC:
Sarcomatoid variant of urothelial carcinoma of the urinary bladder.
J Cancer Res Ther. 9:571–573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mittal V, Rupala KK, Yadav R and
Suryavanshi M: Giant sarcomatoid carcinoma with osseous metaplasia
from urinary bladder diverticulum. Indian J Surg Oncol. 8:436–439.
2017.
|