Introduction
Immune checkpoint inhibitors, such as
anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1
(PD-L1) and anti-cytotoxic T-lymphocyte-associated protein 4
(CTLA-4) antibodies, are widely used for advanced malignant
melanoma, showing clinical efficacy with prolonged survival, and
causing a dramatic change in the treatment strategy for the disease
(1,2). However, there are very few cases of
immune checkpoint inhibitors being used during pregnancy, and their
efficacy and safety in such settings have not yet been established
because they may affect the maternal-to-fetal immune tolerance
system and give rise to some gestational complications as well as
potential harm to the fetus if used during pregnancy (3). In this report, we describe the case of
a patient with advanced malignant melanoma diagnosed at 16 weeks of
gestation who was treated with the anti-PD-1 antibody pembrolizumab
during pregnancy and delivered a live baby at 28 weeks of pregnancy
without serious maternal adverse events, and no neonatal
abnormality was noticed. We also reviewed some reported cases of
the use of immune checkpoint inhibitors during pregnancy for
malignant melanoma in the literature.
Case report
The patient was a 40-year-old woman (gravida 2, para
0). Her medical history included cryotherapy for congenital giant
pigmented nevus in her infancy. There was no family history
warranting special attention. Because of her advanced age, the
patient underwent noninvasive prenatal testing (NIPT) at 10 weeks
of gestation at a local hospital. Chromosome 13 aneuploidy was
detected, and chromosomes 21 and 18 were not reportable, leading to
‘inconclusive result’. Accordingly, the possibility of maternal
malignant tumors was pointed out, and she was referred to our
hospital. A detailed interview and a whole-body examination
revealed that she had noticed a mass in her buttocks. The patient
then visited the dermatology department. On visual examination,
there were scattered pigmented lesions with bluish tones all over
the buttocks and brownish tones on the trunk and extremities.
Palpation revealed a mass on the buttocks. Magnetic resonance
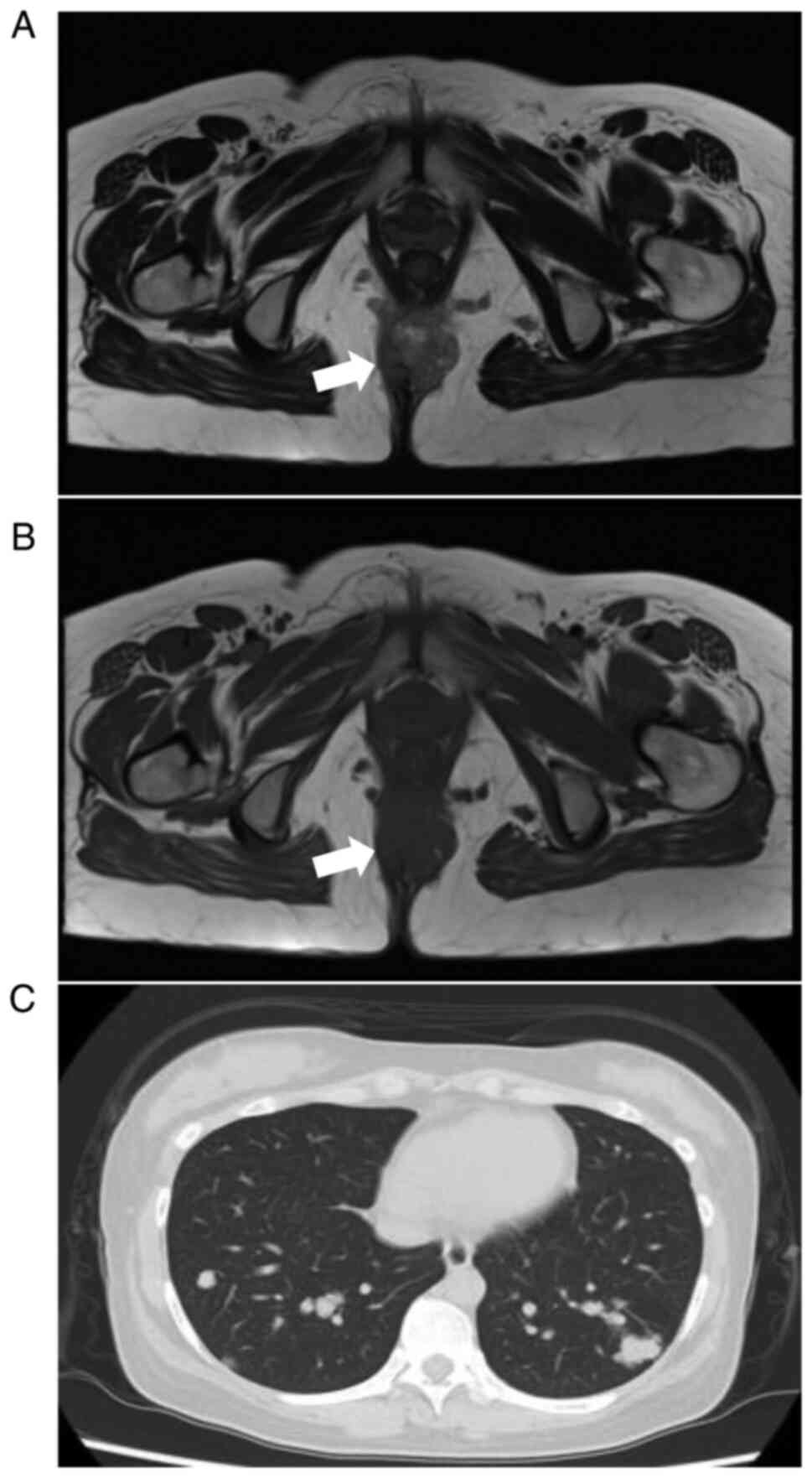
imaging (MRI) of the pelvis at 15 weeks of gestation showed a
lobulated mass with partial high intensity on T1- and T2-weighted
images, high intensity on a diffusion-weighted image, and low
intensity on apparent diffusion coefficient maps within the
subcutaneous fat tissue of the buttocks (Fig. 1A and B), and swelling of the left inguinal lymph
node was also noted. A biopsy of the buttock mass was performed at
16 weeks of gestation, and the pathological findings led to the
diagnosis of malignant melanoma. The amniotic fluid examination at
16 weeks of gestation showed 46XY normal karyotype, with no
chromosomal abnormalities in the fetus. Upon being given an
adequate explanation about the prognosis and future treatment plan
by an obstetrician and a dermatologist, the patient strongly
desired to proceed with pregnancy. On hematological examination,
tumor markers, such as 5-S-CD, CEA, CA19-9, CYFRA, SCC, were all
within the normal range, and there were no abnormal findings in
other parameters. Plain computed tomography (CT) at 20 weeks of
gestation revealed small and large nodules in both lungs and
enlarged lymph nodes at the tracheal bifurcation, indicating lung
and subcarinal lymph node metastases (Fig. 1C). No other distant metastases were
observed.
Based on the above, the patient was diagnosed as
having stage IV primary malignant melanoma of the buttocks
(cT4aN2bM1b) with multiple lung metastases, left inguinal lymph
node metastases, and subcarinal lymph node metastases. Taking into
consideration both the gestational weeks for fetal growth and lung
maturation and the suppression of maternal melanoma progression, we
decided to treat the patient with pembrolizumab alone during
pregnancy, attempting to maintain pregnancy until 28 weeks of
gestation, and institute optimal melanoma treatment after delivery
by cesarean section. Three courses of pembrolizumab therapy (200
mg/body, every 3 weeks) were administered from 21 weeks and 0 days
to 27 weeks of gestation. During the treatment with pembrolizumab,
fetal growth was uneventful and no maternal adverse events were
observed, and the patient underwent cesarean section at 28 weeks
and 0 days of gestation. The newborn was a boy weighing 1,291 g,
with an arterial cord blood gas pH of 7.339, and the 1- and 5-min
Apgar scores were 7 and 8, respectively. The postnatal course was
good, although the newborn was managed in the neonatal intensive
care unit because of preterm birth. Umbilical cord blood showed a
slightly high XX signal of 4.8% on cross-sex fluorescence in situ
hybridization, and the possibility of metastasis of melanoma to the
newborn could not be ruled out. However, there was no evidence of
metastasis in the newborn and his growth was good. The placenta
weighed 316 g, with no grossly obvious melanotic macules being
observed, and pathological findings did not show any tumor
metastasis. At the time of the cesarean section, intraperitoneal
observation showed that the right ovary swelled to 7 cm in size,
and the capsule of the tumor ruptured spontaneously, resulting in
bleeding. There were some black lesions on the posterior surface of
the uterus, but no disseminated lesions were found in the abdominal
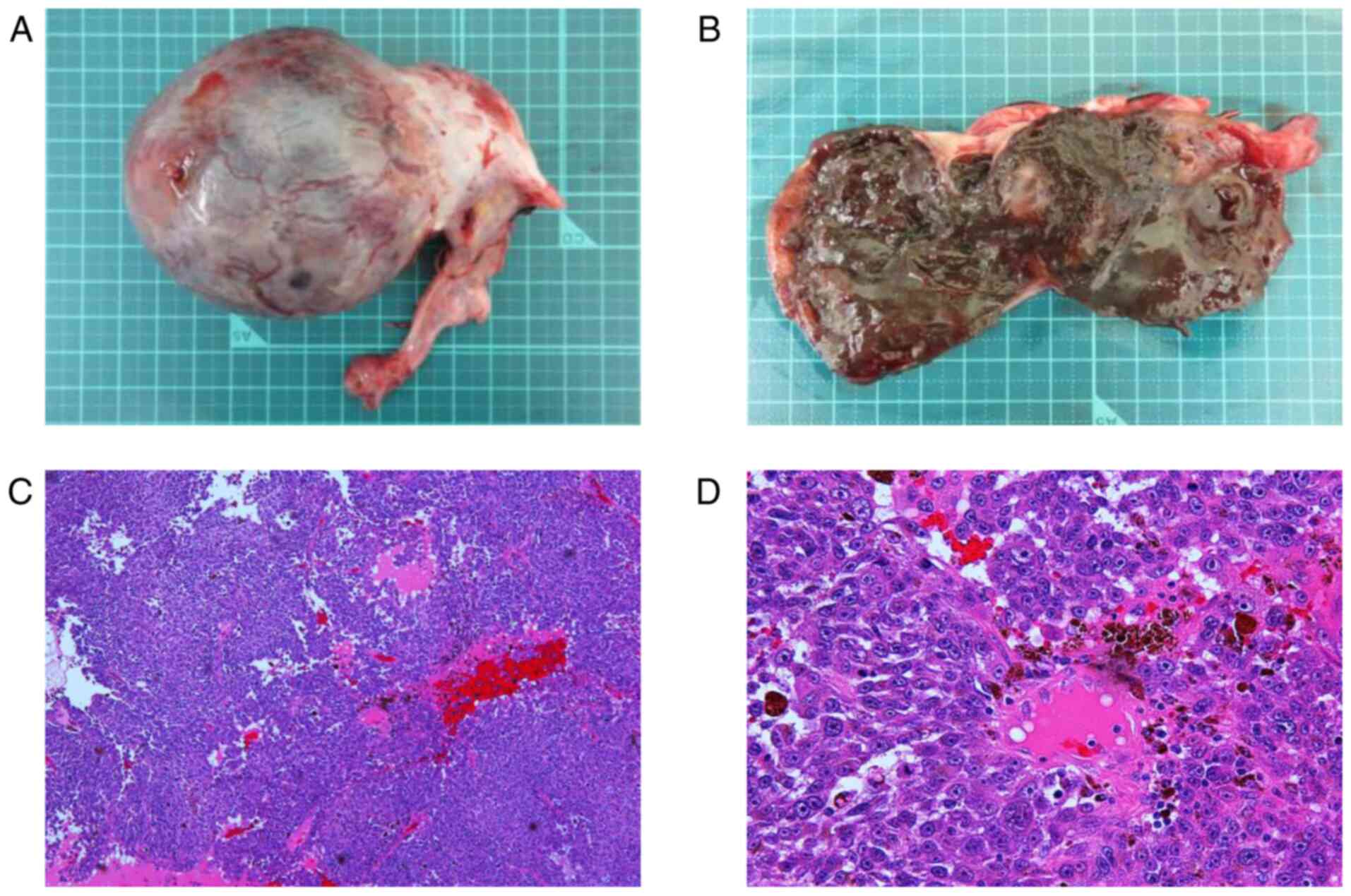
cavity. Right salpingo-oophorectomy was performed. The resected
right adnexa weighed 195 g, and gross examination revealed a black
area and hematoma in the tumor section (Fig. 2A and B). Pathological findings showed that the
tumor was composed of medullary proliferation of atypical cells
with amphophilic cytoplasm and some brown pigment (Fig. 2C and D). These histological images were
congruent with the appearance of malignant melanoma on a biopsy of
the buttocks, and the diagnosis of right ovarian metastasis of
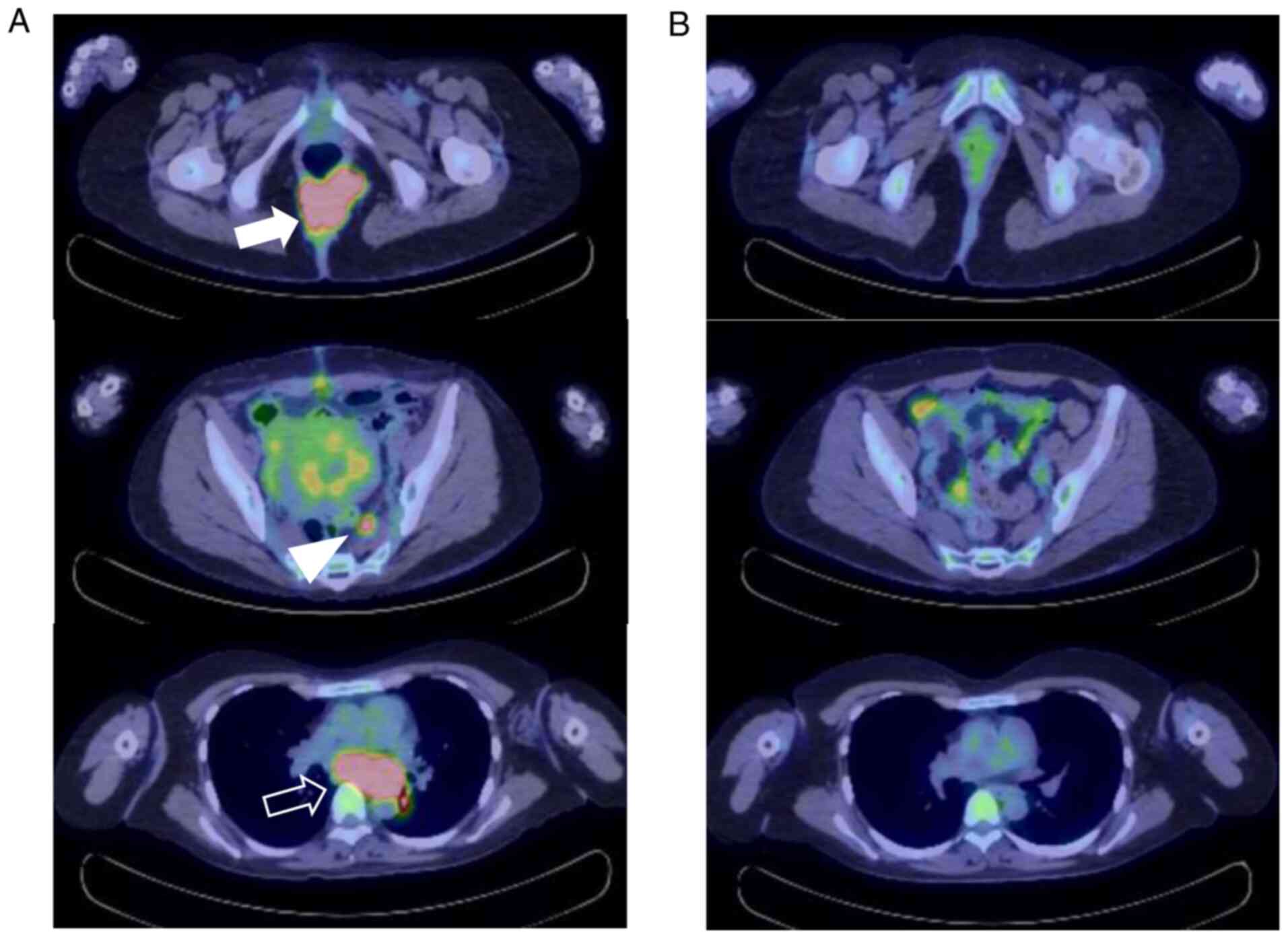
malignant melanoma was made. Postoperative positron emission
tomography-computed tomography (PET/CT) showed accumulation of
fluorodeoxyglucose (FDG) with an SUVmax of 12.09 in the buttock
tumor, the main lesion. FDG accumulation was also observed in the
mediastinal lymph nodes with an SUVmax of 14.61, in the myometrium
with an SUVmax of 7.30, and in the sacral lymph nodes with an
SUVmax of 5.68 (Fig. 3). On
postoperative day 13, administration of the anti-PD-1 antibody
nivolumab (80 mg/body) was started in combination with ipilimumab
(1 mg/kg), an anti-CTLA-4 antibody. This was followed by nivolumab
monotherapy, which was highly effective. Now that 20 months have
elapsed since the cesarean section, the patient is alive with
partial response (PR) being maintained.
Discussion
The incidence of malignant melanoma is on the rise,
and malignant melanoma is reported to account for 8% of
malignancies associated with pregnancy (4). With the advent of molecular-targeted
agents and immune checkpoint inhibitors, the treatment of malignant
melanoma has undergone drastic changes in recent years, with
response rates exceeding 50% (1,2).
Currently, immune checkpoint inhibitors are the first choice of
drug therapy in the absence of BRAF mutations, while combination
therapy with a BRAF inhibitor and a MEK inhibitor or an immune
checkpoint inhibitor is used in the presence of BRAF mutations
(1). Because our patient did not
have a BRAF mutation and the PD-L1 expression rate was 1 to 4%, an
immune checkpoint inhibitor was selected for first-line therapy. At
the time of the initial diagnosis, she was already pregnant and
hoping to have a baby. Due to her stage IV disease, however, early
treatment during pregnancy was considered necessary to restrain
disease progression, which made it difficult for us to figure out
the optimal treatment. While chemotherapy during the first
trimester of pregnancy may increase the risk of fetal malformation,
miscarriage, and stillbirth, it has been reported that the
incidence of fetal malformation is not significantly different
between women on chemotherapy and those with normal pregnancies in
the second and third trimesters (5). On the other hand, it has been reported
that chemotherapy after the second trimester may increase the risk
of fetal growth restriction, premature birth, and complications due
to the prematurity of the newborn (6). After due informed consent, we started
treatment from 21 weeks of gestation, aiming at maintaining the
pregnancy until 28 weeks for the sake of fetal maturation.
In the maternal immune system during pregnancy,
immune tolerance to paternally derived antigens carried by the
fetus is important for maintaining pregnancy. It is known that
immune checkpoint molecules, such as PD-1, CTLA-4, and TIM-3, have
a major impact on maternal immune system (7). Furthermore, regulatory T cells (Treg)
play an important role in maternal immune tolerance, and the
CTLA-4/B7 and PD-1/PD-L1 pathways are involved in the activation of
Treg (8). It has therefore been
discussed that immune checkpoint inhibitors targeting CTLA-4 and
PD-1, which are used in the treatment of malignant melanoma, may
affect the maternal immune system and give rise to some gestational
complications if used during pregnancy. While the US FDA pregnancy
risk category of both pembrolizumab (anti-PD-1) and ipilimumab
(anti-CTLA-4) is ‘Not assigned’ (Use is not recommended) because of
their potential harm to the fetus, no conclusion has been reached
yet. As for animal models, a reproductive and developmental
toxicity study of ipilimumab in pregnant cynomolgus monkeys showed
higher incidences of miscarriage, stillbirth, premature birth, low
birth weight baby, and infant mortality in ipilimumab-treated
animals than in control animals (9).
Reports on the use of immune checkpoint inhibitors
for malignant melanoma in humans during pregnancy, of which there
are few, are summarized in Table I
(10-13).
All four patients received ipilimumab or nivolumab alone or in
combination. Deterioration of the maternal or fetal condition
resulted in premature birth in most cases, but there was no
metastasis to the newborn. One of the four mothers died early after
delivery. In our case, three cycles of pembrolizumab monotherapy
were given during pregnancy, and no fetal effects, such as fetal
malformation or fetal growth restriction, were noted until 28 weeks
of gestation, with no maternal adverse events being observed.
Neither was there any evidence of metastasis of malignant melanoma
to the placenta, and postnatal development of the newborn has been
uneventful so far, with no metastasis being observed. Alexander
et al showed the risk of transplacental metastasis to the
infant in cases with metastatic melanoma during pregnancy (14). In their report, the fetal risk of
melanoma metastasis was 22% when tumor involvement into the
placenta was observed; therefore, the placentas of women with
suspected metastatic melanoma should be carefully examined grossly
and histologically. In our case, the patient actually had the risk
of transplacental melanoma metastasis to her fetus; however, we
chose to maintain pregnancy at least until 28 weeks of gestation
for considering fetal maturation and development.
 | Table IReported cases of the use of immune
checkpoint inhibitors for malignant melanoma during pregnancy. |
Table I
Reported cases of the use of immune
checkpoint inhibitors for malignant melanoma during pregnancy.
| Author (year) | Patient age | Disease stage | Drug used | Mode of delivery and
reason for delivery | Metastasis to the
fetus and placenta | Maternal outcome | Neonatal outcome | (Refs) |
|---|
| Mehta et al
(2018) | 31 | III | Ipilimumab IL-2 | Not reported | None | PD | Normal development,
no metastases | (10) |
| Menzer et al
(2018) | 34 | IV | Ipilimumab
Nivolumab | Cesarean section at
24 weeks; Deterioration of maternal condition | Metastasis to the
placenta No metastasis to the fetus | Death (postoperative
day 1) | Mild motor
development delay, no metastasis | (11) |
| Burotto et al
(2018) | 34 | IV | Ipilimumab
Nivolumab | Cesarean section at
32 weeks; Placental insufficiency | None | PR | Normal development,
no metastases | (12) |
| Xu et al
(2019) | 32 | IV | Nivolumab | Cesarean section at
33 weeks; Fetal growth restriction | None | CR | Congenital
hypothyroidism; Normal development, no metastases | (13) |
In the present case, pembrolizumab therapy during
pregnancy may not have been effective in controlling the
progression of the disease, given the fact that metastatic lesions
were found in the ovaries removed during cesarean section, that the
postpartum PET/CT scan showed no shrinkage of the buttock tumor
compared with the MRI scan during pregnancy, and that the disease
spread to the mediastinal lymph nodes and myometrium. Nevertheless,
the use of immune checkpoint inhibitors may be considered as a
treatment option for advanced malignant melanoma during pregnancy,
because our patient was able to continue pregnancy without adverse
events.
Finally, it is of interest that melanoma could be
diagnosed during pregnancy as a result of NIPT in our case. NIPT is
a test for inferring fetal chromosomal aneuploidy using cell-free
fetal DNA (cfDNA) fragments in maternal blood, and it is primarily
used to detect trisomy 13, trisomy 18, and trisomy 21. The results
of NIPT may be positive, negative, or inconclusive, and the
incidence of inconclusive results is 0.32 to 5.4% (15). In our case, aneuploidy of chromosome
13 was detected, whereas chromosomes 21 and 18 were not reportable,
turning out inconclusive results. The most common reason for
inconclusive NIPT results is a lack of cfDNA in the maternal blood,
but other factors, such as autoimmune disease, chromosomal
aneuploidy other than trisomy 13, 18, and 21, and maternal
malignancy, have also been suggested (16). In fact, there are reports of
incidental detection of occult maternal malignancies by NIPT
(17,18). The present case is rare in that,
while NIPT yielded inconclusive results, amniotic fluid examination
did not show any chromosomal abnormality in the fetus, and further
examination taking into account the possibility of maternal
malignancy led to the diagnosis of advanced malignant melanoma
during pregnancy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA, SM and KI conceived and designed the case
report, and wrote the initial draft of the report. AK, HM, KN, MM
and SN collected the clinical data. TN, NI, NO, YM, SY and YY
analyzed the data from images and pathological examinations. YA and
KI confirmed the authenticity of all the raw data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent for surgery and tissue
collection was obtained from the patient.
Patient consent for publication
Written informed consent for the publication of the
present report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnson DB and Sosman JA: Therapeutic
advances and treatment options in metastatic melanoma. JAMA Oncol.
1:380–386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weiss SA, Wolchok JD and Sznol M:
Immunotherapy of melanoma: Facts and hopes. Clin Cancer Res.
25:5191–5201. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Johnson DB, Sullivan RJ and Menzies AM:
Immune checkpoint inhibitors in challenging populations. Cancer.
123:1904–1911. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stensheim H, Møller B, van Dijk T and
Fosså SD: Cause-specific survival for women diagnosed with cancer
during pregnancy or lactation: A registry-based cohort study. J
Clin Oncol. 27:45–51. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hahn KM, Johnson PH, Gordon N, Kuerer H,
Middleton L, Ramirez M, Yang W, Perkins G, Hortobagyi GN and
Theriault RL: Treatment of pregnant breast cancer patients and
outcomes of children exposed to chemotherapy in utero. Cancer.
107:1219–1226. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cardonick E and Iacobucci A: Use of
chemotherapy during human pregnancy. Lancet Oncol. 5:283–291.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miko E, Meggyes M, Doba K, Barakonyi A and
Szereday L: Immune checkpoint molecules in reproductive immunology.
Front Immunol. 10(846)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang YH and Sun HX: Immune checkpoint
molecules in pregnancy: Focus on regulatory T cells. Eur J Immunol.
50:160–169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Poulet FM, Wolf JJ, Herzyk DJ and Degeorge
JJ: An evaluation of the impact of PD-1 pathway blockade on
reproductive safety of therapeutic PD-1 inhibitors. Birth Defects
Res B Dev Reprod Toxicol. 107:108–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mehta A, Kim KB and Minor DR: Case report
of a pregnancy during ipilimumab therapy. J Glob Oncol. 4:1–3.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Menzer C, Beedgen B, Rom J, Duffert CM,
Volckmar AL, Sedlaczek O, Richtig E, Enk A, Jäger D and Hassel JC:
Immunotherapy with ipilimumab plus nivolumab in a stage IV melanoma
patient during pregnancy. Eur J Cancer. 104:239–242.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Burotto M, Gormaz JG, Samtani S, Valls N,
Silva R, Rojas C, Portino S and de la Jara C: Viable pregnancy in a
patient with metastatic melanoma treated with double checkpoint
immunotherapy. Semin Oncol. 45:164–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu W, Moor RJ, Walpole ET and Atkinson VG:
Pregnancy with successful foetal and maternal outcome in a melanoma
patient treated with nivolumab in the first trimester: Case report
and review of the literature. Melanoma Res. 29:333–337.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alexander A, Samlowski WE, Grossman D,
Bruggers CS, Harris RM, Zone JJ, Noyes D, Bowen GM and Leachman SA:
Metastatic melanoma in pregnancy: Risk of transplacental metastases
in the infant. J Clin Oncol. 21:2179–2186. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mackie FL, Hemming K, Allen S, Morris RK
and Kilby MD: The accuracy of cell-free fetal DNA-based
non-invasive prenatal testing in singleton pregnancies: A
systematic review and bivariate meta-analysis. BJOG. 124:32–46.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Samura O and Okamoto A: Causes of aberrant
non-invasive prenatal testing for aneuploidy: A systematic review.
Taiwan J Obstet Gynecol. 59:16–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bianchi DW, Chudova D, Sehnert AJ, Bhatt
S, Murray K, Prosen TL, Garber JE, Wilkins-Haug L, Vora NL, Warsof
S, et al: Noninvasive prenatal testing and incidental detection of
occult maternal malignancies. JAMA. 314:162–169. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Miyagami K, Matsuoka R, Tokunaka M,
Shirato N, Izumi M, Hirose T and Sekizawa A: A case of Ewing's
sarcoma identified via noninvasive prenatal testing. Clin Case Rep.
8:867–871. 2020.PubMed/NCBI View Article : Google Scholar
|